Abstract
Age-related differences in cortical microstructure are used to understand the neuronal mechanisms that underlie human brain ageing. The cerebral vasculature contributes to cortical ageing, but its precise interaction with cortical microstructure is poorly understood. In a cross-sectional study, we combine venous imaging with vessel distance mapping to investigate the interaction between venous distances and age-related differences in the microstructural architecture of the primary somatosensory cortex, the primary motor cortex and additional areas in the frontal cortex as non-sensorimotor control regions. We scanned 18 younger adults and 17 older adults using 7 Tesla MRI to measure age-related changes in longitudinal relaxation time (T1) and quantitative susceptibility mapping (QSM) values at 0.5 mm isotropic resolution. We modelled different cortical depths using an equi-volume approach and assessed the distance of each voxel to its nearest vein using vessel distance mapping. Our data reveal a dependence of cortical quantitative T1 values and positive QSM values on venous distance. In addition, there is an interaction between venous distance and age on quantitative T1 values, driven by lower quantitative T1 values in older compared to younger adults in voxels that are closer to a vein. Together, our data show that the local venous architecture explains a significant amount of variance in standard measures of cortical microstructure and should be considered in neurobiological models of human brain organisation and cortical ageing.
Keywords: laminar imaging, ultra-high field MRI, iron, arteries, neurodegeneration
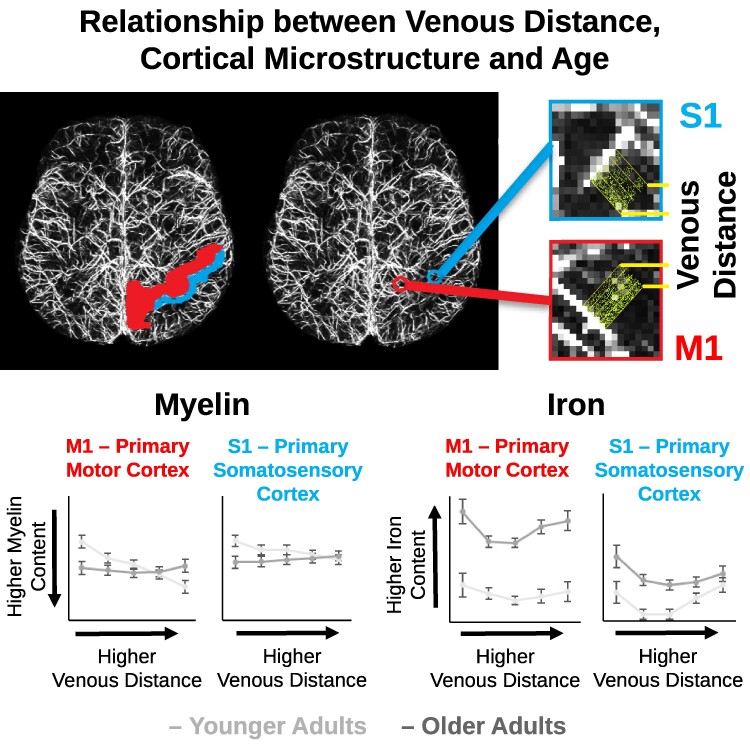
Markers of cortical ageing vary across studies and cohorts. Knoll, Doehler et al. use 7 Tesla MRI and report that markers of microstructural cortical ageing show a dependence on the distance to the nearest vein. This identifies venous distance as a hidden variable of microstructural ageing.
Graphical Abstract
Graphical Abstract.
Introduction
Age-related differences in cortical microstructure are used to understand the neuronal mechanisms that underlie human brain ageing and neurodegeneration. Vascular supply patterns in the brain vary between individuals,1,2 interact with cortical microstructure and functional decline3,4 and contribute to brain pathology.5 However, the interaction between the local vascular architecture and age-related changes in cortical microstructure is poorly understood. This knowledge gap prevents us from understanding the mechanisms that underlie human cortical ageing and potential protective factors.
The human cortex is permeated by a dense net of blood vessels that is highly variable in location and diameter.2 Vascular changes are a risk factor for all-cause dementia,6 and anti-hypertensive treatment can lower dementia incidence.7 A human MRI study reported that participants with cerebral small vessel disease, who show a double-supply pattern of hippocampal vascularisation, perform better in cognitive tests compared to those with a single-supply pattern.3 Vascular health is regarded as a key driver for resilience in ageing, for example by maintaining blood flow and neuronal metabolism.8
However, it is still unclear how individual vascular supply patterns interact with age-related cortical degeneration. Cortical locations that are more distant to larger blood vessels might be more prone to age-related structural alterations, such as myelin loss9-11 and iron accumulation,10,12-14 in case ageing blood vessels reduce supply to locations farther away from major branches (Hypothesis 1, H1). In this view, cortical locations that are closer to a vein may be more protected from age-related decline. Alternatively, myelin loss and iron accumulation might be more pronounced at cortical locations that are closer to blood vessels, because age-related substance accumulation and calcification occur near or within blood vessels15 (Hypothesis 2, H2). In this view, cortical locations that are farther away from a vein may be more protected from age-related decline. Finally, cortical degeneration may occur independently of the distance to blood vessels (Hypothesis 3, H3).
To test these hypotheses, we compared two extreme age groups (healthy younger adults < 30 years, healthy older adults > 65 years) using ultra-high resolution magnetic resonance imaging at 7 Tesla (7T-MRI). Quantitative T1 values (qT1) were used as validated proxy for cortical myelin content.16-18 Quantitative susceptibility mapping (QSM) was used to quantify cortical iron12,13,19-21 (positive QSM, pQSM) and calcium/proteins20,22-26 (negative QSM, nQSM). We focus on the primary somatosensory cortex (S1) and the primary motor cortex (M1) as both areas play an important role in ageing and neurodegeneration, and venous density in S1 and M1 has been associated with myelin loss and declined sensorimotor function in older adults.5 Investigations of S1 and M1 microstructure remain rare, but may explain functional loss in hands and feet in older age, which may impact daily life routines.27 In addition, we investigate several areas in the frontal cortex [superior frontal gyrus (SFG), caudal middle frontal cortex (CMF) and rostral middle frontal cortex (RMF)] as non-sensorimotor control regions (results shown in Supplementary material). QSM images were used for vein identification. Vessel distance mapping (VDM) was used to link age-related changes in cortical microstructure to the distance to the nearest vein.28,29
We combine MRI proxies of cortical myelin, iron and calcium/proteins with VDM to test whether age-related changes in S1 and M1 microstructure show a relation to the distance to the nearest vein. This work contributes to our understanding of how the individual vascular architecture relates to microstructural variance in the ageing cortex, and provides critical insights into the mechanisms of cortical ageing.
Materials and methods
Participants
35 healthy volunteers (n = 18 younger adults: M = 25 years, SD = 3 years, range = 21–29 years, 9 females; n = 17 older adults: M = 70 years, SD = 4 years, range = 65–77 years, 10 females) were recruited from the database of the German Center for Neurodegenerative Diseases, Magdeburg, Germany and participated in our cross-sectional study. All participants were right-handed (laterality index ranging from +40 to +100).30
7T-MRI contraindications, chronic illness, psychiatric and neurological disorders, central acting medications and impaired hand function were exclusion criteria. 1/17 older adults underwent carpal tunnel surgery on both hands. However, no complaints were reported and no outliers were detected in sensorimotor tasks (see Supplementary Table 1).
Regarding vascular health, 6/17 older adults reported a history of hypertension that was well-controlled with specific medication in all cases. Two experienced neurologists evaluated vascular health of older adults according to the Standards for Reporting Vascular Changes on Neuroimaging (STRIVE-2)31,32 and the guideline by Chen et al.33 We detected microbleeds, cortical superficial siderosis, lacunae and/or enlarged perivascular spaces in 6/17 older adults (see Table 1). All anomalies were detected outside S1 and M1. In total, 9/17 older adults showed vascular health risk factors (hypertension and/or signs of cerebral small vessel diseases). Besides, participants showed no medical illnesses. As indicated by the ‘Montreal Cognitive Assessment’ (MoCA),34 there were no signs of cognitive impairments (mean score 28.33 ± 1.45), except for one older adult with a MoCA score of 21 (MoCA ≥ 26 is considered normal34). Because this participant performed equally well in sensorimotor tasks compared with all other older adults (see Supplementary Table 1), we kept the data in the main analysis and performed additional control analyses excluding this participant. There were no professional musicians among the participants.35-37
Table 1.
MoCA scores and vascular health rating for older adults
| Participant | MoCA | Hypertension | Thrombosis | STRIVE-2 criteria | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cerebral microbleeds | cSS | Microinfarcts | Lacunae | EPVS | Composite score | ||||
| 1 | 26 | n | n | n | n | n | n | n | 1 |
| 2 | 21 | n | n | n | n | n | n | n | 1 |
| 3 | 29 | y | n | n | n | n | Subcortical frontal L (1) | n | 2 |
| 4 | 28 | n | n | n | n | n | n | n | 1 |
| 5 | 28 | n | n | Temporal R (1) | Frontal R | n | Frontal L, parietal L + R (3) | n | 4 |
| 6 | 29 | n | n | n | n | n | n | n | 1 |
| 7 | 30 | y | n | n | n | n | Deep WM L + R (3) | n | 2 |
| 8 | 26 | n | n | n | n | n | n | Multiple | 2 |
| 9 | 27 | y | n | n | n | n | n | n | 1 |
| 10 | 28 | y | n | n | n | n | n | n | 1 |
| 11 | 26 | n | n | Temporal R (1) | n | n | n | n | 2 |
| 12 | 26 | n | n | n | n | n | n | n | 1 |
| 13 | - | y | n | n | n | n | n | n | 1 |
| 14 | 28 | n | n | n | n | n | n | n | 1 |
| 15 | 29 | y | n | Occipital R (1) | Parietal R, frontal L + R | n | n | Multiple | 4 |
| 16 | 26 | n | n | n | n | n | n | n | 1 |
| 17 | 30 | n | n | n | n | n | n | n | 1 |
The ‘Montreal Cognitive Assessment’ (MoCA) score is an indicator of cognitive impairment. A MoCA score ≥ 26 is considered normal. Vascular health is given as self-reported history of hypertension (y = yes, n = no), visually detected thrombosis following the guideline by Chen et al.33 and the STRIVE-2 criteria32 (cSS = cortical superficial siderosis; EPVS = enlarged perivascular spaces). In parentheses, the numbers of lesions are detailed. The composite score reflects the sum of positive STRIVE-2 criteria (1 = no positive criterion, 2 = one positive criterion, 3 = two positive criteria, 4 = three positive criteria).
All participants were paid for their attendance and provided written informed consent. The study was approved by the local Ethics Committee of the Otto von Guericke University Magdeburg, Germany. Structural S1 and M1 data were published previously.14,38 However, the venous architecture was not investigated before.
MRI data acquisition
All participants took part in one structural 7T-MRI session. Data were acquired at a Siemens MAGNETOM scanner located in Magdeburg, Germany, using a 32-channel head coil. First, MP2RAGE39 whole-brain images were acquired at 0.7 mm isotropic resolution (240 sagittal slices, field-of-view = 224 mm, repetition time = 4800 ms, echo time = 2.01 ms, inversion time TI1/TI2 = 900/2750 ms, flip angle = 5°/3°, bandwidth = 250 Hz/Px, GRAPPA 2). Second, MP2RAGE part-brain images (optimized for M1 and S1) were acquired at 0.5 mm isotropic resolution (208 transversal slices, field-of-view read = 224 mm, repetition time = 4800 ms, echo time = 2.62 ms, inversion time TI1/TI2 = 900/2750 ms, flip angle = 5°/3°, bandwidth = 250 Hz/Px, GRAPPA 2, slab oversampling = 7.7%). Finally, part-brain susceptibility-weighted images (SWI) were acquired at 0.5 mm isotropic resolution using a 3D GRE sequence40 (208 transversal slices, field-of-view read = 192 mm, repetition time = 22 ms, echo time = 9.00 ms, flip angle = 10°, bandwidth = 160 Hz/Px, GRAPPA 2 and slice oversampling = 7.7%). The total scanning time was around 60 min.
Image pre-processing
We used the same pre-processing pipeline as described previously.14,38 In short, data quality were ensured by two independent raters. QSM images were reconstructed using the Bayesian multiscale dipole inversion algorithm (qsmbox v2.0, https://gitlab.com/acostaj/QSMbox).41 MP2RAGE and non-normalized QSM data13 were processed using JIST42 and CBS Tools43 as plug-ins for MIPAV (https://mipav.cit.nih.gov/).
Slab qT1 images were registered to upsampled whole-brain qT1 images (ANTs v1.9 × embedded in CBS Tools)44 in one single step before slab and whole-brain images were merged. Extra-cranial tissue and dura mater were removed.43 QSM images were registered to the merged whole-brain qT1 images using ITK-SNAP (v3.6.0, www.itksnap.org).
Cortical segmentation was calculated on the UNI image, using the TOADS algorithm.45 To estimate the boundaries between white matter (WM) and grey matter (GM) and between GM and cerebrospinal fluid, the CRUISE algorithm46 was used. Resulting level set surfaces47 were optimized to precisely match M1 and S1 (based on empirical optimisation).14,38
The cortex was divided into 21 cortical depths using the validated equi-volume model.48 After removing the three most superficial and the two deepest cortical depths to reduce partial volume effects,49,50 the remaining 16 depths were averaged into four equally spaced compartments (SF = superficial, OM = outer-middle, IM = inner-middle and DP = deep).49 The resulting cortical depths do not correspond to anatomical layers. Finally, the non-merged high-resolution qT1 and QSM slab data were used to extract qT1 values, pQSM values and nQSM values.
Vessel segmentation and VDM
The Filtered Vessels module (v3.0.4; CBS Tools) was used to extract vessels from the QSMs.43 The vasculature was segmented using a Markov random field diffusion technique. Previous validation studies revealed that this technique captures venous architectures with high precision.51 Resulting vessel probability maps represent the probability of each voxel belonging to a vessel.
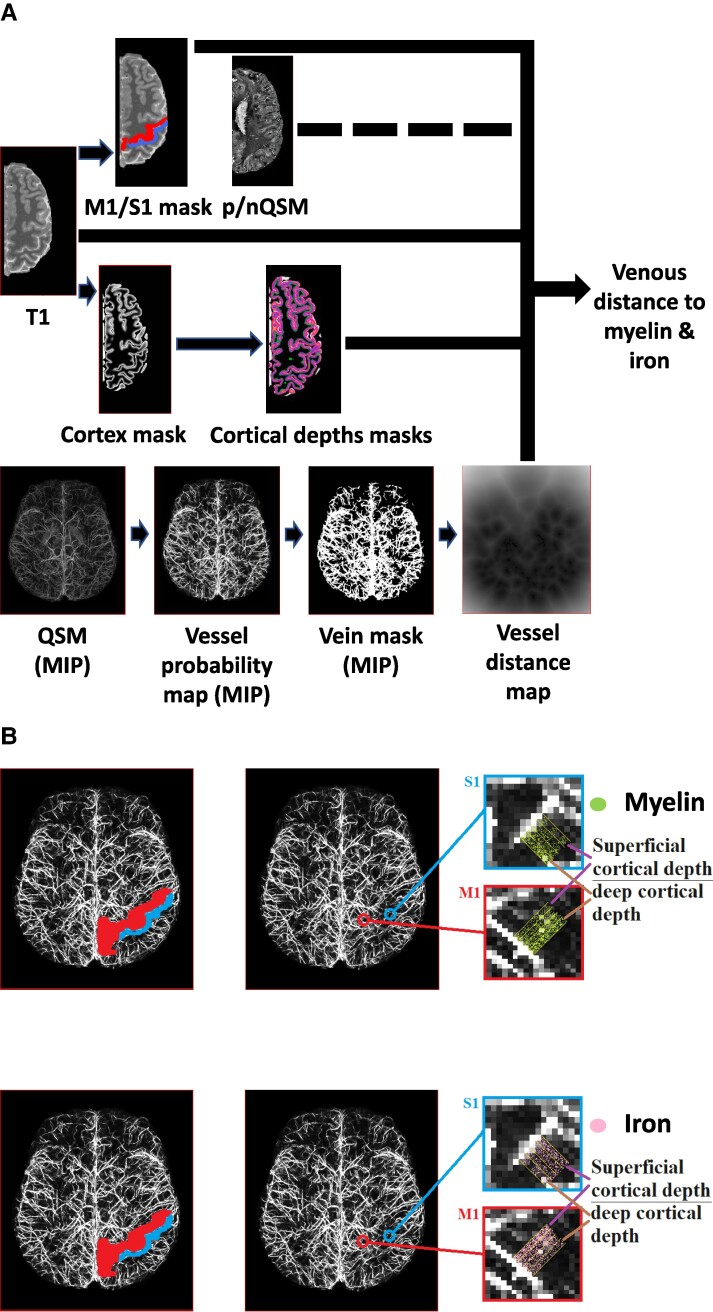
For computing VDMs,29 individual vessel probability maps were binarized. For thresholding, we used the openly available small vessel segmentation pipeline (OMELETTE, https://gitlab.com/hmattern/omelette).52 Thresholds were set using a 3-class Otsu histogram analysis (returning a lower and upper threshold). Voxels above the upper threshold were considered as vessels, voxels below the lower threshold were considered as background. To classify vessels between the lower and upper threshold, hysteresis thresholding was used,53 which considers a voxel belonging to a vessel only if it is connected to a voxel with a value above the upper threshold. A Euclidean distance transformation54 was applied to the thresholded data to compute the distance to the closest segmented vein per voxel. Resulting VDMs were multiplied with binarized cortical depth masks and brain area masks (S1, M1, SFG, CMF and RMF), generating one VDM per cortical depth, brain area and participant (see Fig. 1). qT1, pQSM and nQSM images were multiplied with inverted binarized vessel probability masks (extracted values not overlapping with veins).
Figure 1.
Overview methodology and analysis. (A) Quantitative T1 (qT1) and quantitative susceptibility mapping (QSM) images were sampled at different cortical depths (cortical depths masks) after cortex segmentation (cortex masks). Masks were applied to extract data from the primary motor cortex (M1, red mask) and primary somatosensory cortex (S1, blue mask). As a non-sensorimotor control region, data was extracted from the pre-frontal cortex (covering the superior frontal gyrus, SFG; the caudal middle frontal cortex, CMF; the rostral middle frontal cortex, RMF; not shown). QSM images (displayed as maximum intensity projections, MIP) were used to extract vessel probability maps (including intensity values between 0 and 1) to identify venous vasculature. A hysteresis filter was used to generate binarized vein masks before a Euclidean distance transformation was applied to compute the vessel distance map (VDM). Individual VDMs were multiplied with binarized cortical depth masks and then applied to the qT1 image before resulting parameter maps were multiplied with binarized M1/S1/SFG/CMF/RMF masks (analysis pathway shown as black lines). Please note that the same analysis pathway was applied to the positive QSM (pQSM) and negative QSM (nQSM) data (black dotted line). B: Shown are masks covering left M1 (red) and left S1 (blue) together with the vessel probability map of one example participant. Magnified images show extracted cortical depth compartments in relation to the distance to the nearest vein.
Definition of brain areas
M1 and S1 masks were manually generated using anatomical landmarks,55-58 following a standardized procedure.14,38,59,60 We masked all slices in M1 in which the hand knob was visible,56 and extended the masks until the pre-central gyrus was completely covered.14,59 S1 masks were drawn from the crown of the postcentral gyrus to the fundus of the central sulcus,38 covering area 3b and parts of areas 1 and 3a.55 M1 masks also include medial M1, whereas S1 masks mainly cover lateral S1. Resulting masks were plotted in reference to co-registered Freesurfer labels to ensure overlap with automated approaches.
The masks for SFG, CMF and RMF were generated using Freesurfer (v7.4.1). Whole-brain T1-weighted images (0.7 mm isotropic) were used as input for the recon-all command to segment the cortex based on the Desikan–Killiany atlas61 in individual subject space. Resulting labels were upsampled and registered to the slab images (0.5 mm isotropic). Binarized labels were used as SFG, CMF and RMF masks.
Distance binning
Each cortical VDM was discretized into five bins that represent the distance to the nearest vein (bin 0–2: 0.01–2 mm distance to the nearest vein, bin 2–4: 2.01–4 mm, bin 4–6: 4.01–6 mm, bin 6–8: 6.01–8 mm, bin 8–10: 8.01–10 mm). This was calculated for each voxel in each brain area. Binning was used to obtain different distance conditions for statistical analysis. We chose five bins to include a minimum of 4 voxels per distance condition. Resulting binned data (five VDMs per cortical depth and brain region) was used to extract qT1, pQSM and nQSM values.
Vascular health evaluation
Two experienced neurologists evaluated vascular health of older adults based on visual inspection of MR scans. Following the guideline by Chen et al.,33 thromboses were investigated using part-brain QSM and SWI data. According to the STRIVE-2 recommendations, microbleeds and cortical superficial siderosis were investigated based on part-brain SWI data, whereas lacunae and microinfarcts were examined using whole-brain T1-weighted images.
Behavioural tasks
Outside of the MRI scanner, tactile detection thresholds were assessed using fine hair stimuli, tactile 2-point discrimination performance was assessed using a standard procedure,62-64 and sensorimotor integration performance was assessed with a custom made pressure sensor65 (see Supplementary Methods for details).
Statistical analysis
To test for a main effect of venous distance and an interaction between venous distance and age on cortical microstructure, we calculated mixed-effects ANOVAs on qT1, pQSM and nQSM values with age group (younger adults and older adults) as between-subjects factor, and brain area (M1 and S1), cortical depth (SF, OM, IM and DP) and distance to the nearest vein (bin 0–2, bin 2–4, bin 4–6, bin 6–8 and bin 8–10; the same excluding bin 0–2) as within-subjects factors. The same mixed-effects ANOVAs were calculated with the SFG, CMF and RMF included as additional brain areas; results of these additional analyses are shown in the Supplementary material. Statistical analyses were performed using SPSS (v21.0.) and R (v4.2.2). Sample distributions were tested for normality using Shapiro–Wilk’s test in combination with visual inspections (see Supplementary Figs 1 and 2). Homogeneity of variances was tested with Levene’s test. In case of sphericity violations, Greenhouse-Geisser-corrected results were used. Post hoc tests were performed as two-tailed paired-samples t-tests to follow up within-subjects effects and as two-tailed independent samples Welch t-tests to follow up between-subjects effects. Given the bin representing the closest distance to the nearest vein (bin 0–2) contained no qT1 values in 12/18 younger adults and 4/17 older adults, no pQSM values in 11/18 younger and 8/17 older adults and no nQSM values in 13/18 younger and 8/17 older adults, we report statistical analyses both for five distance conditions with imputed missing values using the method of multiple monotone imputation based on linear regression, and for four distance conditions (excluding bin 0–2). The significance level was set to P ≤ 0.05. In addition to uncorrected P-values, we report Holm–Bonferroni corrected P-values. Generalized Eta-squared (η²G) was calculated as an effect size estimator for ANOVAs.66 Cohen’s benchmarks of 0.06 and 0.14 for medium and large effects, respectively, were applied.67 Hedges-corrected Cohen’s d was calculated as an effects size measure for post hoc t-tests68 to account for small sample sizes (rstatix package v0.7.2). Confidence intervals for Cohen’s d were bootstrapped with the percentile method.69 Cohen’s benchmarks of 0.2, 0.5 and 0.8 for small, moderate and large effects, respectively, were applied.67
To control for a possible effect of vascular health risk on qT1, pQSM and nQSM values in older adults, we calculated random intercept models using the lmer function (lme 4 package v1.1.33). We compared two models (model 1: structural measure ∼ distance to nearest vein + vascular risk + 1/participant; model 2: structural measure ∼ distance to nearest vein + vascular risk + distance to nearest vein: vascular risk + 1/participant) with a null model (structural measure ∼ distance to nearest vein + 1/participant).
Permutation Welch two sample t-tests were calculated using the perm.t.test function (MKinfer package v1.1) with a number of 100 000 Monte-Carlo permutations. Finally, we correlated pQSM and qT1 values for all area-by-distance conditions separately for younger and older adults using Pearson correlation coefficients (misty package v0.6.2).
Results
Age-related differences in cortical myelination interact with the distance to the nearest vein
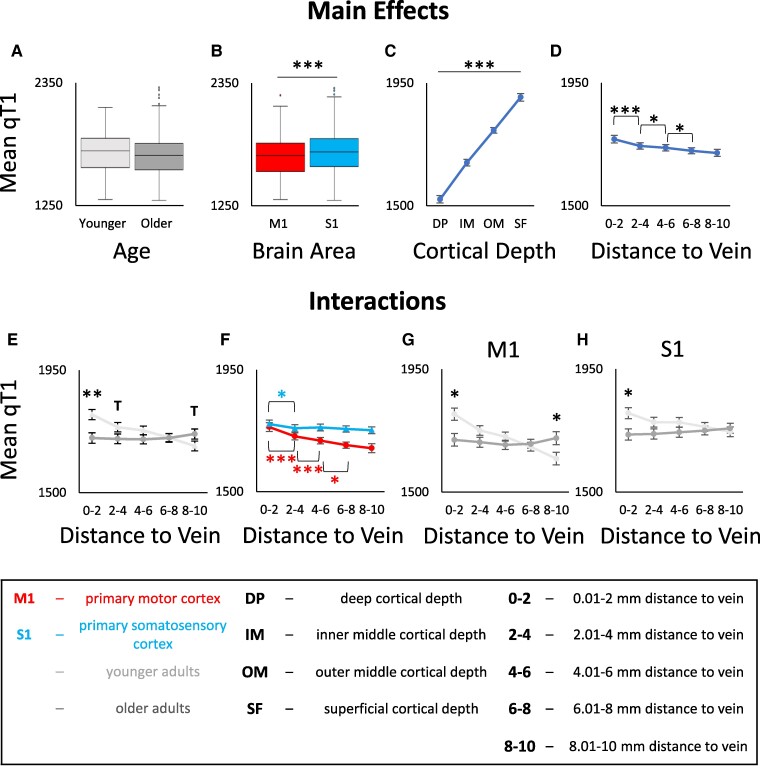
To test for the main effect of venous distance on qT1 values and a possible interaction with age, we computed an ANOVA with the factors age (younger adults and older adults), brain area (S1 and M1), cortical depth (SF, OM, IM and DP) and venous distance (bin 0–2, bin 2–4, bin 4–6, bin 6–8 and bin 8–10) on qT1 values. As expected, there is a significant main effect of brain area driven by lower qT1 values (higher myelination) in M1 compared to S1, and a significant main effect of cortical depth driven by lower qT1 values (higher myelination) in deeper compared to superficial depths in M1 and S1 (see Table 2 and Fig. 2).
Table 2.
ANOVA results for quantitative T1 (qT1) values
| Effect | Conditions | Mean (ms) | SEM (ms) | Statistics | ||||
|---|---|---|---|---|---|---|---|---|
| DFn | DFd | F | P | ηG² | ||||
| Age | Younger | 1725.97 | 15.94 | 1 | 33 | 1.092 | 0.304 | 0.018 |
| Older | 1702.08 | 16.40 | ||||||
| Brain area | M1 | 1692.42 | 12.71 | 1 | 33 | 24.856 | 1.924*10−5 | 0.056 |
| S1 | 1735.63 | 11.73 | ||||||
| Cortical depth | Deep | 1523.86 | 13.13 | 3 | 31 | 909.332 | 2.285*10−37 | 0.708 |
| Inner middle | 1658.11 | 11.65 | ||||||
| Outer middle | 1776.34 | 10.22 | ||||||
| Superficial | 1897.80 | 13.98 | ||||||
| Distance | 0.01–2 mm | 1743.83 | 13.46 | 4 | 30 | 16.289 | 9.651*10−6 | 0.036 |
| 2.01–4 mm | 1719.03 | 12.16 | ||||||
| 4.01–6 mm | 1712.38 | 11.53 | ||||||
| 6.01–8 mm | 1701.47 | 10.89 | ||||||
| 8.01–10 mm | 1693.41 | 12.87 | ||||||
| Age × Distance | Younger/0.01–2 mm | 1786.91 | 18.76 | 4 | 30 | 25.657 | 7.668*10−8 | 0.056 |
| Younger/2.01–4 mm | 1740.50 | 16.95 | ||||||
| Younger/4.01–6 mm | 1728.96 | 16.06 | ||||||
| Younger/6.01–8 mm | 1702.08 | 15.17 | ||||||
| Younger/8.01–10 mm | 1671.42 | 17.94 | ||||||
| Older/0.01–2 mm | 1700.76 | 19.31 | ||||||
| Older/2.01–4 mm | 1697.57 | 17.44 | ||||||
| Older/4.01–6 mm | 1695.80 | 16.53 | ||||||
| Older/6.01–8 mm | 1700.87 | 15.61 | ||||||
| Older/8.01–10 mm | 1715.39 | 18.46 | ||||||
| Age × Brain area | Younger/M1 | 1700.53 | 17.71 | 1 | 33 | 0.783 | 0.382 | 0.002 |
| Older/M1 | 1684.31 | 18.23 | ||||||
| Younger/S1 | 1751.41 | 16.36 | ||||||
| Older/S1 | 1719.85 | 16.83 | ||||||
| Brain area × Distance | M1/0.01–2 mm | 1738.28 | 16.18 | 4 | 30 | 8.628 | 2.325*10−4 | 0.013 |
| M1/2.01–4 mm | 1704.16 | 12.67 | ||||||
| M1/4.01–6 mm | 1687.94 | 11.69 | ||||||
| M1/6.01–8 mm | 1671.56 | 11.81 | ||||||
| M1/8.01–10 mm | 1660.16 | 16.50 | ||||||
| S1/0.01–2 mm | 1749.39 | 14.49 | ||||||
| S1/2.01–4 mm | 1733.91 | 12.96 | ||||||
| S1/4.01–6 mm | 1736.82 | 12.58 | ||||||
| S1/6.01–8 mm | 1731.39 | 11.47 | ||||||
| S1/8.01–10 mm | 1726.65 | 12.68 | ||||||
| Age × Brain Area × Distance | Younger/M1/0.01–2 mm | 1785.29 | 22.55 | 4 | 30 | 3.714 | 0.024 | 0.006 |
| Younger/M1/2.01–4 mm | 1725.63 | 17.66 | ||||||
| Younger/M1/4.01–6 mm | 1702.89 | 16.30 | ||||||
| Younger/M1/6.01–8 mm | 1666.23 | 16.46 | ||||||
| Younger/M1/8.01–10 mm | 1622.62 | 23.00 | ||||||
| Younger/S1/0.01–2 mm | 1788.53 | 20.20 | ||||||
| Younger/S1/2.01–4 mm | 1755.36 | 18.06 | ||||||
| Younger/S1/4.01–6 mm | 1755.03 | 17.53 | ||||||
| Younger/S1/6.01–8 mm | 1737.92 | 15.99 | ||||||
| Younger/S1/8.01–10 mm | 1720.23 | 17.68 | ||||||
| Older/M1/0.01–2 mm | 1691.27 | 23.21 | ||||||
| Older/M1/2.01–4 mm | 1682.68 | 18.17 | ||||||
| Older/M1/4.01–6 mm | 1672.99 | 16.77 | ||||||
| Older/M1/6.01–8 mm | 1676.88 | 16.94 | ||||||
| Older/M1/8.01–10 mm | 1697.71 | 23.66 | ||||||
| Older/S1/0.01–2 mm | 1710.25 | 20.79 | ||||||
| Older/S1/2.01–4 mm | 1712.45 | 18.58 | ||||||
| Older/S1/4.01–6 mm | 1718.60 | 18.04 | ||||||
| Older/S1/6.01–8 mm | 1724.86 | 16.45 | ||||||
| Older/S1/8.01–10 mm | 1733.07 | 18.19 | ||||||
Results for the mixed-effects ANOVA on quantitative T1 (qT1) values with factors age (n = 18 younger adults, n = 17 older adults), cortical depth (4 depths), brain area (primary somatosensory cortex, S1; primary motor cortex, M1) and venous distance (5 distances). Given are mean qT1 values in milliseconds (Mean in ms) and standard errors of the mean (SEM), degrees of freedom of the numerator (DFn), degrees of freedom of the denominator (DFd), P-values (P) and effect sizes (nG²). P-values ≤ 0.05 are considered as significant. A multiplication operation is marked by an asterisk (*).
Figure 2.
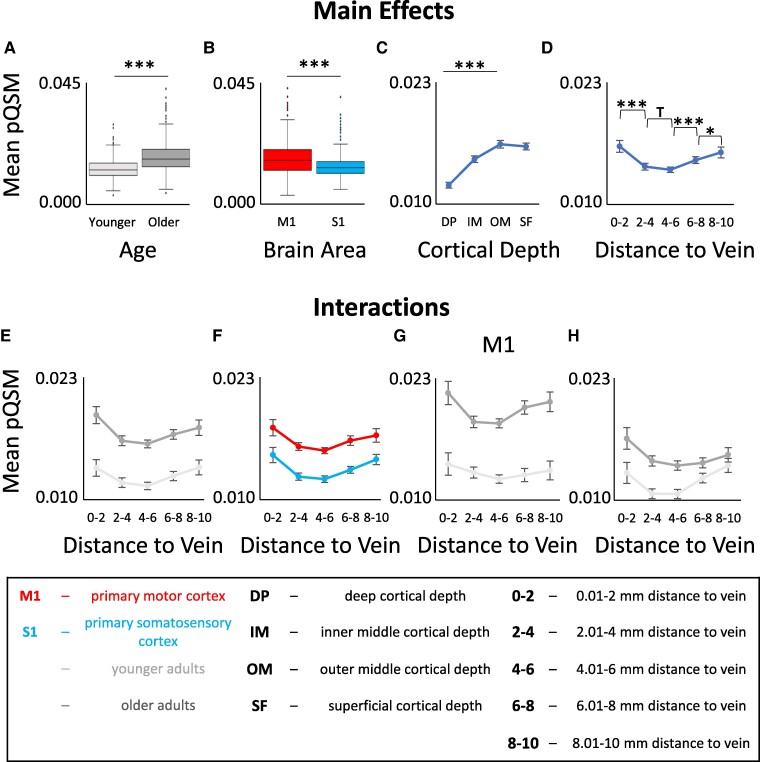
Interaction between age, brain area, cortical depth and venous distance for cortical quantitative T1 (qT1) values. qT1 values are given in milliseconds (lower values indicate higher myelin content). A: No significant main effect of age on qT1 values. Shown are medians, interquartile ranges and lower and upper quartiles for younger (n = 18, light grey) and older (n = 17, dark grey) adults. Dots above a box mark outliers. B: Significant main effect of brain area (i.e. primary motor cortex, M1 (red) and primary somatosensory cortex, S1 (blue)) on qT1 values. C: Significant main effect of cortical depth on qT1 values; dots represent mean qT1 values for the different cortical depths averaged across age groups, distances and brain areas. Error bars show standard errors of the mean (SEM). D: Significant main effect of venous distance on qT1 values (0–2 = 0.01–2 mm, 2–4 = 2.01–4 mm, 4–6 = 4.01–6 mm, 6–8 = 6.01–8 mm, 8–10 = 8.01–10 mm). E: Significant interaction effect between venous distance and age on qT1 values. F: Significant interaction effect between venous distance and brain area on qT1 values. G/H: Significant interaction effect between venous distance, age and brain area (G: data for M1, H: data for S1). Significant results of post hoc t-tests to follow-up mixed-effects ANOVA results (see Table 2 for statistical results) are marked by asterisks: * P ≤ 0.05, ** P ≤ 0.005, *** P ≤ 0.0005 (uncorrected). Trends above P = 0.05 are marked by a T.
There is also a significant main effect of venous distance driven by significantly higher qT1 values (reduced myelination) closer compared to farther away from a vein (significant between bin 0–2 and bin 2–4 (t(34) = 4.428, P = 9.353*10−5, PHolm–Bonferroni = 3.74*10−4, d = 0.74), between bin 2–4 and bin 4–6 (t(34) = 2.710, P = 0.010, PHolm–Bonferroni = 0.03, d = 0.43) and between bin 4–6 and bin 6–8 (t(34) = 2.371, P = 0.024, PHolm–Bonferroni = 0.048, d = 0.38; see Table 2 and Fig. 2D). The venous distance explains 3.6% of the variance in the cortical qT1 signal.
The significant interaction between brain area and venous distance (see Table 2) reveals that this effect is mainly driven by M1 rather than by S1. Post hoc t-tests show that in M1, qT1 values are significantly higher in bin 0–2 compared to bin 2–4 (t(34) = 4.190, P = 1.871*10−5, PHolm–Bonferroni = 7.484*10−5, d = 0.69), in bin 2–4 compared to bin 4–6 (t(34) = 4.443, P = 8.952*10−5, PHolm–Bonferroni = 2.686*10−4, d = 0.73) and in bin 4–6 compared to bin 6–8 (t(34) = 2.640, P = 0.012, PHolm–Bonferroni = 0.024, d = 0.45). In S1, qT1 values are only higher in bin 0–2 compared to bin 2–4, however, this result does not survive Holm–Bonferroni correction (t(34) = 2.402, P = 0.022, PHolm–Bonferroni = 0.088, d = 0.40). All other comparisons are not significant (bin 2–4 versus bin 4–6: t(34)=−0.823, P = 0.416, d = −0.14; bin 4–6 versus 6–8: t(34) = 1.063, P = 0.295, d = 0.18; bin 6–8 versus bin 8–10: t(34) = 0.790, P = 0.435, d = 0.13).
We then explored potential age effects and detected no significant main effect of age and no significant interaction between age and brain area (see Table 2 and Fig. 2). However, there is a significant interaction between age and venous distance on qT1 values (see Table 2). Post hoc t-tests reveal significantly lower qT1 values (higher myelination) in older adults compared to younger adults in bin 0–2 (t(23.345) =−3.145, P = 0.004, PHolm–Bonferroni = 0.02, d = −1.05) and a trend in the same direction in bin 2–4 (t(22.878) =−1.756, P = 0.092, d = −0.58), but a reversed trend in bin 8–10 (t(26.499) = 1.745, P = 0.093, d = 0.56; see Fig. 2E).
Finally, a significant three-way interaction between age, brain area and venous distance (see Table 2) reveals that the interaction between age and venous distance is mainly driven by M1 rather than by S1 (see also Fig. 3). In M1, post hoc t-tests reveal significantly lower qT1 values (higher myelination) in older compared to younger adults in bin 0–2 (t(21.015) = −2.845, P = 0.010, PHolm–Bonferroni = 0.05, d = −0.95) and the reverse effect in bin 8–10 (t(33) = 2.276, P = 0.029, PHolm–Bonferroni = 0.116, d = 0.75). The latter does not survive Holm–Bonferroni correction, but presents with a moderate effect size (see Fig. 3). In S1, post hoc t-tests reveal significantly lower qT1 values (higher myelination) in older compared to younger adults in bin 0–2 (t(33) = −2.701, P = 0.011, PHolm–Bonferroni = 0.05, d = −0.89, see Fig. 2G and H), but not at other distances. These results are confirmed by permutation t-tests (see Supplementary Table 2).
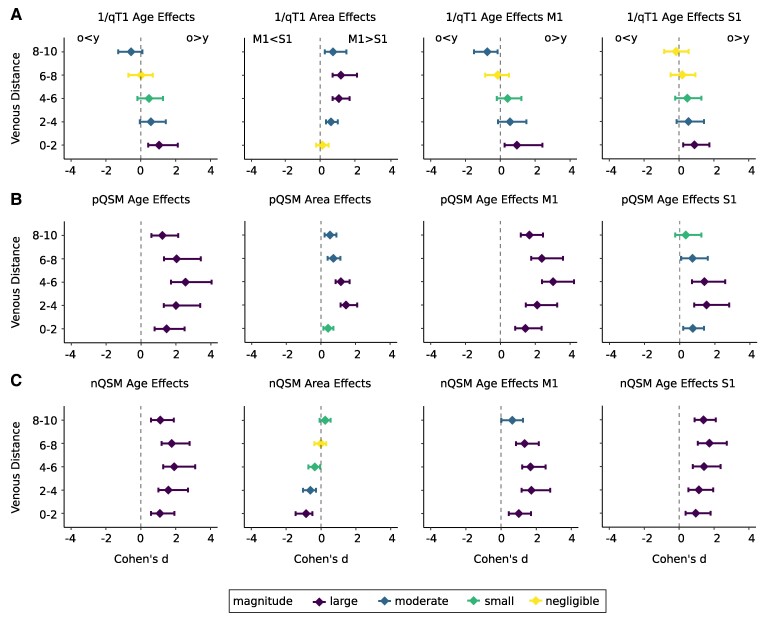
Figure 3.
Effect sizes for the effects of age and brain area on quantitative T1 (qT1), positive QSM (pQSM) and negative QSM (nQSM) values plotted in relation to venous distances. Effect sizes (coloured dots) are given as Cohen’s d (standardized difference between group means), venous distances are given in millimetres. qT1 and nQSM values were reversed before effect size calculations, so that larger effect sizes always indicate higher substance in older compared to younger adults (o > y) and in primary motor cortex (M1) compared to primary somatosensory cortex (S1) (M1 > S1). Different colours indicate the magnitude of the effect sizes (large effects: purple, moderate effects: blue, small effects: green, negligible effects: yellow). Horizontal lines indicate 95% confidence intervals. A: Effect sizes shown for 1/qT1 values. From left to right: age effects averaged across areas (S1 and M1), area effects averaged across age groups (younger and older adults), age effects for M1, age effects for S1. B: Same as in A but effect sizes shown for pQSM values. C: Same as in A but effect sizes shown for nQSM values.
The interaction effects remain significant when excluding the closest bin (0–2, see Supplementary Table 3) and when removing one older participant with a MoCA score below the cut-off for healthy ageing (see Supplementary Table 4).
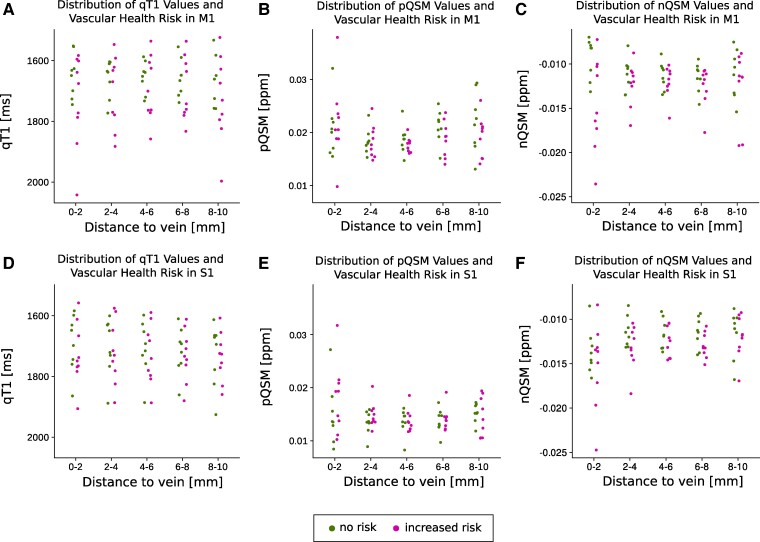
Controlling for effects of vascular health, comparisons of random intercept models reveal no significant effect of vascular health risk on qT1 values (M1: χ2(1) = 1.35, P = 0.245; S1: χ2(1) = 0.25, P = 0.621) and no significant interaction effect between vascular health risk and distance to the nearest vein in older adults (M1: χ2(5) = 5.22, P = 0.389; S1: χ2(5) = 1.70, P = 0.889) (see Fig. 4 for individual values).
Figure 4.
Distribution of vascular health risks in older adults. Individual vascular health risks (i.e. binary variable indicating the presence of hypertension and/or signs of cerebral small vessel diseases according to the STRIVE-2 criteria; green: no risk, magenta: increased risk) shown in relation to the distance to the nearest vein (given in millimetres). A: Distribution of vascular health risks for older adults (n = 17) in primary motor cortex (M1) in relation to quantitative T1 values (qT1, given in milliseconds, i.e. ms). B: Same as in A but for positive QSM values (pQSM, given in parts per million, i.e. ppm). C: Same as in A but for negative QSM values (nQSM, given in ppm). D: Distribution of vascular health risks for older adults in primary somatosensory cortex (S1) in relation to qT1 values. E: Same as in D but for pQSM values. F: Same as in D but for nQSM values.
Cortical iron shows a relationship to the distance to the nearest vein
To test for the main effect of venous distance on pQSM values and a possible interaction with age, we computed the same ANOVA as reported above on pQSM values. As expected, there is a significant main effect of age with higher pQSM values (more iron) in older compared to younger adults, a significant main effect of brain area with higher pQSM values in M1 compared to S1, and a significant main effect of cortical depth driven by reduced pQSM values in deeper cortical depths (see Table 3 and Fig. 5).
Table 3.
ANOVA results for positive QSM (pQSM) values
| Effect | Conditions | Mean | SEM | Statistics | ||||
|---|---|---|---|---|---|---|---|---|
| (ppm) | (ppm) | DFn | DFd | F | P | ηG² | ||
| Age | Younger | 0.0125 | 0.00046 | 1 | 33 | 48.395 | 5.954*10−8 | 0.270 |
| Older | 0.0172 | 0.00048 | ||||||
| Brain area | M1 | 0.0163 | 0.00039 | 1 | 33 | 69.291 | 1.292*10−9 | 0.131 |
| S1 | 0.0134 | 0.00036 | ||||||
| Cortical depth | Deep | 0.0120 | 0.00032 | 3 | 31 | 152.340 | 1.384*10−24 | 0.172 |
| Inner middle | 0.0148 | 0.00035 | ||||||
| Outer middle | 0.0164 | 0.00041 | ||||||
| Superficial | 0.0162 | 0.00036 | ||||||
| Distance | 0.01–2 mm | 0.0162 | 0.00063 | 4 | 30 | 8.366 | 0.002 | 0.056 |
| 2.01–4 mm | 0.0140 | 0.00037 | ||||||
| 4.01–6 mm | 0.0137 | 0.00029 | ||||||
| 6.01–8 mm | 0.0147 | 0.00036 | ||||||
| 8.01–10 mm | 0.0155 | 0.00057 | ||||||
| Age × Distance | Younger/0.01–2 mm | 0.0134 | 0.00088 | 4 | 30 | 0.612 | 0.508 | 0.004 |
| Younger/2.01–4 mm | 0.0118 | 0.00051 | ||||||
| Younger/4.01–6 mm | 0.0115 | 0.00040 | ||||||
| Younger/6.01–8 mm | 0.0125 | 0.00050 | ||||||
| Younger/8.01–10 mm | 0.0134 | 0.00079 | ||||||
| Older/0.01–2 mm | 0.0190 | 0.00090 | ||||||
| Older/2.01–4 mm | 0.0163 | 0.00052 | ||||||
| Older/4.01–6 mm | 0.0159 | 0.00042 | ||||||
| Older/6.01–8 mm | 0.0169 | 0.00051 | ||||||
| Older/8.01–10 mm | 0.0177 | 0.00082 | ||||||
| Age × Brain area | Younger/M1 | 0.0129 | 0.00055 | 1 | 33 | 33.449 | 1.825*10−6 | 0.068 |
| Older/M1 | 0.0197 | 0.00056 | ||||||
| Younger/S1 | 0.0121 | 0.00050 | ||||||
| Older/S1 | 0.0147 | 0.00052 | ||||||
| Brain area × Distance | M1/0.01–2 mm | 0.0176 | 0.00087 | 4 | 30 | 0.185 | 0.790 | 0.001 |
| M1/2.01–4 mm | 0.0156 | 0.00042 | ||||||
| M1/4.01–6 mm | 0.0152 | 0.00032 | ||||||
| M1/6.01–8 mm | 0.0163 | 0.00050 | ||||||
| M1/8.01–10 mm | 0.0168 | 0.00072 | ||||||
| S1/0.01–2 mm | 0.0147 | 0.00080 | ||||||
| S1/2.01–4 mm | 0.0124 | 0.00038 | ||||||
| S1/4.01–6 mm | 0.0122 | 0.00035 | ||||||
| S1/6.01–8 mm | 0.0132 | 0.00037 | ||||||
| S1/8.01–10 mm | 0.0143 | 0.00053 | ||||||
| Age × Brain Area × Distance | Younger/M1/0.01–2 mm | 0.0138 | 0.00121 | 4 | 30 | 2.293 | 0.120 | 0.011 |
| Younger/M1/2.01–4 mm | 0.0129 | 0.00059 | ||||||
| Younger/M1/4.01–6 mm | 0.0122 | 0.00045 | ||||||
| Younger/M1/6.01–8 mm | 0.0127 | 0.00070 | ||||||
| Younger/M1/8.01–10 mm | 0.0132 | 0.00101 | ||||||
| Younger/S1/0.01–2 mm | 0.0129 | 0.00112 | ||||||
| Younger/S1/2.01–4 mm | 0.0107 | 0.00052 | ||||||
| Younger/S1/4.01–6 mm | 0.0107 | 0.00049 | ||||||
| Younger/S1/6.01–8 mm | 0.0124 | 0.00051 | ||||||
| Younger/S1/8.01–10 mm | 0.0137 | 0.00074 | ||||||
| Older/M1/0.01–2 mm | 0.0214 | 0.00125 | ||||||
| Older/M1/2.01–4 mm | 0.0183 | 0.00061 | ||||||
| Older/M1/4.01–6 mm | 0.0182 | 0.00046 | ||||||
| Older/M1/6.01–8 mm | 0.0199 | 0.00072 | ||||||
| Older/M1/8.01–10 mm | 0.0205 | 0.00104 | ||||||
| Older/S1/0.01–2 mm | 0.0166 | 0.00115 | ||||||
| Older/S1/2.01–4 mm | 0.0142 | 0.00054 | ||||||
| Older/S1/4.01–6 mm | 0.0137 | 0.00051 | ||||||
| Older/S1/6.01–8 mm | 0.0140 | 0.00053 | ||||||
| Older/S1/8.01–10 mm | 0.0148 | 0.00077 | ||||||
Results for the ANOVA on positive QSM (pQSM) values with factors age (n = 18 younger adults, n = 17 older adults), cortical depth (4 depths), brain area (primary somatosensory cortex, S1; primary motor cortex, M1) and venous distance (5 distances). Given are mean pQSM values in parts per million (Mean in ppm) and standard error of the mean (SEM), degrees of freedom of the numerator (DFn), degrees of freedom of the denominator (DFd), P-values (P) and effect sizes (nG²). P-values ≤ 0.05 are considered as significant. A multiplication operation is marked by an asterisk (*).
Figure 5.
Interaction between age, brain region, cortical depth and venous distance for positive QSM (pQSM) values. A: Significant main effect of age on pQSM values. Shown are medians, interquartile ranges and lower and upper quartiles for younger (n = 18, light grey) and older (n = 17, dark grey) adults. Dots mark outliers. pQSM values are given in parts per million (higher values indicate higher iron content). B: Significant main effect of brain area (primary motor cortex, M1 (red); primary somatosensory cortex, S1 (blue)) on pQSM values. C: Significant main effect of cortical depth on pQSM values. Dots indicate mean pQSM values for different cortical depths averaged across age groups, distances and brain areas. Error bars indicate standard errors of the mean (SEM). D: Significant main effect of venous distance on pQSM values (0–2 = 0.01− 2 mm, 2–4 = 2.01–4 mm, 4–6 = 4.01–6 mm, 6–8 = 6.01–8 mm, 8–10 = 8.01–10 mm). E: No significant interaction effect between venous distance and age on pQSM values. F: No significant interaction effect between venous distance and brain area on pQSM values. G/H: No significant interaction effect between venous distance, age and brain area (G: data for M1, H: data for S1). Significant results of post hoc t-tests to follow-up mixed-effects ANOVA results (see Table 3 for statistical results) are marked by asterisks: * P ≤ 0.05, ** P ≤ 0.005, *** P ≤ 0.0005 (uncorrected). Trends above P = 0.05 are marked by a T.
There is also a significant main effect of venous distance (see Table 3 and Fig. 5D) driven by significantly higher pQSM values (more iron) in bin 0–2 compared to bin 2–4 (t(34) = 4.388, P = 1.052*10−4, PHolm–Bonferroni = 4.208*10−4, d = 0.73) and significantly lower pQSM values (less iron) in bin 4–6 compared to bin 6–8 (t(34)=−6.132, P = 5.799*10−7, PHolm–Bonferroni = 1.740*10−6, d = −1.01) and in bin 6–8 compared to bin 8–10 (t(34)=−2.289, P = 0.028, PHolm–Bonferroni = 0.056, d = −0.38). However, the latter result does not survive Holm–Bonferroni correction. In addition, there is a trend towards higher pQSM values in bin 2–4 compared to bin 4–6 (t(34) = 1.715, P = 0.095, d = 0.28). This shows that in both younger and older adults, there is a U-shaped relationship between cortical iron content and venous distance. Other than for qT1 values, this effect is not significantly modulated by brain area (see Table 3 and Fig. 5F). The venous distance explains 5.6% of the variance in the cortical pQSM signal.
Other than for qT1 values, there is no significant interaction between age and venous distance (see Table 3). These results are confirmed by permutation t-tests (see Supplementary Table 5). Instead, age effects differ significantly between brain areas (see Table 3). There are more pronounced age effects in M1 compared to S1 (M1: younger adults minus older adults = −0.0067 ppm, t(33)=−8.860, P = 4.505*10−11, PHolm–Bonferroni = 9.01*10−11, d = −2.80; S1: younger adults minus older adults = −0.0026 ppm, t(33)=−3.416, P = 0.008, PHolm–Bonferroni = 0.008, d = −1.18; see also Fig. 3).
These results are confirmed when removing one older participant with a MoCA score below the cut-off for healthy ageing (see Supplementary Table 6) and when excluding the closest distance (bin 0–2), except that for the latter there is a significant three-way interaction between age, brain area and venous distance (see Supplementary Table 7). This interaction is driven by the less pronounced U-shaped relationship between pQSM values and venous distance when the closest distance is removed. There are age-related differences in cortical iron content that depend on the distance to the nearest vein where this effect is different in M1 versus S1 (M1: more iron in older adults with increasing venous distances (older adults minus younger adults): 2.01–4 mm = 0.0054 ppm, 4.01–6 mm = 0.0059 ppm, 6.01–8 mm = 0.0072 ppm, 8–10 = 0.0073 ppm; S1: more iron in older adults with decreasing venous distances (older adults minus younger adults): 2.01–4 mm = 0.0035 ppm, 4.01–6 mm = 0.0030 ppm, 6.01–8 mm = 0.0016 ppm, 8.01–10 mm = 0.0011 ppm). Taken together, age effects on iron content are stronger in M1 compared to S1. When excluding the closest distance, it becomes apparent that higher iron content in older adults is more pronounced at distances farther away from a vein in M1 and closer to a vein in S1.
Controlling for effects of vascular health, comparisons of random intercept models reveal no significant effect of vascular health risk on pQSM values (M1: χ2(1) = 0.64, P = 0.423; S1: χ2(1) = 0.66, P = 0.418), and no significant interaction effect between vascular health risk and distance to the nearest vein in older adults (M1: χ2(5) = 4.43, P = 0.489; S1: χ2(5) = 5.13, P = 0.400) (see also Fig. 4 for individual values).
Finally, we correlated pQSM values with qT1 values for all area-by-distance conditions separately for younger and older adults (see Supplementary Fig. 3, Supplementary Table 8). Particularly at distances close to the nearest vein (bin 0–2), the results indicate only weak correlations in both age groups.
Cortical nQSM values show a relationship to the distance to the nearest vein
To test for the main effect of venous distance on nQSM values, and a possible interaction with age, we computed the same ANOVA as reported for qT1 and pQSM on nQSM values. As expected, there is a significant main effect of age with more negative nQSM values (higher calcium/protein content) in older adults compared to younger adults (see Fig. 3 for effect sizes). This is confirmed by permutation t-tests (see Supplementary Table 9). There is also a significant main effect of brain area with more negative nQSM values (higher calcium/protein content) in S1 compared to M1 (see Supplementary Table 10, Supplementary Fig. 4G) and a significant main effect of cortical depth driven by more negative nQSM values in deep and superficial compared to middle cortical depth (inverted U-shape, see Supplementary Fig. 4C).
There is also a significant interaction between brain area and venous distance on nQSM values (see Supplementary Table 10). This is driven by a significant effect of venous distance in S1, showing more negative nQSM values in bin 0–2 compared to bin 2–4 (t(34) = −4.196, P = 1.841*10−5, PHolm–Bonferroni = 7.364*10−5, d = −0.69). All other comparisons are not significant or offer trends (more negative nQSM values in bin 6–8 compared to bin 8–10 in S1, t(34)=−1.936, P = 0.061, d = −0.32; less negative nQSM in bin 4–6 compared to bin 6–8 in M1, t(34) = 1.943, P = 0.060, d = 0.32). There is neither a significant interaction between venous distance and age nor between venous distance, age and brain area (see Supplementary Table 10). When excluding the closest distance (bin 0–2), there is still a significant main effect of cortical depth and an interaction between brain area and venous distance, but no main effect of brain area (see Supplementary Table 11).
Controlling for effects of vascular health, comparisons of random intercept models revealed no significant effect of vascular health risk on nQSM values (M1: χ2(1) = 3.21, P = 0.073; S1: χ2(1) = 1.75, P = 0.19). However, there was a significant interaction effect between vascular health risk and distance to the nearest vein on nQSM values in M1, but not in S1 in older adults (M1: χ2(5) = 18.02, P = 0.003; S1: χ2(5) = 3.67, P = 0.598), indicating that older adults with vascular health risk factors exhibit more negative nQSM values in M1 at distances close to the nearest vein (0.01–2 mm) compared to older adults without vascular health risk factors (see also Fig. 4 for individual values).
Interaction between brain microstructure and venous distance is not restricted to sensorimotor cortex
To investigate whether the above reported effects - occur more widely throughout the frontal cortex or are restricted to the sensorimotor system, we conducted additional analyses incorporating the SFG, CMF and RMF. Please note that additional brain areas, for example in the temporal cortex, could not be integrated into these analyses given our slab acquisition did not cover the entire brain. We computed an ANOVA with the factors, age (younger adults and older adults), brain area (S1, M1, SFG, CMF and RMF), cortical depth (SF, OM, IM and DP) and venous distance (bin 0–2, bin 2–4, bin 4–6, bin 6–8 and bin 8–10) on qT1 and pQSM values. We replicate the main effect of venous distance on qT1 values (see Supplementary Fig. 5, Supplementary Table 12) and pQSM values (see Supplementary Fig. 6, Supplementary Table 13), and the significant interaction between age and venous distance on qT1 values (see Supplementary Fig. 5, Supplementary Table 12) when three additional brain areas were integrated into the analyses. Whereas similarities and differences between brain areas for each structural marker can be inspected in detail in Supplementary Figs 5–7 and Supplementary Tables 12–14, the replication of the main effects and interactions show that an influence of the venous architecture on brain microstructure is not restricted to the sensorimotor system.
Discussion
Our data shows a dependence of standard measures of cortical microstructure on the distance to the nearest vein both in younger and older adults. In addition, with respect to ageing, we tested three hypotheses: Cortical locations that are more distant to veins are more prone to age-related degeneration, and cortical locations that are closer to a vein are more protected from age-related decline (H1), cortical locations that are closer to veins are more prone to age-related degeneration and cortical locations that are farther away from a vein are more protected from age-related decline (H2), finally, cortical degeneration occurs independently of the distance to the nearest vein (H3). Whereas H3 is supported for age-related differences in QSM values, an interaction between venous distance and age occurs for qT1 values. When interpreting low qT1 values as markers of high myelin,17 and high qT1 values as markers of low myelin (hence neurodegeneration59), our data supports H1 for cortical myelin. Other interpretations of the lower qT1 values that older adults show compared to younger adults in voxels closer to veins are discussed below. Together, this study shows that the local venous architecture explains a significant amount of variance in standard measures of cortical microstructure and needs to be considered in neurobiological models of cortical ageing and microstructural brain organisation.
We report a significant interaction effect between age and venous distance on qT1 values. The interaction is driven by older adults showing lower qT1 values (interpreted as more myelin)17 in voxels closer to a vein compared to younger adults. In addition, in M1, older adults show higher qT1 values (interpreted as less myelin)59 in voxels farther away from a vein compared to younger adults. This pattern of results, and the interpretation of qT1 values representing intact cortical myelination, confirms H1 for cortical qT1 values. Prior studies show either reduced or higher cortical myelination in older compared to younger adults9-11,70,71 or no significant differences at all.59 Those contrary findings may relate to hidden variables that explain a significant amount of variance but have been disregarded so far. Our data indicate that the venous architecture, specifically the distance to the nearest vein, acts as such a hidden variable and may explain prior contradictory findings on age-related changes in cortical T1 values.
Possible neuronal mechanisms are that increased metabolism close to blood vessels may prevent age-related myelin loss, and/or ageing blood vessels particularly reduce supply to locations that are farther away from major branches. Myelin itself can be a driver for increased metabolism, since it is a highly demanding structure.72 The venous architecture would in this view be a protective structure for older adults’ cortical microstructure, specifically with respect to myelin loss. Perosa et al.3 hypothesized that in the hippocampus, a steady arterial vascular supply constitutes a ‘vascular reserve’ mechanism in cases of pathology, relating to larger hippocampal volume4 and preserved cognitive abilities in patients with cerebral small vessel disease.73 Our results indicate that a similar vascular reserve mechanism can be assigned to veins.
Our study cannot clarify if the lower qT1 values in older adults in cortical locations close to veins indeed reflect higher myelination, and if this is an adaptive or maladaptive process, and/or a marker of vascular reserve, compensation or even pathology. Our finding that older adults with vascular health risk factors exhibit higher nQSM values in M1 at distances close to the nearest vein compared to older adults without vascular health risk factors indicates that the substance close to the vein may also contain markers of calcification and/or protein accumulation. To clarify whether increased substances close to a vein in older adults relate to better or worse cognitive performance and brain health, a larger cohort needs to undergo ultra-high resolution imaging in combination with assessments of cognitive functions and markers of neurodegeneration, such as amyloid and tau PET imaging.74 In case future research would identify the lower qT1 signal in older adults close to veins as maladaptive or as marker of neurodegeneration, H2 would be accepted for qT1. Additionally, investigating patients with exceptionally high or low myelin content close to vessels in a longitudinal study may allow addressing its effect on brain health.
Both younger and older adults show a U-shaped relationship between pQSM values (iron) and venous distances, driven by highest pQSM values at cortical voxels closest and farthest away from a vein. This effect is not significantly modulated by age, confirming H3. However, methodological limitations need to be considered: Whereas we here focus on veins at the mesoscopic scale, it is possible that microvessels located close to larger veins increase pQSM values. Increased pQSM values at distances close to a vein need to be treated with caution and could potentially be driven by partial volume artefacts. Therefore, we recalculated all analyses without the closest distance. When doing so, the cortical myelin effects as described above remain, but there is a significant interaction between age, brain area and venous distance on pQSM values. This is driven by opposing relationships between age-related iron content and venous distance in M1 versus S1: Whereas age-related differences in iron content are larger at greater venous distances in M1, they are larger at smaller venous distances in S1. This would confirm H1 for M1 and H2 for S1, rather than confirming H3. Given the resolution was 0.5 mm isotropic, which limits the amount of small veins that can be classified, the present data cannot clarify the relationship between age-related iron accumulation and venous distance, but warrants further research using higher resolutions (0.35 mm or higher).75
When brain areas are inspected in isolation, differences become apparent. The U-shaped relationship between venous distance and pQSM values is present in the M1, S1, RMF and CMF, but is not present in the SFG. The interaction between venous distance and age on qT1 values is driven by the M1, S1 and CMF, but is not present in the SFG and RMF. The reason for these differences is at present unclear. Given the sensorimotor cortex shows higher myelination than the other three investigated cortical areas, effects of venous distances may be easier to detect in the sensorimotor cortex. However, given the CMF shows an interaction between age and cortical distance, this cannot be the only explanatory factor. Alternatively, the effect of venous architecture might be particularly strong in areas with pronounced age effects, which are almost absent in the RMF and weak in the SFG. In addition, in the SFG, the effect of venous distance on pQSM values shows an opposing direction far away from veins compared to the other investigated areas in younger adults, which may be explained by differences in vascular plasticity76 or connectivity.77 Whereas our results therefore show that the reported effects are not restricted to the sensorimotor cortex, future research should clarify the factors that contribute to areal differences presented in this study.
With respect to further study limitations, the participant number was relatively low but motivated by previous layer-dependent 7T-MRI studies using quantitative in vivo proxies to describe the microstructural cortex architecture,16,49 and is above previously reported sample sizes. To reduce type I and type II errors, we applied corrections for multiple comparisons and reported statistical trends. In addition, we conducted permutation tests, known to be superior to conventional tests when investigating small samples,78,79 and we reported effect sizes. 7T-MRI has superior statistical power compared to MRI at lower field strengths, showing effects at the single participant level80,81 (see Supplementary Figs 1 and 2). Nevertheless, the inclusion of more participants would have benefitted the study with respect to generalisability and robustness. Moreover, the image resolution of 0.5 mm isotropic does not allow identifying the entire venous network. For a comprehensive investigation of the neuronal mechanisms that underlie the interaction between blood vessels and cortical microstructure, also the arterial blood supply patterns need to be investigated, and should be complemented by blood flow assessments. Analyses of time-of-flight MRI measurements would benefit future studies. Moreover, previous studies pointed out that hypertension and alterations in brain vasculature relate to myelin loss and M1 pathology,5,82-85 and 9/17 older adults show global signs of vascular health risk. However, apart from nQSM values at the closest distance in M1, these indicators do not significantly predict S1 and M1 microstructure in older adults, and may therefore not account for the main findings. Assessing the potential impact of hypertension and other vascular disorders on cortical microstructure is an important topic for future studies.
Taken together, we show that the venous architecture interacts with cortical microstructure and age-related changes thereof. This is an important insight as most studies do not analyse cortical alterations as a function of venous or arterial distance, which may lead to misinterpretations of present (or absent) effects. Our study provides evidence for the importance of the human venous architecture in understanding cortical function in health and disease.
Supplementary Material
Contributor Information
Christoph Knoll, Institute of Cognitive Neurology and Dementia Research (IKND), Otto von Guericke University Magdeburg, Magdeburg 39120, Germany; German Center for Neurodegenerative Diseases (DZNE) Magdeburg, Magdeburg 39120, Germany.
Juliane Doehler, Institute of Cognitive Neurology and Dementia Research (IKND), Otto von Guericke University Magdeburg, Magdeburg 39120, Germany; German Center for Neurodegenerative Diseases (DZNE) Magdeburg, Magdeburg 39120, Germany.
Alicia Northall, Institute of Cognitive Neurology and Dementia Research (IKND), Otto von Guericke University Magdeburg, Magdeburg 39120, Germany; German Center for Neurodegenerative Diseases (DZNE) Magdeburg, Magdeburg 39120, Germany.
Stefanie Schreiber, German Center for Neurodegenerative Diseases (DZNE) Magdeburg, Magdeburg 39120, Germany; Center for Behavioral Brain Sciences (CBBS), Otto von Guericke University Magdeburg, Magdeburg 39106, Germany; Department of Neurology, Otto von Guericke University of Magdeburg, Magdeburg 39120, Germany.
Johanna Rotta, Department of Neurology, Otto von Guericke University of Magdeburg, Magdeburg 39120, Germany; Department of Neurology, Katharinenhospital, Klinikum Stuttgart, Stuttgart 70174, Germany.
Hendrik Mattern, German Center for Neurodegenerative Diseases (DZNE) Magdeburg, Magdeburg 39120, Germany; Center for Behavioral Brain Sciences (CBBS), Otto von Guericke University Magdeburg, Magdeburg 39106, Germany; Department Biomedical Magnetic Resonance (BMMR), Otto von Guericke University Magdeburg, Magdeburg 39120, Germany.
Esther Kuehn, Institute of Cognitive Neurology and Dementia Research (IKND), Otto von Guericke University Magdeburg, Magdeburg 39120, Germany; Hertie Institute for Clinical Brain Research (HIH), Tübingen 72076, Germany; German Center for Neurodegenerative Diseases (DZNE) Tübingen, Tübingen 72076, Germany.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
CK was supported by the European Structural and Investment Fund (ESIF)/European Social Fund (ESF) 2014–2020, funding code: ZS/2020/05/141591, purpose: ZOOM-IN. The project was supported by the German Research Foundation (DFG) project number 425899996—SFB 1436. JD was funded by the German Research Foundation (DFG) project number KU 3711/2–1 (423633679) and project number 425899996—SFB 1436. AN was supported by the Else Kröner Fresenius Stiftung: 2019-A03. HM was supported by the German Research Foundation (DFG) project number MA 9235/1–1 (446268581) and MA 9235/3–1 (501214112).
Competing interests
The authors report no competing interests.
Data availability
MRI data available upon request, requirements are a formal data sharing agreement and need to submit a formal project outline. Code for small vessel segmentation hysteresis thresholding: https://gitlab.com/hmattern/omelette
References
- 1. Bernier M, Cunnane SC, Whittingstall K. The morphology of the human cerebrovascular system. Hum Brain Mapp. 2018;39(12):4962–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huck J, Wanner Y, Fan AP, et al. High resolution atlas of the venous brain vasculature from 7 T quantitative susceptibility maps. Brain Struct Funct. 2019;224(7):2467–2485. [DOI] [PubMed] [Google Scholar]
- 3. Perosa V, Priester A, Ziegler G, et al. Hippocampal vascular reserve associated with cognitive performance and hippocampal volume. Brain. 2020;143(2):622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vockert N, Perosa V, Ziegler G, et al. Hippocampal vascularization patterns exert local and distant effects on brain structure but not vascular pathology in old age. Brain Commun. 2021;3(3):fcab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schreiber S, Bernal J, Arndt P, et al. Brain vascular health in ALS is mediated through motor cortex microvascular integrity. Cells. 2023;12(6):957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ungvari Z, Toth P, Tarantini S, et al. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat Rev Nephrol. 2021;17(10):639–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peters R, Xu Y, Fitzgerald O, et al. Blood pressure lowering and prevention of dementia: An individual patient data meta-analysis. Eur Heart J. 2022;43(48):4980–4990. [DOI] [PubMed] [Google Scholar]
- 8. Arenaza-Urquijo EM, Przybelski SA, Lesnick TL, et al. The metabolic brain signature of cognitive resilience in the 80+: Beyond Alzheimer pathologies. Brain. 2019;142(4):1134–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho S, Jones D, Reddick WE, Ogg RJ, Steen RG. Establishing norms for age-related changes in proton T1 of human brain tissue in vivo. Magn Reson Imaging. 1997;15(10):1133–1143. [DOI] [PubMed] [Google Scholar]
- 10. Callaghan MF, Freund P, Draganski B, et al. Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiol Aging. 2014;35(8):1862–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grydeland H, Vértes PE, Váša F, et al. Waves of maturation and senescence in micro-structural MRI markers of human cortical myelination over the lifespan. Cereb Cortex. 2019;29(3):1369–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Acosta-Cabronero J, Betts MJ, Cardenas-Blanco A, Yang S, Nestor PJ. In Vivo MRI mapping of brain iron deposition across the adult lifespan. J Neurosci. 2016;36(2):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Betts MJ, Acosta-Cabronero J, Cardenas-Blanco A, Nestor PJ, Düzel E. High-resolution characterisation of the aging brain using simultaneous quantitative susceptibility mapping (QSM) and R2* measurements at 7T. Neuroimage. 2016;138:43–63. [DOI] [PubMed] [Google Scholar]
- 14. Northall A, Doehler J, Weber M, Vielhaber S, Schreiber S, Kuehn E. Layer-specific vulnerability is a mechanism of topographic map aging. Neurobiol Aging. 2023;128:17–32. [DOI] [PubMed] [Google Scholar]
- 15. Farkas E, de Vos RAI, Donka G, Jansen Steur EN, Mihály A, Luiten PGM. Age-related microvascular degeneration in the human cerebral periventricular white matter. Acta Neuropathol. 2006;111(2):150–157. [DOI] [PubMed] [Google Scholar]
- 16. Dinse J, Härtwich N, Waehnert MD, et al. A cytoarchitecture-driven myelin model reveals area-specific signatures in human primary and secondary areas using ultra-high resolution in-vivo brain MRI. Neuroimage. 2015;114:71–87. [DOI] [PubMed] [Google Scholar]
- 17. Stueber C, Morawski M, Schäfer A, et al. Myelin and iron concentration in the human brain: A quantitative study of MRI contrast. Neuroimage. 2014;93(Pt 1):95–106. [DOI] [PubMed] [Google Scholar]
- 18. Haast RAM, Ivanov D, Formisano E, Uludaǧ K. Reproducibility and reliability of quantitative and weighted T(1) and T(2)(∗) mapping for myelin-based cortical parcellation at 7 Tesla. Front Neuroanat. 2016;10:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012;62(3):1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deistung A, Schäfer A, Schweser F, Biedermann U, Turner R, Reichenbach JR. Toward in vivo histology: A comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. Neuroimage. 2013;65:299–314. [DOI] [PubMed] [Google Scholar]
- 21. Hametner S, Endmayr V, Deistung A, et al. The influence of brain iron and myelin on magnetic susceptibility and effective transverse relaxation—A biochemical and histological validation study. Neuroimage. 2018;179:117–133. [DOI] [PubMed] [Google Scholar]
- 22. Deistung A, Schweser F, Reichenbach JR. Overview of quantitative susceptibility mapping. NMR Biomed. 2017;30(4):e3569. [DOI] [PubMed] [Google Scholar]
- 23. Schweser F, Deistung A, Lehr BW, Reichenbach JR. Differentiation between diamagnetic and paramagnetic cerebral lesions based on magnetic susceptibility mapping. Med Phys. 2010;37(10):5165–5178. [DOI] [PubMed] [Google Scholar]
- 24. Jang J, Nam Y, Jung SW, Riew TR, Kim SH, Kim IB. Paradoxical paramagnetic calcifications in the globus pallidus: An ex vivo MR investigation and histological validation study. NMR Biomed. 2021;34(10):e4571. [DOI] [PubMed] [Google Scholar]
- 25. Kim H, Jang J, Kang J, et al. Clinical implications of focal mineral deposition in the globus Pallidus on CT and quantitative susceptibility mapping of MRI. Korean J Radiol. 2022;23(7):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y, Spincemaille P, Liu Z, et al. Clinical quantitative susceptibility mapping (QSM): Biometal imaging and its emerging roles in patient care. J Magn Reson Imaging. 2017;46(4):951–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kuehn E, Perez-Lopez MB, Diersch N, Döhler J, Wolbers T, Riemer M. Embodiment in the aging mind. Neurosci Biobehav Rev. 2018;86:207–225. [DOI] [PubMed] [Google Scholar]
- 28. Mattern H. Vessel distance mapping of the aging subcortical venous vasculature. Magn Reson Mater Phys Biol Med. 2021;34(Suppl. 1):S190. [Google Scholar]
- 29. Garcia-Garcia B, Mattern H, Vockert N, et al. Vessel distance mapping: A novel methodology for assessing vascular-induced cognitive resilience. Neuroimage. 2023;274:120094. [DOI] [PubMed] [Google Scholar]
- 30. Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 31. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duering M, Biessels GJ, Brodtmann A, et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol. 2023;22(7):602–618. [DOI] [PubMed] [Google Scholar]
- 33. Chen J, Zhang Z, Nie X, et al. Thrombus magnetic susceptibility is associated with recanalization and clinical outcome in patients with ischemic stroke. Neuroimage Clin. 2022;36:103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 35. Ragert P, Schmidt A, Altenmüller E, Dinse HR. Superior tactile performance and learning in professional pianists: Evidence for meta-plasticity in musicians. Eur J Neurosci. 2004;19(2):473–478. [DOI] [PubMed] [Google Scholar]
- 36. Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270(5234):305–307. [DOI] [PubMed] [Google Scholar]
- 37. Schwenkreis P, El Tom S, Ragert P, Pleger B, Tegenthoff M, Dinse HR. Assessment of sensorimotor cortical representation asymmetries and motor skills in violin players. Eur J Neurosci. 2007;26(11):3291–3302. [DOI] [PubMed] [Google Scholar]
- 38. Doehler J, Northall A, Liu P, et al. The 3D structural architecture of the human hand area is nontopographic. J Neurosci. 2023;43(19):3456–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49(2):1271–1281. [DOI] [PubMed] [Google Scholar]
- 40. Haacke EM, Xu Y, Cheng YCN, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004;52(3):612–618. [DOI] [PubMed] [Google Scholar]
- 41. Acosta-Cabronero J, Milovic C, Mattern H, Tejos C, Speck O, Callaghan MF. A robust multi-scale approach to quantitative susceptibility mapping. Neuroimage. 2018;183:7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lucas BC, Bogovic JA, Carass A, et al. The Java Image Science Toolkit (JIST) for rapid prototyping and publishing of neuroimaging software. Neuroinformatics. 2010;8(1):5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bazin PL, Weiss M, Dinse J, Schäfer A, Trampel R, Turner R. A computational framework for ultra-high resolution cortical segmentation at 7Tesla. Neuroimage. 2014;93(Pt 2):201–209. [DOI] [PubMed] [Google Scholar]
- 44. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bazin PL, Pham DL. Topology-preserving tissue classification of magnetic resonance brain images. IEEE Trans Med Imaging. 2007;26(4):487–496. [DOI] [PubMed] [Google Scholar]
- 46. Han X, Pham DL, Tosun D, Rettmann ME, Xu C, Prince JL. CRUISE: Cortical reconstruction using implicit surface evolution. Neuroimage. 2004;23(3):997–1012. [DOI] [PubMed] [Google Scholar]
- 47. Sethian JA. Level set methods and fast marching methods: Evolving interfaces in computational geometry, fluid mechanics, computer vision, and materials science. Cambridge University Press; 1999. https://books.google.de/books?id=ErpOoynE4dIC [Google Scholar]
- 48. Waehnert MD, Dinse J, Weiss M, et al. Anatomically motivated modeling of cortical laminae. Neuroimage. 2014;93(Pt 2):210–220. [DOI] [PubMed] [Google Scholar]
- 49. Kuehn E, Dinse J, Jakobsen E, et al. Body topography parcellates human sensory and motor Cortex. Cereb Cortex. 2017;27(7):3790–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tardif CL, Schäfer A, Waehnert M, Dinse J, Turner R, Bazin PL. Multi-contrast multi-scale surface registration for improved alignment of cortical areas. Neuroimage. 2015;111:107–122. [DOI] [PubMed] [Google Scholar]
- 51. Bazin PL, Plessis V, Fan AP, Villringer A, Gauthier CJ. Vessel segmentation from quantitative susceptibility maps for local oxygenation venography. In: 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI); April 13-16, 2016; Prague, Czech Republic. IEEE; 2016:1135-1138.
- 52. Mattern H. Openly available sMall vEsseL sEgmenTaTion pipelinE (OMELETTE). In: Poster presented at: 29th Annual Meeting of the International Society of Magnetic Resonance in Medicine (ISMRM); May 15-20, 2021; Virtual Meeting. ISMRM; 2021:3745.
- 53. Fraz MM, Basit A, Remagnino P, Hoppe A, Barman SA. Retinal vasculature segmentation by morphological curvature, reconstruction and adapted hysteresis thresholding. In: 2011 7th International Conference on Emerging Technologies (ICET); September 5-6, 2011; Islamabad, Pakistan. IEEE; 2011:1-6. [Google Scholar]
- 54. Maurer CR, Qi R, Raghavan V. A linear time algorithm for computing exact Euclidean distance transforms of binary images in arbitrary dimensions. IEEE Trans Pattern Anal Mach Intell. 2003;25(2):265–270. [Google Scholar]
- 55. Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory Cortex: 1. Microstructural organization and interindividual variability. Neuroimage. 1999;10(1):63–83. [DOI] [PubMed] [Google Scholar]
- 56. Yousry TA, Schmid UD, Alkadhi H, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(Pt 1):141–157. [DOI] [PubMed] [Google Scholar]
- 57. Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci. 2011;31(32):11597–11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Northall A, Doehler J, Weber M, et al. Multimodal layer modelling reveals in vivo pathology in amyotrophic lateral sclerosis. Brain. 2024;147(3):1087–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. [DOI] [PubMed] [Google Scholar]
- 61. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 62. Kalisch T, Tegenthoff M, Dinse HR. Improvement of sensorimotor functions in old age by passive sensory stimulation. Clin Interv Aging. 2008;3(4):673–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kuehn E, Doehler J, Pleger B. The influence of vision on tactile Hebbian learning. Sci Rep. 2017;7(1):9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pleger B, Wilimzig C, Nicolas V, et al. A complementary role of intracortical inhibition in age-related tactile degradation and its remodelling in humans. Sci Rep. 2016;6:27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vieluf S, Godde B, Reuter EM, Voelcker-Rehage C. Age-related differences in finger force control are characterized by reduced force production. Exp Brain Res. 2013;224(1):107–117. [DOI] [PubMed] [Google Scholar]
- 66. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York: Routledge; 1988. [Google Scholar]
- 68. Wasserman S, Hedges L, Olkin I. Statistical methods in meta-analysis. In: Stat Med. 1985;20:75. [Google Scholar]
- 69. Carpenter J, Bithell J. Bootstrap confidence intervals: When, which, what? A practical guide for medical statisticians. Stat Med. 2000;19(9):1141–1164. [DOI] [PubMed] [Google Scholar]
- 70. Seiler A, Schöngrundner S, Stock B, et al. Cortical aging—New insights with multiparametric quantitative MRI. Aging (Albany NY). 2020;12(16):16195–16210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. Intracortical myelin links with performance variability across the human lifespan: Results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci. 2013;33(47):18618–18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hirrlinger J, Nave KA. Adapting brain metabolism to myelination and long-range signal transduction. Glia. 2014;62(11):1749–1761. [DOI] [PubMed] [Google Scholar]
- 73. Pohlack ST, Meyer P, Cacciaglia R, Liebscher C, Ridder S, Flor H. Bigger is better! Hippocampal volume and declarative memory performance in healthy young men. Brain Struct Funct. 2014;219(1):255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Maass A, Berron D, Harrison TM, et al. Alzheimer’s pathology targets distinct memory networks in the ageing brain. Brain. 2019;142(8):2492–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van Gelderen P, Li X, de Zwart JA, et al. Effect of motion, cortical orientation and spatial resolution on quantitative imaging of cortical R(2)* and magnetic susceptibility at 0.3 mm in-plane resolution at 7 T. Neuroimage. 2023;270:119992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kirst C, Skriabine S, Vieites-Prado A, et al. Mapping the fine-scale organization and plasticity of the brain vasculature. Cell. 2020;180(4):780–795.e25. [DOI] [PubMed] [Google Scholar]
- 77. Pandya DN, Yeterian EH. Comparison of prefrontal architecture and connections. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1423–1432. [DOI] [PubMed] [Google Scholar]
- 78. Ludbrook J, Dudley H. Why permutation tests are superior to t and F tests in biomedical research. Am Stat. 1998;52(2):127–132. [Google Scholar]
- 79. Noguchi K, Konietschke F, Marmolejo-Ramos F, Pauly M. Permutation tests are robust and powerful at 0.5% and 5% significance levels. Behav Res Methods. 2021;53(6):2712–2724. [DOI] [PubMed] [Google Scholar]
- 80. Viessmann O, Polimeni JR. High-resolution fMRI at 7 Tesla: Challenges, promises and recent developments for individual-focused fMRI studies. Curr Opin Behav Sci. 2021;40:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dumoulin SO, Knapen T. Chapter 25—The power of ultra-high field for cognitive neuroscience: Gray-matter optimized fMRI. In: Bloch KM, Guye M, Poser BA, eds. Ultra-High field neuro MRI. Vol 10. Advances in magnetic resonance technology and applications. Academic Press; 2023:407–418. [Google Scholar]
- 82. Jacków-Nowicka J, Podgórski P, Bladowska J, et al. The impact of common epidemiological factors on gray and white matter volumes in magnetic resonance imaging-is prevention of brain degeneration possible? Front Neurol. 2021;12:633619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on T2*-weighted gradient-echo MR images. AJNR Am J Neuroradiol. 2003;24(1):88–96. [PMC free article] [PubMed] [Google Scholar]
- 84. Dobrynina LA, Zabitova MR, Kalashnikova LA, Gnedovskaya EV, Piradov MA. Hypertension and cerebral microangiopathy (cerebral small vessel disease): Genetic and epigenetic aspects of their relationship. Acta Naturae. 2018;10(2):4–15. [PMC free article] [PubMed] [Google Scholar]
- 85. Li H, Jacob MA, Cai M, et al. Regional cortical thinning, demyelination and iron loss in cerebral small vessel disease. Brain. 2023;146(11):4659–4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
MRI data available upon request, requirements are a formal data sharing agreement and need to submit a formal project outline. Code for small vessel segmentation hysteresis thresholding: https://gitlab.com/hmattern/omelette