Abstract
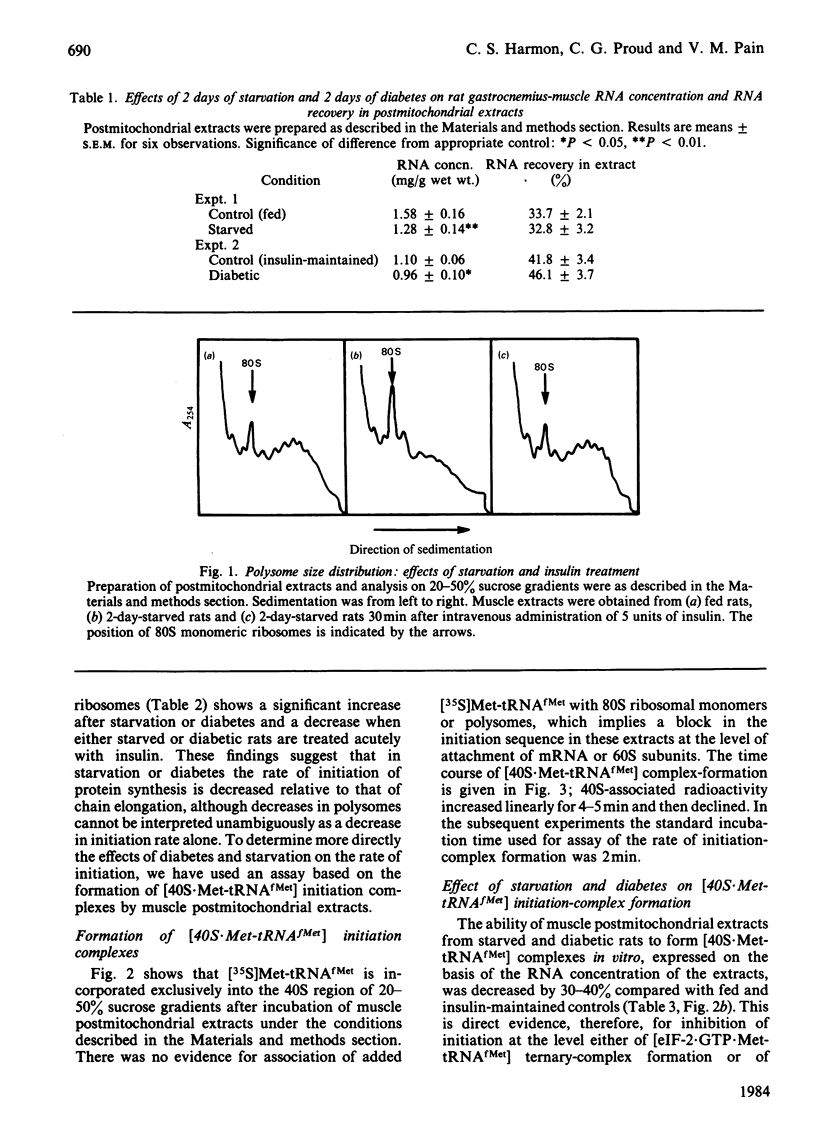
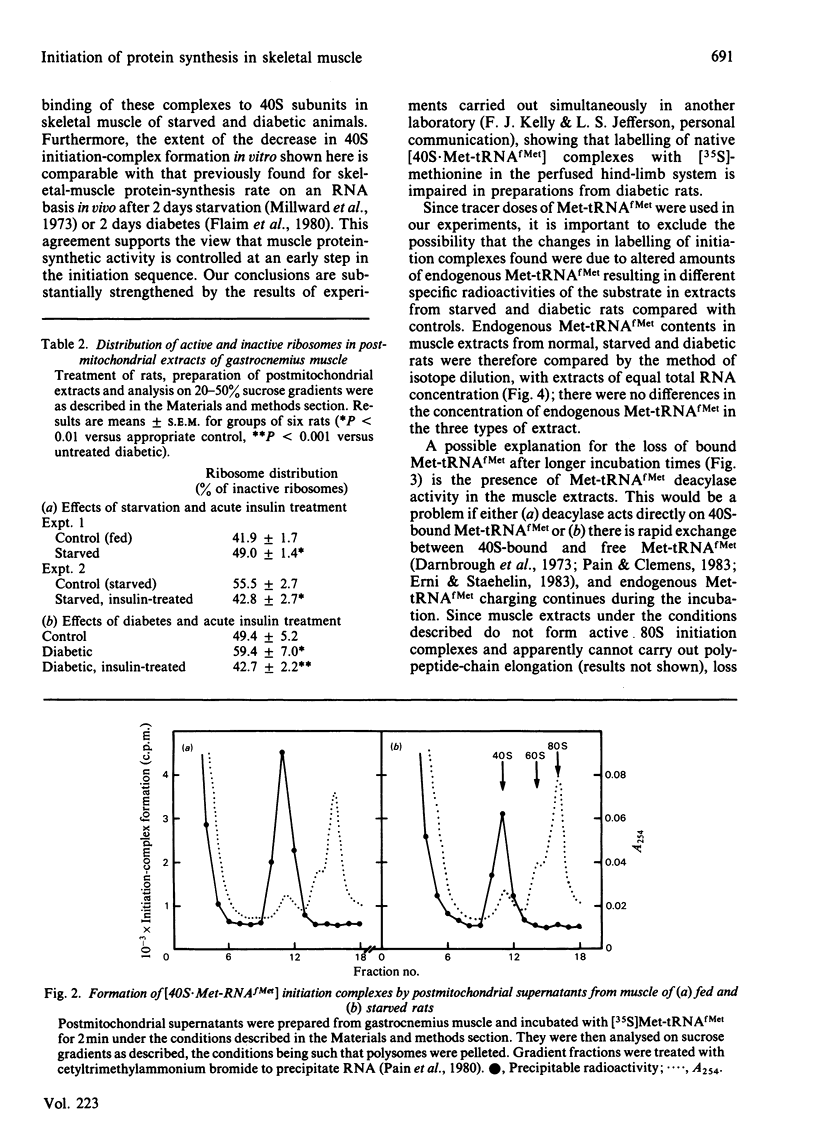
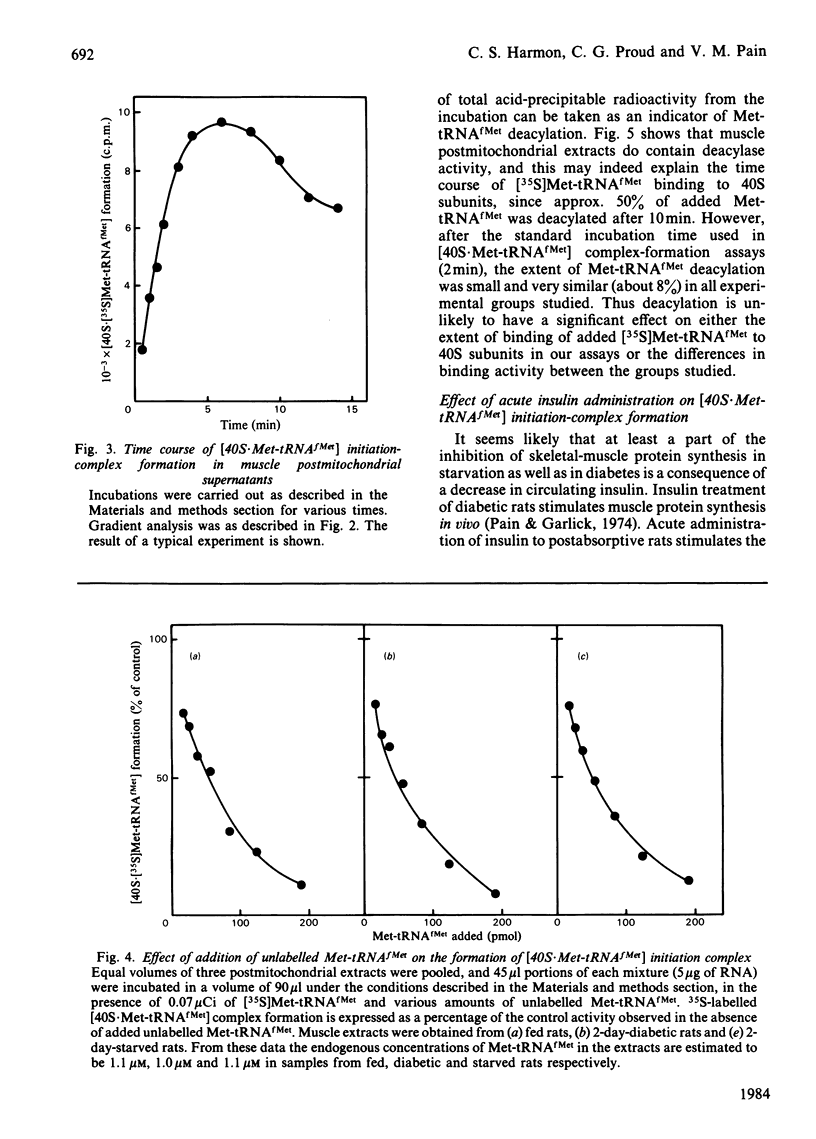
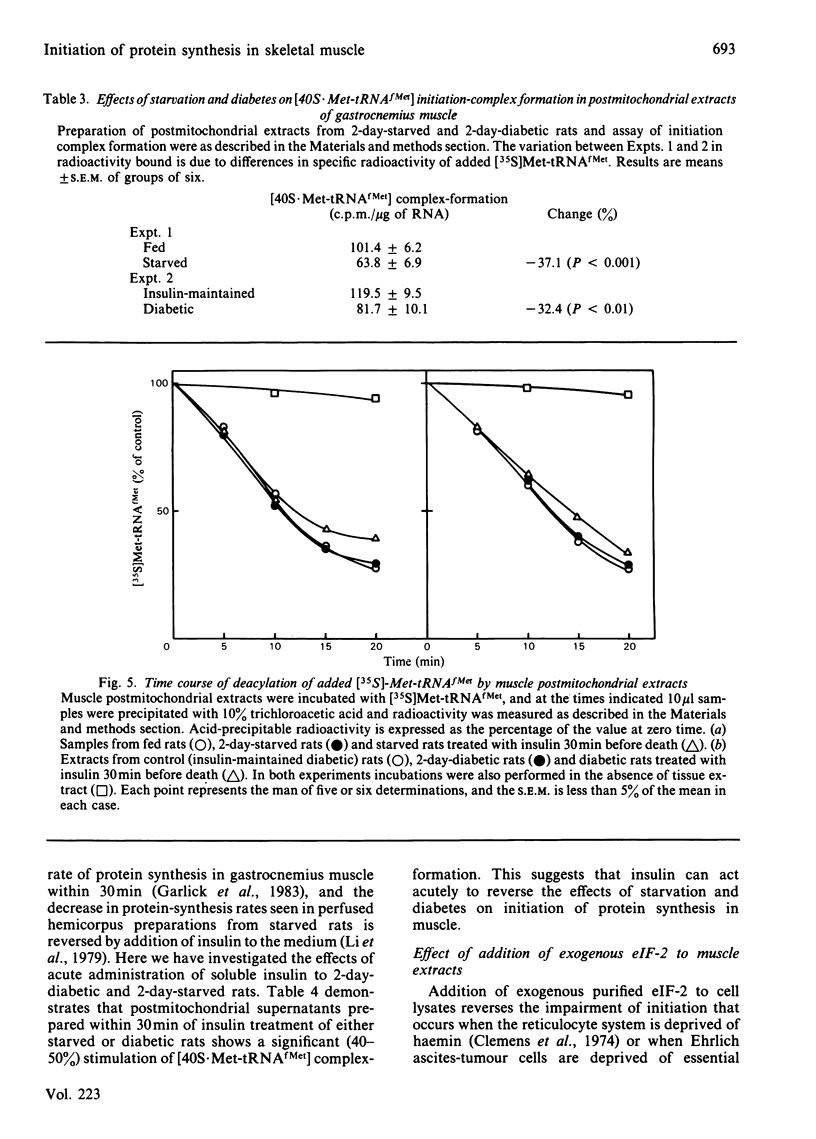
The rate of protein synthesis in skeletal muscle is greatly decreased in response to diabetes and starvation. Analysis of polyribosome profiles indicates that polypeptide-chain initiation is impaired under these conditions. To identify the step in initiation that is affected, we assayed the incorporation of [35S]methionyl-tRNAfMet into [35S]methionyl-tRNAfMet . 40S-ribosomal-subunit initiation complexes in cell-free extracts based on postmitochondrial supernatants prepared from gastrocnemius muscle. Extracts from either starved or diabetic rats were 30-40% less active in forming these complexes compared with those derived from fed or insulin-maintained controls respectively. This change could be reversed by treatment of either starved or diabetic rats with insulin in vivo 30 min before death. Formation of 40S initiation complexes by extracts from either fed or starved rats could be stimulated by the addition of exogenous purified initiation factor eIF-2, but extracts from starved or diabetic rats were more sensitive than controls to stimulation by low concentrations of the factor. These results provide evidence for the acute regulation by insulin of protein synthesis in skeletal muscle at the level of polypeptide-chain initiation, and suggest that in this tissue, as in certain other eukaryotic systems, control of initiation appears to be mediated by changes in the activity of initiation factor eIF-2.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benne R., Salimans M., Goumans H., Amesz H., Voorma H. O. Regulation of protein synthesis in rabbit reticulocyte lysates. Phosphorylation of eIF-2 does not inhibit its capacity to recycle. Eur J Biochem. 1980 Mar;104(2):501–509. doi: 10.1111/j.1432-1033.1980.tb04452.x. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Henshaw E. C., Rahamimoff H., London I. M. Met-tRNAfMet binding to 40S ribosomal subunits: a site for the regulation of initiation of protein synthesis by hemin. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2946–2950. doi: 10.1073/pnas.71.8.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J., Pain V. M., Wong S. T., Henshaw E. C. Phosphorylation inhibits guanine nucleotide exchange on eukaryotic initiation factor 2. Nature. 1982 Mar 4;296(5852):93–95. doi: 10.1038/296093a0. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Elson N. A., Anderson W. F. Initiation of globin synthesis: assays. Methods Enzymol. 1974;30:101–127. doi: 10.1016/0076-6879(74)30014-6. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Duncan R., McConkey E. H. Rapid alterations in initiation rate and recruitment of inactive RNA are temporally correlated with S6 phosphorylation. Eur J Biochem. 1982 Apr;123(3):539–544. doi: 10.1111/j.1432-1033.1982.tb06565.x. [DOI] [PubMed] [Google Scholar]
- Erni B., Staehelin T. Initiation of mammalian protein synthesis: dynamic properties of the assembly process in vitro. Biochim Biophys Acta. 1983 Sep 9;740(4):373–378. doi: 10.1016/0167-4781(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Flaim K. E., Copenhaver M. E., Jefferson L. S. Effects of diabetes on protein synthesis in fast- and slow-twitch rat skeletal muscle. Am J Physiol. 1980 Jul;239(1):E88–E95. doi: 10.1152/ajpendo.1980.239.1.E88. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., Fern M., Preedy V. R. The effect of insulin infusion and food intake on muscle protein synthesis in postabsorptive rats. Biochem J. 1983 Mar 15;210(3):669–676. doi: 10.1042/bj2100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw E. C., Hirsch C. A., Morton B. E., Hiatt H. H. Control of protein synthesis in mammalian tissues through changes in ribosome activity. J Biol Chem. 1971 Jan 25;246(2):436–446. [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 1. Classification and substrate specificities. Eur J Biochem. 1983 May 2;132(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07357.x. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leader D. P., Thomas A., Voorma H. O. The protein synthetic activity in vitro of ribosomes differing in the extent of phosphorylation of their ribosomal proteins. Biochim Biophys Acta. 1981 Nov 27;656(1):69–75. doi: 10.1016/0005-2787(81)90028-9. [DOI] [PubMed] [Google Scholar]
- Legon S., Jackson R. J., Hunt T. Control of protein synthesis in reticulocyte lysates by haemin. Nat New Biol. 1973 Jan 31;241(109):150–152. doi: 10.1038/newbio241150a0. [DOI] [PubMed] [Google Scholar]
- Li J. B., Higgins J. E., Jefferson L. S. Changes in protein turnover in skeletal muscle in response to fasting. Am J Physiol. 1979 Mar;236(3):E222–E228. doi: 10.1152/ajpendo.1979.236.3.E222. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., James W. P., Nnanyelugo D. O., Ryatt J. S. Relationship between protein synthesis and RNA content in skeletal muscle. Nature. 1973 Jan 19;241(5386):204–205. doi: 10.1038/241204a0. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., Manchester K. L., Towbin H., Gordon J., Thomas G. The phosphorylation of ribosomal protein S6 in rat tissues following cycloheximide injection, in diabetes, and after denervation of diaphragm. A simple immunological determination of the extent of S6 phosphorylation on protein blots. J Biol Chem. 1982 Oct 25;257(20):12316–12321. [PubMed] [Google Scholar]
- Pain V. M., Clemens M. J. Assembly and breakdown of mammalian protein synthesis initiation complexes: regulation by guanine nucleotides and by phosphorylation of initiation factor eIF-2. Biochemistry. 1983 Feb 15;22(4):726–733. doi: 10.1021/bi00273a003. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Garlick P. J. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974 Jul 25;249(14):4510–4514. [PubMed] [Google Scholar]
- Pain V. M., Henshaw E. C. Initiation of protein synthesis in Ehrlich ascites tumour cells. Evidence for physiological variation in the association of methionyl-tRNAf with native 40-S ribosomal subunits in vivo. Eur J Biochem. 1975 Sep 15;57(2):335–342. doi: 10.1111/j.1432-1033.1975.tb02306.x. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Lewis J. A., Huvos P., Henshaw E. C., Clemens M. J. The effects of amino acid starvation on regulation of polypeptide chain initiation in Ehrlich ascites tumor cells. J Biol Chem. 1980 Feb 25;255(4):1486–1491. [PubMed] [Google Scholar]
- Proud C. G., Clemens M. J., Pain V. M. Regulation of binding of initiator tRNA to eukaryotic initiation factor eIF-2. Effects of the haem-controlled repressor on the kinetics of ternary complex formation. FEBS Lett. 1982 Nov 8;148(2):214–220. doi: 10.1016/0014-5793(82)80810-7. [DOI] [PubMed] [Google Scholar]
- Proud C. G., Pain V. M. Purification and phosphorylation of initiation factor eIF-2 from rabbit skeletal muscle. FEBS Lett. 1982 Jun 21;143(1):55–59. doi: 10.1016/0014-5793(82)80273-1. [DOI] [PubMed] [Google Scholar]
- Rannels D. E., Pegg A. E., Rannels S. R., Jefferson L. S. Effect of starvation on initiation of protein synthesis in skeletal muscle and heart. Am J Physiol. 1978 Aug;235(2):E126–E133. doi: 10.1152/ajpendo.1978.235.2.E126. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and initial characterization of the cyclic 3':5'-AMP independent protein kinase of the heme-regulated translational inhibitor. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4349–4353. doi: 10.1073/pnas.73.12.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mauser L., Ochoa S. Mechanism of polypeptide chain initiation in eukaryotes and its control by phosphorylation of the alpha subunit of initiation factor 2. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2537–2540. doi: 10.1073/pnas.79.8.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]



