Abstract
Rat liver endothelial cells in primary cultures at 7 degrees C bind radioactively labelled sodium hyaluronate (HA; Mr 400 000) specifically and with high affinity (Kd = 6 X 10(-11) M). Maximal binding capacity is approx. 10(4) molecules per cell. Inhibition experiments with unlabelled HA and oligosaccharides from HA indicate that each molecule is bound by several receptors acting co-operatively and that the single receptor recognizes a tetra- or hexa-saccharide sequence of the polysaccharide. At 37 degrees C the liver endothelial cells endocytose the HA. The process combines the features of a receptor-mediated and a fluid-phase endocytosis. The rate of internalization does not show any saturation with increasing HA concentration, but is approximately proportional to the polysaccharide concentration at and above the physiological concentration. At 50 micrograms of free HA/l each liver endothelial cell accumulates 0.1 fg of the polysaccharide/min. Fluorescent HA accumulates in perinuclear granules, presumably lysosomes. Degradation products from HA appear in the medium about 30 min after addition of the polysaccharide to the cultures. The radioactivity from HA containing N-[3H]acetyl groups or 14C in the sugar rings is recovered mainly as [3H]acetate and [14C]acetate respectively. Estimations of the capacity of liver endothelial cells to internalize and degrade HA in vitro indicate that these cells may be primarily responsible for the clearance of HA from human blood in vivo.
Full text
PDF



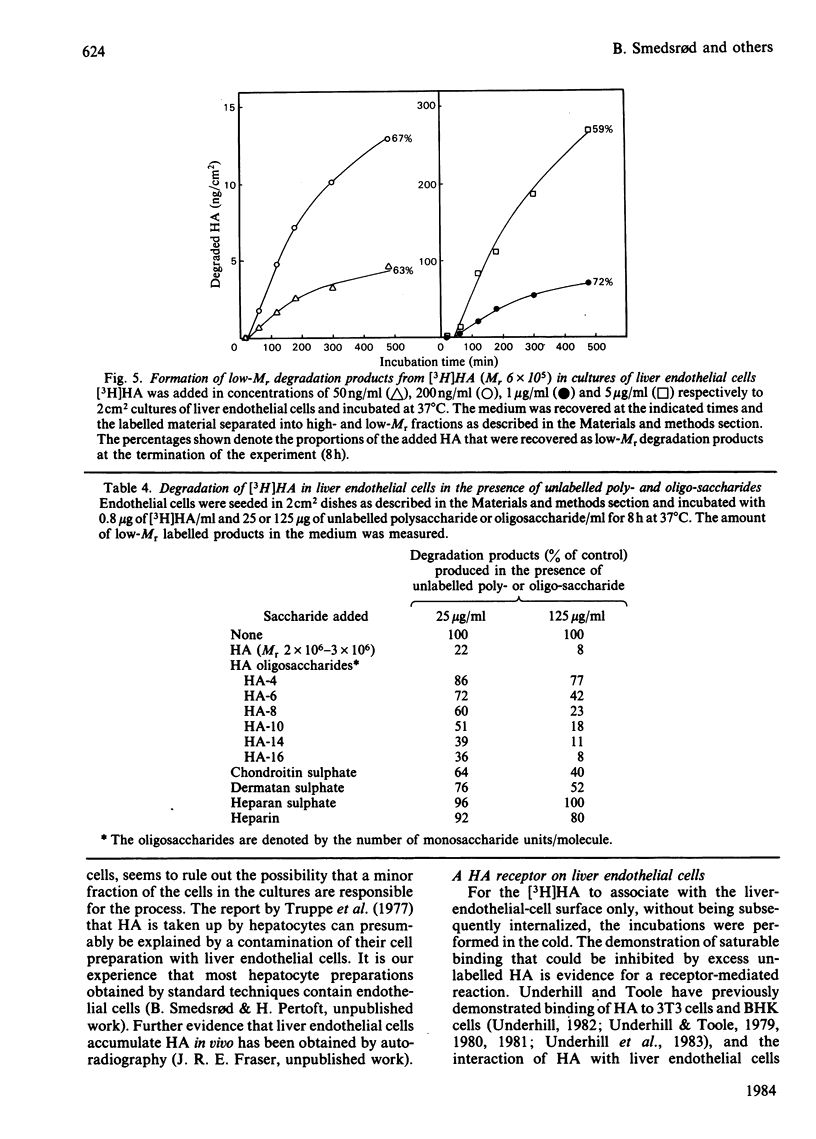
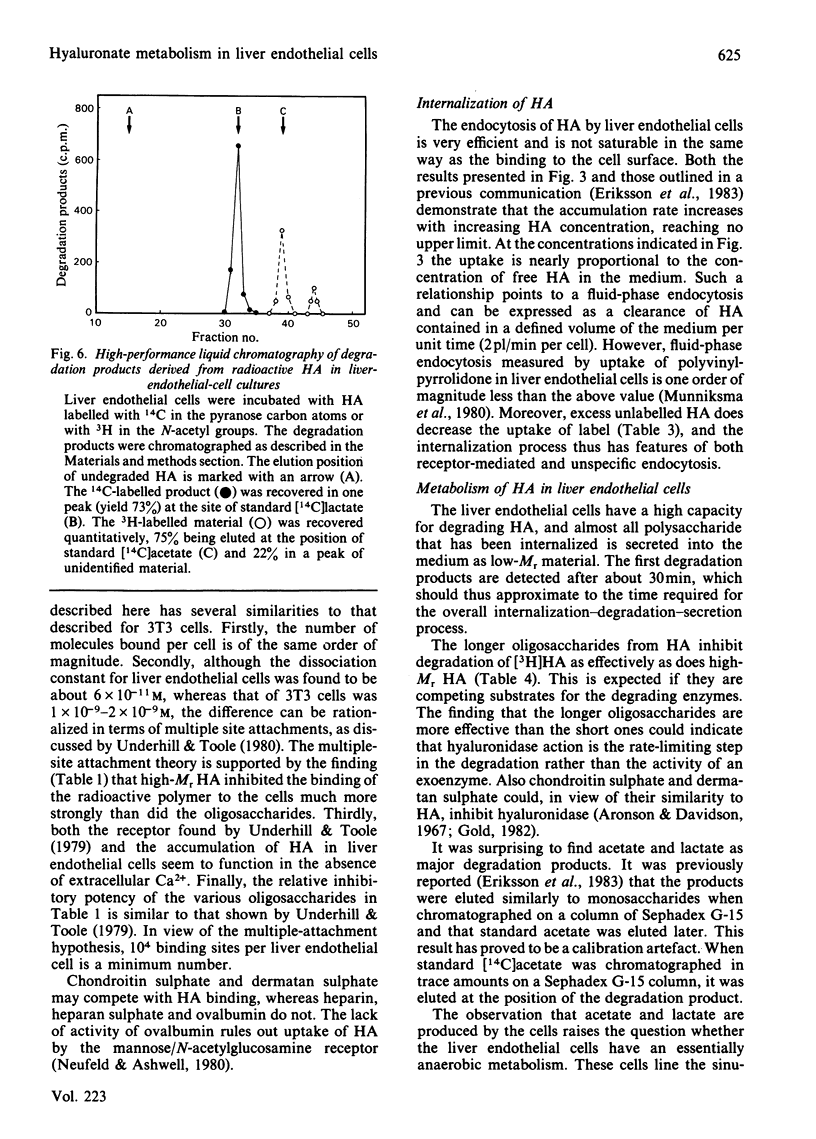

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Davidson E. A. Lysosomal hyaluronidase from rat liver. II. Properties. J Biol Chem. 1967 Feb 10;242(3):441–444. [PubMed] [Google Scholar]
- Eriksson S., Fraser J. R., Laurent T. C., Pertoft H., Smedsrød B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp Cell Res. 1983 Mar;144(1):223–228. doi: 10.1016/0014-4827(83)90458-5. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Appelgren L. E., Laurent T. C. Tissue uptake of circulating hyaluronic acid. A whole body autoradiographic study. Cell Tissue Res. 1983;233(2):285–293. doi: 10.1007/BF00238296. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Laurent T. C., Engström-Laurent A., Laurent U. G. Elimination of hyaluronic acid from the blood stream in the human. Clin Exp Pharmacol Physiol. 1984 Jan-Feb;11(1):17–25. doi: 10.1111/j.1440-1681.1984.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Laurent T. C., Pertoft H., Baxter E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem J. 1981 Nov 15;200(2):415–424. doi: 10.1042/bj2000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold E. W. Purification and properties of hyaluronidase from human liver. Differences from and similarities to the testicular enzyme. Biochem J. 1982 Jul 1;205(1):69–74. doi: 10.1042/bj2050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Iverius P. H. Coupling of glycosaminoglycans to agarose beads (sepharose 4B). Biochem J. 1971 Oct;124(4):677–683. doi: 10.1042/bj1240677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knook D. L., Sleyster E. C. Isolated parenchymal, Kupffer and endothelial rat liver cells characterized by their lysosomal enzyme content. Biochem Biophys Res Commun. 1980 Sep 16;96(1):250–257. doi: 10.1016/0006-291x(80)91207-3. [DOI] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Laurent U. B., Granath K. A. The molecular weight of hyaluronate in the aqueous humour and vitreous body of rabbit and cattle eyes. Exp Eye Res. 1983 Apr;36(4):481–492. doi: 10.1016/0014-4835(83)90042-8. [DOI] [PubMed] [Google Scholar]
- Laurent U. B., Tengblad A. Determination of hyaluronate in biological samples by a specific radioassay technique. Anal Biochem. 1980 Dec;109(2):386–394. doi: 10.1016/0003-2697(80)90665-x. [DOI] [PubMed] [Google Scholar]
- Munniksma J., Noteborn M., Kooistra T., Stienstra S., Bouma J. M., Gruber M., Brouwer A., Praaning-van Dalen Dalen D., Knook D. L. Fluid endocytosis by rat liver and spleen. Experiments with 125I-labelled poly(vinylpyrrolidone) in vivo. Biochem J. 1980 Nov 15;192(2):613–621. doi: 10.1042/bj1920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya T., Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970 Mar 18;198(3):607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Teien A. N., Abildgaard U., Hök M. The anticoagulant effect of heparan sulfate and dermatan sulfate. Thromb Res. 1976 Jun;8(6):859–867. doi: 10.1016/0049-3848(76)90014-1. [DOI] [PubMed] [Google Scholar]
- Truppe W., Basner R., von Figura K., Kresse H. Uptake of hyaluronate by cultured cells. Biochem Biophys Res Commun. 1977 Sep 23;78(2):713–719. doi: 10.1016/0006-291x(77)90237-6. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Chi-Rosso G., Toole B. P. Effects of detergent solubilization on the hyaluronate-binding protein from membranes of simian virus 40-transformed 3T3 cells. J Biol Chem. 1983 Jul 10;258(13):8086–8091. [PubMed] [Google Scholar]
- Underhill C. B. Interaction of hyaluronate with the surface of simian virus 40-transformed 3T3 cells: aggregation and binding studies. J Cell Sci. 1982 Aug;56:177–189. doi: 10.1242/jcs.56.1.177. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Toole B. P. Binding of hyaluronate to the surface of cultured cells. J Cell Biol. 1979 Aug;82(2):475–484. doi: 10.1083/jcb.82.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill C. B., Toole B. P. Physical characteristics of hyaluronate binding to the surface of simian virus 40-transformed 3T3 cells. J Biol Chem. 1980 May 25;255(10):4544–4549. [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. Properties of fractionated chondroitin sulphate from ox nasal septa. Biochem J. 1971 May;122(4):477–485. doi: 10.1042/bj1220477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970 Apr;31(1):125–150. doi: 10.1016/s0022-5320(70)90150-4. [DOI] [PubMed] [Google Scholar]
- de Belder A. N., Wik K. O. Preparation and properties of fluorescein-labelled hyaluronate. Carbohydr Res. 1975 Nov;44(2):251–257. doi: 10.1016/s0008-6215(00)84168-3. [DOI] [PubMed] [Google Scholar]