ABSTRACT
The incidence of diphtheria has been rising over the past decade, particularly in its cutaneous form. A clinical review of the case series was therefore required. We reviewed the epidemiological, clinical, microbiological and therapeutic data of cutaneous diphtheria cases, in adult patients living in metropolitan France with a skin sample positive for corynebacteria of the diphtheriae complex between 2018 and 2022. Of the 132 cases identified, 63 met the inclusion criteria. The mean age was 53.8 years, 68.3% were men and 56.7% had travelled outside mainland France. Immunization rate was 44%. Lesions involved the lower limbs (86.9%), corresponded to ulcerations in 82% of cases. Two species were identified in the study: C. diphtheriae (77%) and C. ulcerans (23%). 39% were toxigenic. Other bacteria were present in 88.9% of cases: Staphylococcus aureus (54.7%) and Streptococcus pyogenes (49.1%). 17.5% of clinicians ignored the presence of Corynebacteria of the diphtheriae species complex. Clinicians seem to be unfamiliar with this disease due to under-reporting and a lack of knowledge and awareness among clinicians, and rarely mention it, which explains the frequent failure to comply with French recommendations. Clinical data are consistent with the literature. Continued epidemiological surveillance, increased vaccination coverage in high-risk populations and better information of clinicians are essential to prevent and control this preventable disease.
KEYWORDS: Cutaneous diphtheria, epidemiology, metropolitan France, microbiology, traveller, emergent
Introduction
Corynebacteria of the diphtheriae species complex (CdSC) include the species Corynebacterium diphtheriae (Cd), C. ulcerans (Cu), C. pseudotuberculosis, C. belfantii, C. rouxii, C. silvaticum and C. ramonii. Some strains of Cd and Cu are characterized by their ability to express the diphtheria toxin gene (tox), acquired through infection with a corynebacteriophage, and are called toxigenic [1].
While the strict definition of diphtheria corresponds to infection by a Cd or Cu toxigenic strain, the pathogenicity of non-toxigenic strains (tox-), and the need to use reference laboratories to identify toxigenicity has led some national health organizations, including France's High Committee of Public Health (HCSP), to take into account tox – bacteria and to implement specific measures (antibiotherapy) against them, as soon as CdSC is identified.
Although the classical and most severe clinical presentation of diphtheria is respiratory and ear nose and throat (ENT) damages (pseudomembranous angina and laryngeal respiratory distress known as “croup”) which represent 10–30% of cases in literature, cutaneous infections are more common. They represent 57-86% of cases in literature and play an important role in the epidemiology of the disease [2, 3]. These two presentations are not mutually exclusive [3] and may be associated with cutaneous or pharyngeal colonization.
The severity of diphtheria is linked to the presence of diphtheria toxin, responsible for cardiac and neurological complications after its diffusion away from the infectious site. This toxin syndrome is particularly common in ENT infections but also occurs in cutaneous ones [4, 5]. Fortunately, the tox – strains are the most common in France (90%) [6].
The main mode of transmission of respiratory diphtheria is the respiratory route (droplets): the probability of contamination depends on the clinical presentation and carriage. Nevertheless, contact transmission via skin lesions appears to be the most important in terms of frequency [4], but transmission by indirect contact is also possible with contaminated objects [5, 7]. In contrast, C. ulcerans is a zoonosis, transmitted to humans by animals (typically domestic cats and dogs), and rarely by consumption of unpasteurized milk [8].
Diphtheria was a major cause of morbidity and mortality before the invention of diphtheria antitoxin (DAT); and later vaccination (lethality >10%) [9], particularly in the paediatric populations. It often occurred as an epidemic, with a highly contagious nature favoured by promiscuity and poor hygiene.
Mass vaccination against diphtheria drastically reduced the incidence of this disease, starting after the Second World War in developed countries and through the expanded programme of immunization (EPI) in most countries. No cases were recorded in France between 1990 and 2002. The rarity of diphtheria cases has led to a loss of clinical expertise in this disease in developed countries. Current medical teaching in France addresses this disease only through its ENT presentation (more characteristic and more often associated with the toxin syndrome), while cutaneous involvement is never or rarely mentioned.
However, the last two decades have seen a re-emergence of diphtheria infections in most developed countries, including France [10, 11]. These cases mainly concern people in precarious situations with comorbidities, as well as travellers from endemic areas, such as India, Madagascar, Indonesia, Africa, or former USSR [11–13]. In the context of this re-emergence, cutaneous infections are now more frequent than respiratory forms [14].
This study aimed to review cases of cutaneous diphtheria in French mainland and provide an overview of its epidemiological, microbiological, clinical, and therapeutic characteristics. We also aimed to assess clinicians’ practices in relation to this disease, something that has never been done before.
Material and methods
Population
A retrospective multicentric observational cross-sectional study, which included cases reported in Mainland France from January 1, 2018, to December 31, 2022. All cutaneous forms had a skin sample sent to the Corynebacteria National Reference Center (CNR) for tox gene detection with multiplex polymerase chain reaction (PCR). The laboratories that had sent the samples to the CNR were contacted one by one, to retrospectively contact the clinicians who had managed the patients with CdSC-positive skin samples. We included adults living in mainland France with skin samples positive for CdSC in culture or PCR. Patients were then contacted by their clinicians to express their consent, after having received information regarding the study. The patients with whom it has not been possible to contact (migrants, homeless people, etc.) and for whom a regulatory agreement for health establishments was not possible were excluded from this study in accordance with French and European data legislation and ethical laws. Patient were considered as immunized if booster doses were in line with the French vaccination schedule, Alcohol use disorder is characterized by compulsive heavy alcohol use and loss of control over alcohol intake, using drug included intravenous drug only.
We then sent a questionnaire (see S1 in supplementary materials) to clinicians in computerized format (e-CRF) (Appendix1), to gather epidemiological data (age, gender, comorbidities, vaccination status), clinical data (hypothesis before diagnosis, pre-existing lesions before the diagnosis of cutaneous diphtheria, description of lesions, location, size, microbiological data (identification of species, toxigenicity, co-infection, nasopharyngeal carriage) and therapeutic data (antibiotic therapy, serotherapy, duration of treatment). We also included questions allowing the verification of compliance with French guidelines recommendations: duration of antibiotic therapy, control of vaccination status and post-infection vaccination, microbiological follow-up (search for eradication of the bacterium in the case of a positive nasopharyngeal carriage, serological sampling before serotherapy or 1 month after infection if no serotherapy).
Statistical analysis
A description of the various sociodemographic, microbiological, clinical, and therapeutic variables of the study population was carried out and a 95% confidence interval was provided for qualitative and quantitative data. Qualitative variables were analysed by frequency, percentages and confidence intervals. Quantitative variables were analysed by mean (p), and 95% confidence interval (95% CI).
The Fisher exact test was used to compare qualitative variables, and the Kruskal–Wallis rank sum test to compare quantitative variables. All tests were performed with a significance level of 5%.
All analyses were performed with R software version 4.0.3.1
Ethical consideration
This study was approved by the Centre Hospitalier Intercommunal Toulon La Seyne sur Mer « Institutional Review Board – N°00012962 » in November 2022 and was registered in ClinicalTrials.gov (NCT05798247) in April 2023. Data collection was analysed by the French Armed Forces Center for Epidemiology and Public Health (CESPA).
Results
Between January 1, 2018, and December 31, 2022, 132 samples sent to the CNR were classified as “skin sample” with a CdSC isolate. Of these 132 samples corresponding to distinct patients, 69 patients could not be included in the study: 11 did not meet the inclusion criteria (3 concerned patients who were minors; 3 corresponded to bone samples, 2 to sinus samples and 2 to hygroma samples; 1 sample was prior to 2018), 9 patients could not be informed of the use of their data), 1 refusal, for 26 cases, no data could be obtained and the regulatory agreement could not be obtained for 22 cases. Finally, 63 patients were included in our study.
Population and epidemiological characteristics
Patient characteristics are given in Table 1. The mean age of the study population was 53.8 years (range: 18–93 years), and 68.3% of patients were male. Among patients 62% had at least one comorbidity, of whom 55% were hypertensive, 50% were diabetic, 25.6% had arteriopathy and 15.1% had alcohol use disorder. Three patients were considered immunocompromised (34 patients (56.7%) had travelled outside mainland France in the year prior to diagnosis, including 67.6% to Africa, 23.5% to Asia, 5.9% to French overseas departments and territories (DROM-COM) and 2.9% to Europe. In terms of diphtheria vaccination status, when it was available, patients were considered immunized in 44% of cases. In more than one patient out of 3, immunization status was not known. Among C. ulcerans cases (n = 14), close contact with an animal prior to diagnosis was reported in 62.4% of cases (77.8% with a dog and 22.2% with a cat) (Table 1).
Table 1.
Epidemiological characteristics of cutaneous diphtheria cases. Mainland France, 2018–2022.
| Epidemiological characteristics | |||
|---|---|---|---|
| N = Total response | n | (p) | |
| Gender | 63 | ||
| Male | 43 | (68.3) | |
| Female | 20 | (31.7) | |
| Age at diagnosis: mean (SD) | 63 | 53.8 | (21) |
| Housing type | 63 | ||
| Individual | 49 | (77.8) | |
| Collective | 7 | (11.1) | |
| Undeclared | 7 | (11.1) | |
| Essential comorbidities | 63 | ||
| No | 24 | (38.1) | |
| Yes | 39 | (61.9) | |
| High blood pressure | 38 | 21 | (55.3) |
| Diabetes | 38 | 19 | (50) |
| Arteriopathy (PAD, ischaemic heart disease) | 39 | 10 | (25.6) |
| Psychological disorders | 38 | 5 | (13.2) |
| Atrial fibrillation | 39 | 4 | (10.2) |
| Venous insufficiency | 39 | 3 | (7.7) |
| Hepatitis B | 39 | 2 | (5.1) |
| Cirrhosis | 39 | 2 | (5.1) |
| Immunosuppression | 60 | 3 | (5) |
| Drug | 57 | 2 | (3.5) |
| Alcohol | 53 | 8 | (15.1) |
| Travel outside France | 60 | 34 | (56.7) |
| Africa | 34 | 23 | (67.6) |
| Asia | 34 | 8 | (23.5) |
| Europe | 34 | 1 | (2.9) |
| French overseas territories | 34 | 2 | (5.9) |
| Vaccination status | 41 | ||
| No | 15 | (36.6) | |
| Yes | 12 | (29.3) | |
| Not wanted | 14 | (34.1) | |
| Animal contact | 55 | 15 | (27.3) |
| Dog | 15 | 10 | |
| Cat | 15 | 5 | |
| Goats | 15 | 2 | |
| Rodents | 15 | 1 | |
| Sheep | 15 | 1 | |
| Fish | 15 | 1 | |
| Chicken | 15 | 1 | |
| Number of C.ulcerans in contact with animals | 9 | ||
| Number of C.diphteriae in contact with animals | 5 | ||
Microbiology
The Corynebacteria strains identified were Cd (77%) and Cu (23%). There were no positive samples from other species. (Table 2).
Table 2.
Microbiological characteristics of cutaneous diphtheria cases. Mainland France, 2018–2022.
| Microbiological characteristics | |||
|---|---|---|---|
| N = Total réponse | n = effectif | (p) | |
| Diagnosis of Corynebacteria of the diphtheriae species complex | 61 | ||
| Fortuitous | 50 | (82) | |
| Oriented | 11 | (18) | |
| Corynebacterium Species | 63 | ||
| C. diphtheriae | 49 | (77,8) | |
| Tox- | 28 | ||
| Tox+ | 14 | ||
| C. ulcerans | 14 | (22,2) | |
| Tox- | 5 | ||
| Tox+ | 9 | ||
| C. pseudo tuberculosis | 0 | (0) | |
| C. belfanti | 0 | (0) | |
| C. rouxii | 0 | (0) | |
| Toxinogen | 56 | ||
| No | 34 | (60.7) | |
| Yes | 22 | (39.3) | |
| Nasopharyngal carriage | 63 | ||
| Not wanted | 30 | (47.6) | |
| Positive | 6 | (9.5) | |
| Negative | 27 | (42.9) | |
| If nasopharyngeal carriage positive: search of eradication after treatment | 6 | ||
| Not wanted | 2 | (33.3) | |
| Positive | 0 | (0) | |
| Negative | 4 | (66.7) | |
| Co-infection | 63 | ||
| No | 7 | (11.1) | |
| Yes | 56 | (88.9) | |
| S. aureus meticilline Sensible | 53 | 25 | (47.2) |
| S.aureus meticilline resistant | 51 | 4 | (7.8) |
| S. pyogenes | 53 | 26 | (49.1) |
| Enterobacteria | 50 | 20 | (40,0) |
| Streptococcus B | 50 | 8 | (16) |
| Streptococcus G | 52 | 6 | (11.5) |
| E. faecalis | 50 | 3 | (6) |
| Pseudomonas aerogenosa | 50 | 3 | (6) |
| Morganella morganii | 50 | 3 | (6) |
| Treponema pallidum | 50 | 2 | (4,0) |
| Neisseria gonorrhoeae | 50 | 2 | (4,0) |
| S. oralis | 50 | 1 | (2,0) |
| Monkeypox | 50 | 1 | (2,0) |
| Scabies | 50 | 1 | (2,0) |
Thirty-nine percent of strains were toxigenic (toxigenic status was only reported for 56 patients): 14 Cd tox + Versus 28 Cd tox – 9 Cu tox + and 5 Cu tox – (Table 2). Six patients of all those studied (9.5%) had associated positive nasopharyngeal carriage. (Table 2) There was no significant statistical difference between the presence of a positive nasopharyngeal carriage and the vaccination status of patients (Table 5).
Table 5.
Clinical appearance of cutaneous diphtheria and complications according to toxigenic status.
| Clinic | Toxinogen | p | |||||
|---|---|---|---|---|---|---|---|
| No N = 34 | Yes N = 22 | ||||||
| n | (%) | [CI95%] | n | (p) | [CI95%] | ||
| Ulcerated lesion | 28 | (62.2) | [46.9-77.5] | 17 | (37.8) | [22.5-53.1] | 0.498 |
| Erythemato purpulish ulceration border | 18 | (69.2) | [49.6-88.9] | 8 | (30.8) | [11.1-50.4] | 0.343 |
| Raised border | 6 | (50.0) | [17.5-82.5] | 6 | (50.0) | [17.5-82.5] | 0.483 |
| Indurated border | 5 | (45.5) | [11.5-79.4] | 6 | (54.5) | [20.6-88.5] | 0.281 |
| Pseudomembrane | 2 | (100.0) | [75.0-100.0] | 0 | (0.0) | [0.0-25.0] | 0.509 |
| Nodular lesion | 2 | (40.0) | [0.0-92.9] | 3 | (60.0) | [7.1-100.0] | 1.000 |
| Papular lesion | 4 | (50.0) | [9.1-90.9] | 4 | (50.0) | [9.1-90.9] | 0.700 |
| Necrotic lesion | 6 | (75.0) | [38.7-100.0] | 2 | (25.0) | [0.0-61.3] | 0.449 |
| Vesicular lesion | 3 | (60.0) | [7.1-100.0] | 2 | (40.0) | [0.0-92.9] | 1.000 |
| Pustular lesion | 4 | (44.4) | [6.4-82.5] | 5 | (55.6) | [17.5-93.6] | 0.457 |
| Crusty lesion | 10 | (55.6) | [29.8-81.3] | 8 | (44.4) | [18.7-70.2] | 0.769 |
| Painful lesion | 19 | (67.9) | [48.8-86.9] | 9 | (32.1) | [13.1-51.2] | 0.171 |
| Pruritic lesion | 2 | (25.0) | [0.0-61.3] | 6 | (75.0) | [38.7-100.0] | 0.052 |
| Edema | 10 | (58.8) | [32.5-85.2] | 7 | (41.2) | [14.8-67.5] | 1.000 |
| Peripheral desquamation | 6 | (54.5) | [20.6-88.5] | 5 | (45.5) | [11.5-79.4] | 0.744 |
| Number of lesions | 0.532 | ||||||
| 1 | 16 | (69.6) | [48.6-90.5] | 7 | (30.4) | [9.5-51.4] | |
| 2–5 | 11 | (52.4) | [28.6-76.1] | 10 | (47.6) | [23.9-71.4] | |
| >5 | 7 | (58.3) | [26.3-90.4] | 5 | (41.7) | [9.6-73.7] | |
| Size of lesions | 0.374 | ||||||
| <1 cm | 7 | (53.8) | [22.9-84.8] | 6 | (46.2) | [15.2-77.1] | |
| 1–5 cm | 15 | (60.0) | [38.8-81.2] | 10 | (40.0) | [18.8-61.2] | |
| >5 cm | 11 | (78.6) | [53.5-100.0] | 3 | (21.4) | [0.0-46.5] | |
| Complications | |||||||
| Arthritis | 4 | (100.0) | [87.5-100.0] | 0 | (0.0) | [0.0-12.5] | 0.539 |
| Bacteraemia | 3 | (100.0) | [83.3-100.0] | 0 | (0.0) | [0.0-16.7] | 0.246 |
| Peripheral neuropathy | 0 | (0.0) | [0.0-16.7] | 3 | (100.0) | [83.3-100.0] | 0.010 |
| Respiratory distress | 1 | (100.0) | [50.0-100.0] | 0 | (0.0) | [0.0-50.0] | 1.000 |
Co-infection of the skin lesion with at least one other identified bacterial species was present in 88.9% of cases: Staphylococcus aureus (54.7%), Streptococcus pyogenes (49.1%), enterobacteria (40%).
Cases of sexually transmitted infections were associated with Treponema pallidum (n = 2), Neisseria gonorrhoeae (n = 2), mpox (n = 1) and scabies (n = 1). All these cases except scabies were detected in the context of genital ulcerations (Table 2).
Four patients immunized against diphtheria were infected with a toxigenic strain, and 7 with a non-toxigenic strain.
Clinical and biological description
Before describing the patients’ clinical and biological characteristics (Table 3), we can notice that the diagnosis of cutaneous diphtheria had not been discussed by the clinician (before the microbiological result) in 82% of cases. The main hypotheses suggested before the microbiological diagnosis of cutaneous diphtheria were ecthyma (22.9%), impetigo (17.6%) and leishmaniasis (14.7%).
Table 3.
Clinical and biological characteristics of cutaneous diphtheria cases. Mainland France, 2018–2022.
| Clinical and microbiological characteristics | |||
|---|---|---|---|
| N = Total response | n = headcount | (p) | |
| Other diagnoses mentioned | 62 | 42 | (67.7) |
| Echtyma | 35 | 8 | (22.9) |
| Burn | 34 | 2 | (5.9) |
| Insect bite | 35 | 3 | (8.6) |
| Impetigo | 34 | 6 | (17.6) |
| Leishmaniasis | 34 | 5 | (14.7) |
| Sporotrichosis | 34 | 1 | (2.9) |
| Pyoderma gangrenosum | 34 | 0 | (0) |
| Buruli ulcer | 34 | 2 | (5.9) |
| Other diagnoses | |||
| Osteitis | 30 | 4 | (13,3) |
| Plantar perforator | 30 | 4 | (13,3) |
| Mycobacteria | 30 | 3 | (10) |
| Syphilis | 30 | 2 | (6,7) |
| Abscess | 30 | 2 | (6,7) |
| Erysipelas | 30 | 2 | (6,7) |
| Necrotizing fasciitis | 30 | 2 | (6,7) |
| Eczema | 30 | 1 | (3,3) |
| Furunculosis | 30 | 1 | (3,3) |
| Pre-existing skin lesion | 60 | ||
| Non | 18 | (30) | |
| Yes | 42 | (70) | |
| Surgical wound | 37 | 10 | (27) |
| Insect bite | 36 | 5 | (13.9) |
| Burn | 36 | 2 | (5.6) |
| Eczema | 35 | 2 | (5.7) |
| Impetigo | 35 | 2 | (5.7) |
| Diabetic foot | 56 | 10 | (17.9) |
| Other pre-existing lesion | |||
| Traumatic wound | 39 | 10 | (25,6) |
| Ulcers (venous/arterial) | 39 | 6 | (15,4) |
| Digital ischaemia | 39 | 1 | (2,6) |
| Pemphigus | 39 | 1 | (2,6) |
| Drug injection point | 39 | 1 | (2,6) |
| Clinical aspect | |||
| Ulcerated lesion | 61 | 50 | (82) |
| Fibrinous | 48 | 34 | (70.8) |
| Clean bottom | 49 | 16 | (32.7) |
| Border of erythematous/purplish ulceration | 48 | 29 | (60.4) |
| Oedema | 55 | 20 | (36.4) |
| Crusty lesion | 56 | 19 | (33.9) |
| Raised border | 47 | 14 | (29.8) |
| Hardened edge | 47 | 14 | (29.8) |
| Peripheral desquamation | 55 | 12 | (21.8) |
| Necrotic lesion | 56 | 9 | (16.1) |
| Pustular lesion | 56 | 9 | (16.1) |
| Papular lesion | 56 | 8 | (14.3) |
| Nodular lesion | 56 | 6 | (10.7) |
| Bed sore | 49 | 5 | (10.2) |
| Vesicular lesion | 56 | 5 | (8.9) |
| Pseudomembrane | 57 | 4 | (7) |
| Panaris | 56 | 1 | (1.8) |
| Other lesion | 50 | ||
| Purulent | 7 | (14) | |
| Bullous | 1 | (2) | |
| Hemorragic | 1 | (2) | |
| Eczema | 1 | (2) | |
| Burgeonning | 1 | (2) | |
| Symptoms associated | |||
| Painful lesion | 57 | 30 | (52.6) |
| Puriginous lesion | 55 | 8 | (14.5) |
| Location of lesions | |||
| Lower limbs | 61 | 53 | (86.9) |
| Upper limbs | 59 | 12 | (20.3) |
| Head | 60 | 6 | (10) |
| Trunk | 59 | 3 | (5.1) |
| Genitals | 59 | 3 | (5.1) |
| Number of lesions | 62 | ||
| 1 | 26 | (41.9) | |
| 2–5 | 22 | (35.5) | |
| >5 | 14 | (22.6) | |
| Size of lesions | 58 | ||
| <1 cm | 15 | (25.9) | |
| 1–5 cm | 28 | (48.3) | |
| >5 cm | 15 | (25.9) | |
| Duration of progression of lesions | 62 | ||
| <1 week | 15 | (24.2) | |
| 1–2 weeks | 9 | (14.5) | |
| 2 weeks to 1 month | 12 | (19.4) | |
| >1 month | 26 | (41.9) | |
| Associated clinical manifestations | 63 | ||
| No | 37 | (58.7) | |
| Yes | 26 | (41.3) | |
| Fever | 26 | 15 | (57.7) |
| Angina/epiglottis | 26 | 0 | (0) |
| Respiratory distress | 26 | 1 | (3.8) |
| Myocarditis | 26 | 0 | (0) |
| Peripheral neuropathy | 26 | 3 | (11.5) |
| Endocarditis | 26 | 0 | (0) |
| Arthritis | 26 | 4 | (15.4) |
| Lymphadenopathy in the drainage area of the lesions | 25 | 7 | (28) |
| Erysipelas | 25 | 3 | (10,1) |
| Osteitis | 25 | 1 | (4) |
| Biological characteristics | |||
| Acute kidney failure | 32 | 4 | (12.5) |
| Neutrophilic polynucleosis | 31 | 12 | (38.7) |
| Increased CRP | 32 | 29 | (90.6) |
| Bacteraemia | 32 | 3 | (9.4) |
Most skin involvement concerned the lower limb (86.9%), the upper limbs in 20.1%, head in 10.1% and trunk and genital location in 5.1%%
Lesions were multiple in 58.1% of cases and measured between 1 and 5 cm in 1 out of 2 cases.
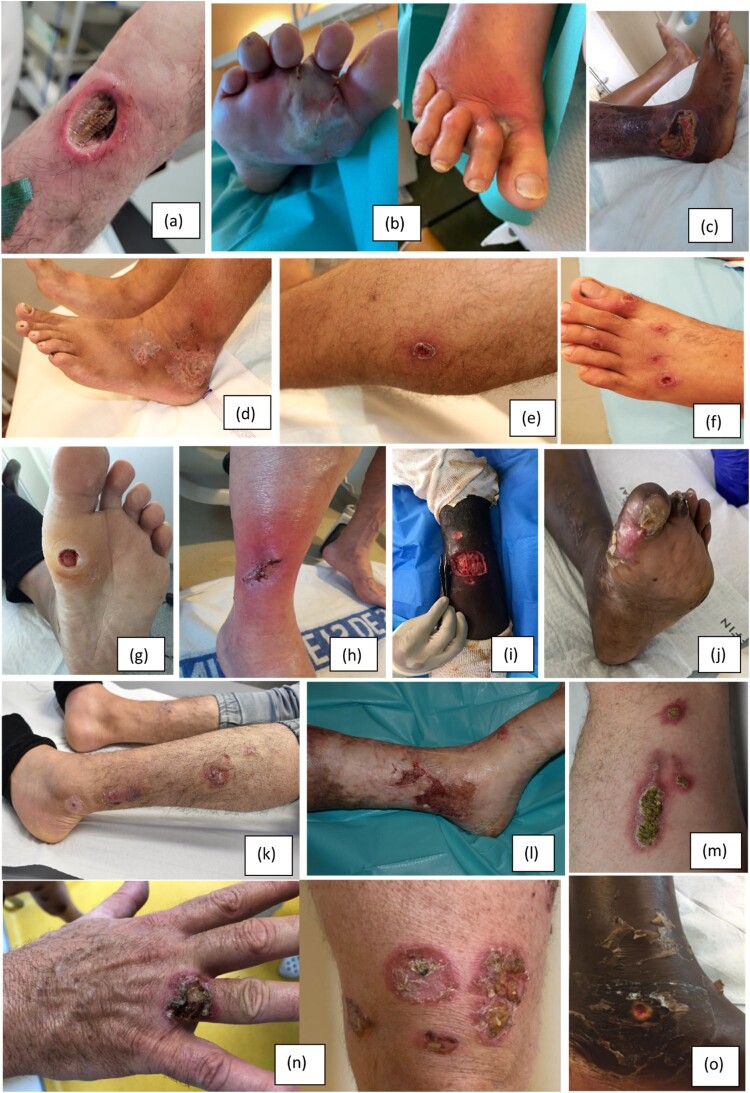
Various clinical aspects were reported in this study (Figure 1). The most common lesion description was ulceration (82%) and in most cases the base was fibrinous (70.8%). The ulcerated edges were most often erythematous-purplish (60.4%), non-indurated and not raised in more than 7 out of 10 patients. The pseudomembranous exudate, which usually evokes the diagnosis, was observed in only 4 patients in our study, two of whom were infected with non-toxigenic strains. Pain was associated with skin involvement in 52.6% of cases, and lesions were predominantly non-pruritic (85.5%)-. There was no significant difference between the clinical presentation, size and number of lesions according to the toxigenic status of the bacteria or the vaccination status (Tables 4 and 5).
Figure 1.
(a) Pseudomembranous exudate (Coll. Dr Lejeune) (b) Purulent blisters complicated by erysipelas (Coll. Dr Lambert de Cursay), (c) Necrotic varicose ulcer (Coll. Dr Hua), (d) Polycyclic erosive lesions (Coll. Dr Durupt), (e) Impetigo-like with desquamative collar (Coll. Dr Durupt), (f) Multiple hollowing ulcerations with violaceous border (Coll. Dr Coudon), (g) Plantar perforating disease (Coll. Dr Moret), (h) Post-surgical lesion complicated by erysipelas(Coll. Dr Wan), (i) Ulcerations with fibrinous base (Coll. Dr Triffault-Fillit), (j) Plantar perforating disease (Coll. Dr Gramont), (k) Coalescence of pustules (Coll. Dr Fenot), (l) Varicose ulcerations (Coll. Dr Birckel), (m) Yellowish crusts on erythematous base (Coll. Fenot), (n) Yellowish crusts on erythematous base(same patient): Coll. Pr Morand, (o)Ulceration, clean base: (Coll. Pr Morand).
Table 4.
Cross sorting between vaccination status, clinical appearance and toxigenicity of strains and nasopharyngeal carriage of diphtheria.
| Clinic | Immunisation status: immunized against diphtheria | p | |||||
|---|---|---|---|---|---|---|---|
| No = 15 | Yes = 12 | ||||||
| n | (%) | [CI95%] | n | (p) | [CI95%] | ||
| Ulcerated lesion | 13 | (61.9) | [38.8-85.1] | 8 | (38.1) | [14.9-61.2] | 0.62 |
| Pseudomembrane | 0 | (NaN) | [NaN-NaN] | 0 | (NaN) | [NaN-NaN] | 1 |
| Nodular lesion | 2 | (66.7) | [0-100] | 1 | (33.3) | [0-100] | 1 |
| Papular lesion | 4 | (100) | [87.5-100] | 0 | (0) | [0-12.5] | 0.113 |
| Necrotic lesion | 1 | (20) | [0-65.1] | 4 | (80) | [34.9-100] | 0.128 |
| Vesicular lesion | 1 | (33.3) | [0-100] | 2 | (66.7) | [0-100] | 0.556 |
| Pustular lesion | 3 | (75) | [20.1-100] | 1 | (25) | [0-79.9] | 0.614 |
| Crusty lesion | 5 | (55.6) | [17.5-93.6] | 4 | (44.4) | [6.4-82.5] | 1 |
| Painful lesion | 6 | (50) | [17.5-82.5] | 6 | (50) | [17.5-82.5] | 0.692 |
| Pruritic lesion | 3 | (75) | [20.1-100] | 1 | (25) | [0-79.9] | 0.614 |
| Edema | 7 | (70) | [36.6-100] | 3 | (30) | [0-63.4] | 0.678 |
| Peripheral desquamation | 3 | (42.9) | [0-86.7] | 4 | (57.1) | [13.3-100] | 0.378 |
| Number of lesions | 0.883 | ||||||
| 1 | 7 | (63.6) | [30.7-96.6] | 4 | (36.4) | [3.4-69.3] | |
| 2–5 | 4 | (57.1) | [13.3-100] | 3 | (42.9) | [0-86.7] | |
| >5 | 4 | (44.4) | [6.4-82.5] | 5 | (55.6) | [17.5-93.6] | |
| Size of lesions | 0.188 | ||||||
| <1 cm | 5 | (71.4) | [30.8-100] | 2 | (28.6) | [0-69.2] | |
| 1–5 cm | 8 | (57.1) | [27.6-86.6] | 6 | (42.9) | [13.4-72.4] | |
| >5 cm | 0 | (0) | [0-16.7] | 3 | (100) | [83.3-100] | |
| Toxinogen Corynebacterium | 0.233 | ||||||
| No | 5 | (41.7) | [9.6-73.7] | 7 | (58.3) | [26.3-90.4] | |
| Yes | 10 | (71.4) | [44.2-98.7] | 4 | (28.6) | [1.3-55.8] | |
| Nasopharyngeal carriage | 0.513 | ||||||
| Positive | 4 | (80) | [34.9-100] | 1 | (20) | [0-65.1] | |
| Negative | 8 | (53.3) | [24.8-81.9] | 7 | (46.7) | [18.1-75.2] | |
In 61.3% of cases, the clinical picture had been evolving for more than two weeks prior to treatment. In 70% of cases, there was a pre-existing lesion: surgical wound (27%), traumatic wound (25.6%) diabetic foot lesion (17.6%) ulceration of venous or arterial origin (15.4%) or insect bite (13.9%).
Associated symptoms were present in 58.7% of patients, mainly in the form of fever (57.7%). Eight patients presented complications: respiratory distress (n = 1) in a patient infected with a non-toxigenic strain, arthritis associated with non-toxigenic strains (n = 4), peripheral neuropathies attributable to toxigenic strains (n = 3).
Three patients had associated bacteraemia in two cases due to a non-toxigenic C.diphtheriae and in one case with Staphylococcus aureus (Table 3).
Individual and collective care
After diagnosis, clinicians disregarded the presence of CdSC in 17.5% of cases.
Concerning immunization, 6 patients of the 22 patients featuring a toxigenic strain identified in this study (27.2%) received an anti-diphtheria serotherapy. None of these patients experienced an anaphylactic reaction following their treatment. All patients whose vaccination was not up to date were vaccinated following infection.
When serotherapy was carried out, five of the six patients concerned had a serological control. Three patients had a serological check-up one month after infection when no serotherapy was performed. Three quarters of patients did not have serology to determine the anti-toxin antibody titre at one month when no serotherapy was performed. Finaly, 47% of patients were not tested for nasopharyngeal carriage, and of those with a positive nasopharyngeal carriage, 33.3% were not tested for eradication (Table 2).
Practitioners looked for contact cases in 42.6% of patients. Patients were isolated using droplet precautions in 54.8% of cases, although this measure is not mandatory for isolated skin infections (Table 6).
Table 6.
Therapeutic characteristics of cutaneous diphtheria cases. Mainland France, 2018–2022.
| Therapeutic characteristics | |||
|---|---|---|---|
| N = Total réponse | n = effectif | (p) | |
| Antibiotic therapy(s) before microbiological diagnostic | 63 | ||
| No | 23 | (36.5) | |
| Yes | 40 | (63.5) | |
| Amoxicilline | 29 | 7 | (24.1) |
| Average in day | (6,3) | ||
| Amoxiclline + clavulanic acid | 35 | 19 | (54.3) |
| Average in day | (4.7) | ||
| Pristinamycine | 30 | 5 | (16.7) |
| Average in day | (8) | ||
| Clindamycine | 33 | 8 | (24.2) |
| Average in day | (5.4) | ||
| Ceftriaxone | 29 | 2 | (6.9) |
| Average in day | 4.5 | ||
| After microbiological diagnostic | 63 | ||
| No | 11 | (17.5) | |
| Yes | 52 | (82.5) | |
| Amoxicilline | 44 | 22 | (50) |
| Average in day | (17.7) | ||
| Amoxiclline + clavulanic acid | 47 | 17 | (36.2) |
| Average in day | (14.5) | ||
| Pristinamycine | 42 | 1 | (2.4) |
| Average in day | (7) | ||
| Clindamycine | 45 | 14 | (31.1) |
| Average in day | (12) | ||
| Roxithromycine | 42 | 1 | (2.4) |
| Average in day | (4) | ||
| Vancomycine | 42 | 2 | (4.8) |
| Average in day | (9) | ||
| Ceftriaxone | 43 | 1 | (2.3) |
| Average in day | (1) | ||
| Multiple antibiotic therapies | 18 | ||
| Post vaccination infection | 43 | ||
| No | 25 | (58.1) | |
| Yes | 18 | (41.9) | |
| Number of non-immune people vaccinated | 15 | (100) | |
| Serotherapy | 60 | ||
| No | 54 | (90) | |
| Yes | 6 | (10) | |
| For which motive? | 6 | ||
| Toxigenic CCD | 6 | (100) | |
| Toxic signs | 0 | (0) | |
| Anaphylactic reaction during serotherapy | 6 | ||
| No | 6 | (100) | |
| Yes | 0 | (0) | |
| Serological sampling before serotherapy (yes) | 6 | 5 | (83.3) |
| At 1 month if no serotherapy carried out | 12 | ||
| No | 9 | (75) | |
| Yes | 3 | (25) | |
| Contact tracking research | 47 | ||
| No | 27 | (57.4) | |
| Yes | 20 | (42.6) | |
| “Droplet” isolation | 62 | ||
| No | 28 | (45.2) | |
| Yes | 34 | (54.8) | |
Discussion
This retrospective multicenter study describes the epidemiological, clinical, microbiological, and therapeutic presentation of 63 cases of cutaneous infection with a Corynebacterium of the diphtheriae species complex that occurred in metropolitan France from 2018 to 2022. This study takes place against a backdrop of epidemiological changes in many developed countries explaining an increase in the number of cases of cutaneous diphtheria: An ageing population with inadequate vaccination coverage, the increase in migratory flows from classic endemic zones, [1, 15] and the improved sensitivity of diagnostic tests, notably with the development of mass spectrometry (MALDI-TOF) for routine microbiological use [2, 16].
Epidemiological characteristics of patients with cutaneous diphtheria described in the literature are consistent with our study: these are, above all, men, travellers, or migrants from endemic areas [1, 12, 15]. In our study, more than half of our population reported a stay in a foreign country where diphtheria is endemic.
In the literature, several risk factors have been identified, both medical and non-medical: immunodepression [3], excessive alcohol consumption [5], injecting drug use [6], alcoholic cirrhosis [11, 15], atopic dermatitis [6], difficult access to healthcare [6] as well as poverty fostered by war and the resulting migratory movements [17]. Our study found only three cases of immunosuppressed patients, but 15.1% of patients were alcoholics, which is not negligible. There was also a high proportion of patients with arteriopathy, a risk factor that has never been described before and which deserves to be explored in a comparative study to determine its role in the occurrence of cutaneous diphtheria.
One of the major risk factors for epidemics of diphtheria is low vaccination coverage [9, 17] To prevent a major epidemic in a community, the threshold of herd immunity against diphtheria is considered at 80-85% [14]. In France, only 44% of people over 65 are immunized according to recommendation. This trend of poor immunization is striking in our study: at least 36.6% of patients were not immunized to diphtheria. Not only does vaccination protect against toxigenic strains and therefore death, [18] but also reduces the risk of transmission by 60% [17]. Moreover, recent work [14] suggested that the vaccine might also protect partially against tox-negative infections and colonization. It is therefore necessary, via the public health systems in France, to encourage physicians to vaccinate with booster doses if needed, in order to prevent new epidemics.
The strength of this study is its clinical description of a large cohort of patients. Indeed, most recently published retrospective studies focus on epidemiological and microbiological data. It is the largest French cohort describing the clinical appearance of cutaneous diphtheria. A recent case series of 14 cases linked with the Indian Ocean was described [19]. The predominance of the lower limbs and the existence of multiple supra-centimetric chronic lesions in our study are also consistent with the literature [20]. Precise detailed clinical descriptions of cutaneous diphtheria date back to descriptive studies of epidemics during and after the Second World War [16, 21]. However, these descriptions were mainly associated with toxigenic strains (up to 84%), reflecting the frequency of pseudomembranous exudate classically described for cutaneous diphtheria. Pseudomembranous exudates were rarely reported in our study, which may be explained by the low number of toxigenic strains and the probable lack of awareness of this aspect among clinicians. In the absence of pseudomembranous exudate, the lesions appear to be polymorphous and difficult to differentiate from a classic pyogenic infection [21]. This can be explained by the high frequency of co-infections with pyogenic bacteria (S. aureus and Streptococcus spp) on samples (88.9%) in our study, and as high as 100% in some authors’ studies [7]. Moreover, both in the literature and in this study, the prior existence of a cutaneous lesion is frequently found, raising questions about the invasive nature of CdSC in generating the lesion, and about the possibility of simple colonization of a wound.
However, certain epidemiological, evolutionary and clinical characteristics should alert the clinician, and allow us to suspect the diagnosis. A return from a travel to an endemic zone, skin lesions that do not heal, or worsening pain in a pre-existing wound should raise suspicion for the diagnosis [21].
Another reason not to ignore this disease is that cutaneous diphtheria can lead to death, even if rarely. According to studies, toxigenic cutaneous diphtheria is associated with a respiratory infection in 20–40% of cases, neurological, cardiac or pulmonary symptoms in 3-5% of cases [4, 5] and up to 28% [22, 23], particularly in people over 60. These figures, which vary considerably from one study to another, depend on the population studied, the country, the study period and access to treatments such as serotherapy or antibiotic therapy. Given the low number of toxigenic strains in our study, these complications are little represented.
Toxigenic complications are well known. Non-toxigenic strains can also cause severe clinical symptoms: pharyngitis, arthritis, bacteraemia [24], endocarditis, osteomyelitis [25], catheter infection [19], also described with a cutaneous origin and therefore should not be underestimated. If left untreated, cutaneous diphtheria can lead to systemic diphtheria with toxin and non-toxin complications and death.
The virulence of non-toxigenic CdSC might be partly explained by the presence of adhesion factors to the body's epithelial cells [26] and the ability to form a biofilm [27]. Various enzymes (phospholipase D, neuraminidase H, and endoglycosidase) may contribute to explain the virulence, particularly that of non-toxigenic C. ulcerans [30].
Even though the virulence of toxigenic and non-toxigenic strains is now well described, and despite the existing recommendations, many clinicians considered a CdSC-positive sample as a simple contamination and did not consider it. More than one in two clinicians did not look for nasopharyngeal carriage, and few checked for eradication of carriage. Contact tracing of cases, even though it is recommended, was largely disregarded, with less than 50% of patients in our study. Two hypotheses may explain these issues: unawareness of French recommendations concerning the management of cutaneous CdSC infections, or lack of knowledge of the pathology, particularly in its cutaneous form. However, there is a significant risk of contagiousness, considered greater in cutaneous forms than in respiratory forms [10, 20]. Indeed, Liebow et al report numerous symptomatic contact cases among caregivers of soldiers hospitalized for Cd: one case of abscessed paronychia, several cases of non-pseudomembranous angina, and skin ulcerations in nurses [16]. CdSC-infected skin lesions are potential reservoirs that can also contaminate the environment of healthy or symptomatic carriers [5]. Diphtheria is a vaccine-preventable disease, but it is also important to be able to detect contact cases to avoid epidemics. In fact, both symptomatic and asymptomatic people can transmit diphtheria by direct contact or by droplet. 9% of patients in our study had a positive nasopharyngeal carriage, which is comparable to other studies [20]. Recognizing, treating and vaccinating patients with cutaneous diphtheria and their contacts thus reduces the risk of epidemics by decreasing patient contagiousness.
The diagnosis is therefore made thanks to a clinician-microbiologist collaboration: on the one hand because the clinicians must alert the microbiologist about the hypothesis of cutaneous diphtheria, on the other hand because the microbiologists must make the clinicians aware of the CdSC‘s pathogenicity which should therefore not be considered as simple contamination.
Nevertheless, this study has several limitations. Although including 63 cases, this work is not representative of the overall epidemiological situation of cutaneous diphtheria in France. Firstly, for administrative reasons and to meet research standards, some populations could not be included in this work: minors, homeless and patients identified as migrants, creating a selection bias.
Due to its retrospective nature, we encountered numerous missing data, which varied depending on the items concerned. These missing data can be explained by a memorization bias associated with delay between the date of onset and the study and sometimes by the non-standardization of data reported in the medical record which was used by clinician for answering the questionnaire. Moreover, the clinicians were not all dermatologists, which could therefore lead to greater variability in clinical descriptions.
The limited number of patients included in this study as well as incomplete data are potentially responsible for a lack of power to demonstrate significant statistical differences in the groups studied where other studies have been able to show differences.
Concerning the antibiotic management, it has been difficult to analyse the sequence of treatment in absence of precision on their temporality and duration before treatment and before or after taking the diagnosis.
Conclusion
This study refined, with certain limitations, the epidemiological, clinical, microbiological and management characteristics of recent cases of skin infection with corynebacteria of the CdSC, toxigenic, or not, occurring in mainland France. Our study offers an overview of practitioners’ practices regarding cutaneous diphtheria.
This work and the related literature, shows that cutaneous diphtheria has unique characteristics. Therefore, we should not consider the presence of Corynebacteria of the diphtheriae complex on a skin lesion as a simple colonization.
It is a rarely mentioned and probably underdiagnosed pathology that could be classified as a “neglected re-emerging infectious disease”. However, from the moment of clinical suspicion or identification, it imposes additional assessment and control measures which seem little known to practitioners.
Although basic research on the knowledge of this infection is still necessary, actions to raise awareness among practitioners and to strengthen vaccination coverage, particularly in endemic areas, seem essential.
Cutaneous diphtheria contributes to the emergence of outbreaks in vulnerable populations, and it is therefore necessary to be aware of this pathology and its recommendations.
Supplementary Material
Acknowledgements
OEDIPE Study Group: We thank the following persons for their help in the study set-up and running: The CHITS, for their investment in this work, and the clinical research associates who participated in the development and administrative aspects of this work: Mrs JOBIC, Mr MITRI, Mr MERCIER, Mrs CESANA CESPA: Mr MARCHI, for their participation in the analysis of statistical data. We thank all clinicians and biologists who responded to the questionnaire: Dr Durupt (Dermatologist, CHU Lyon), Dr Wan (Endocrinologist CHU Lyon), Dr Moret (Endocrinologist, CHU Lyon), Dr Triffault-fillit (Infectious disease specialist, CHU Lyon), Dr Pestre (Infectious disease specialist CH Avignon), Dr Lambert de Cursay (Rheumatologist, CH Brive), Dr Sire (Infectious disease specialist CH De cahors), Dr Martha (Infectious disease specialist, CH Chalon), Dr Guerif (Geriatrician, CH Cholet), Dr Lemaignen (Infectious disease specialist, CHU Tours), Pr Samimi (Dermatologist, CHU Tours), Dr Coudon (General practitioner, CH Bretagne-Atlantique), Dr Fenot (Infectious disease specialist, CHD Vendée), Dr Gramont (Internal medicine physician, CHU Saint-Etienne), Dr Birckel (Dermatologist, CH Colmar),Dr Faure (Infectious disease specialist, CHU Lille),Dr Michon (Infectious disease specialist, CHU Caen),Dr Picard (Dermatologist, CH Saint-Lô), Dr Lansalot-Matras (General Practitioner, CH Tarbes),Dr Liminet (Endocrinologist, CHU Lyon), Dr Djeffal (Endocrinologist, CH Macon), Dr Landais (Internal medicine physician, Hôpital Privé Océane Vannes), Dr Challan-Belval (Internal medicine physician, CH Alpes-Leman), Dr Paris (General Practitioner, CH Briançon), Dr Soubrane (Infectious disease specialist, CHU Bordeaux), Dr Guicheney (Dermatologist, CHU Bordeaux), Dr Seve (Médecin Généraliste, CH Orléans), Dr Cassir (Infectious disease specialist, IHU Marseille), Dr Couzigou (Infectious disease specialist, APHP), Dr Molina (General Practitioner, CHU Toulouse), Dr Lourtet (Biologist, APHP), Dr Vidal (Internal medicine physician, APHP), Dr Soriot-Thomas (General Practitioner, CHU Amiens), Dr Khadher (General Practitioner, CH Guingamp), Pr Piroth (Infectious disease specialist, CHU Dijon), Pr Vedrine (Oncologist, Hôpital Américain de Paris), Dr Paluch (Biologist, CH Valenciennes), Dr Lejeune (Internal medicine physician, CHU Montpellier), Dr Cordel (Infectious disase specialist, APHP), Dr Charvet (Dermatologist, APHP), Dr Herms (Dermatologist, APHP), Dr Surgers (Infectious disease specialist,APHP), Dr Hua (Dermatologist, APHP), Dr Zeller (Internal medicine physician, Hôpital DCSS),Dr Matejka (Endocrinologist, Cabestany), Dr Popescu (Dermatologist, Hôpital Nord-Franche-Comté),Dr Henn (Dermatologist,CH Sud Francilien), Dr Monsel (Infectious disease specialist, APHP), Dr Salle (Dermatologist, APHP), Dr Bentanfous (Infectious disease specialist, APHP), Dr Lefebvre (Infectious disease specialist, CHU Nantes).
Funding Statement
This work was supported by the Centre Hospitalier Intercommunal Toulon La Seyne sur Mer (CHITS) and Hôpital d’Instruction des Armées (HIA) Ste Anne. The National Reference Center for Corynebacteria of the Diphtheriae Complex is supported financially by the Ministry of Health (Public Health France) and Institut Pasteur.
Footnotes
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Contribution of authors
L. Chêne: Conceptualization, Investigation, Methodology, Writing – review & editing. F. Dutasta: Conceptualization, Writing, Manuscript writing. JJ. Morand: Conceptualization, reviewing. A. Valois: Reviewing. F. J anvier: Reviewing. S. Brisse and G. Texier: contributed equally: Supervision, Conceptualization, Data reviewing, Manuscript writing. E. Badell: microbiological strain characterization. J. Toubiana: data reviewing, clinical advising. JP. Suppini: Project administration, Ressources, software, data curation. H. Martinet: Data analysis. Œdipe Study group: Response to the questionnaire.
Ethical approval statement
All parties involved in the act of publishing are treating each other with respect and dignity and without discrimination, harassment, bullying or retaliation.
References
- 1.Sharma NC, Efstratiou A, Mokrousov I, et al. Diphtheria. Nat Rev Dis Primers. 2019;5:81. doi: 10.1038/s41572-019-0131-y [DOI] [PubMed] [Google Scholar]
- 2.Bernard KA, Pacheco AL, Burdz T, et al. Increase in detection of Corynebacterium diphtheriae in Canada: 2006-2019. Can Commun Dis Rep. 2019;45:296–301. doi: 10.14745/ccdr.v45i11a04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gower CM, Scobie A, Fry NK, et al. The changing epidemiology of diphtheria in the United Kingdom, 2009 to 2017. Euro Surveill. 2020;25. doi: 10.2807/1560-7917.ES.2020.25.11.1900462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandit N, Yeshwanth M.. Cutaneous diphtheria in a child. Int J Dermatol. 1999;38:298–299. doi: 10.1046/j.1365-4362.1999.00658.x [DOI] [PubMed] [Google Scholar]
- 5.Harnisch JP. Diphtheria among alcoholic urban adults: A decade of experience in Seattle. Ann Intern Med. 1989;111:71. doi: 10.7326/0003-4819-111-1-71 [DOI] [PubMed] [Google Scholar]
- 6.Patey O, Bimet F, Riegel P, et al. Clinical and molecular study of Corynebacterium diphtheriae systemic infections in France. Coryne Study Group. J Clin Microbiol. 1997;35:441–445. doi: 10.1128/jcm.35.2.441-445.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockcroft WH, Boyko WJ, De A.. Cutaneous infections due to Corynebacterium diphtheriae. Can Med Assoc J. 1973;108:329–331. [PMC free article] [PubMed] [Google Scholar]
- 8.de Benoist A-C, White JM, Efstratiou A, et al. Imported cutaneous diphtheria, United Kingdom. Emerg Infect Dis. 2004;10:511–513. doi: 10.3201/eid1003.030524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen RT, Hardy IRB, Rhodes PH, et al. Ukraine, 1992: first assessment of diphtheria vaccine effectiveness during the recent resurgence of diphtheria in the former Soviet Union. J Infect Dis. 2000;181:S178–S183. doi: 10.1086/315561 [DOI] [PubMed] [Google Scholar]
- 10.Gomes DLR, Martins CAS, Faria LMD, et al. Corynebacterium diphtheriae as an emerging pathogen in nephrostomy catheter-related infection: evaluation of traits associated with bacterial virulence. J Med Microbiol. 2009;58:1419–1427. doi: 10.1099/jmm.0.012161-0 [DOI] [PubMed] [Google Scholar]
- 11.Quick ML, Sutter RW, Kobaidze K, et al. Epidemic diphtheria in the Republic of Georgia, 1993–1996: risk factors for fatal outcome among hospitalized patients. J Infect Dis. 2000;181:S130–S137. doi: 10.1086/315550 [DOI] [PubMed] [Google Scholar]
- 12.Zasada AA, Rzeczkowska M.. Nontoxigenic Corynebacterium diphtheriae infections, Europe. Emerg Infect Dis. 2019;25:1437–1438. doi: 10.3201/eid2507.180995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Björkholm B, Olling S, Larsson P, et al. An outbreak of diphtheria among Swedish alcoholics. Infection. 1987;15:354–358. doi: 10.1007/BF01647738 [DOI] [PubMed] [Google Scholar]
- 14.Coen M, Alberto C, Éperon G, et al. Diphtérie : nouvelle présentation clinique d’une ancienne maladie. Revue Médicale Suisse. 2019;15:786–790. doi: 10.53738/REVMED.2019.15.646.0786 [DOI] [PubMed] [Google Scholar]
- 15.Wojewoda CM, Koval CE, Wilson DA, et al. Bloodstream infection caused by nontoxigenic Corynebacterium diphtheriae in an immunocompromised host in the United States. J Clin Microbiol. 2012;50:2170–2172. doi: 10.1128/JCM.00237-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truelove SA, Keegan LT, Moss WJ, et al. Clinical and epidemiological aspects of diphtheria: A systematic review and pooled analysis. Clin Infect Dis. 2020;71:89–97. doi: 10.1093/cid/ciz808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner KS, White JM, Lucenko I, et al. Diphtheria in the postepidemic period, Europe, 2000–2009. Emerg Infect Dis. 2012;18:217–225. doi: 10.3201/eid1802.110987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martini H, Soetens O, Litt D, et al. Diphtheria in Belgium: 2010–2017. J Med Microbiol. 2019;68:1517–1525. doi: 10.1099/jmm.0.001039 [DOI] [PubMed] [Google Scholar]
- 19.Liebow AA. Tropical ulcers and cutaneous diphtheria. Arch Intern Med. 1946;78:255. doi: 10.1001/archinte.1946.00220030002001 [DOI] [PubMed] [Google Scholar]
- 20.Chaudhary A, Pandey S.. Corynebacterium diphtheriae.. Treasure Island (FL: ): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 21.Gunatillake PD, Taylor G.. The role of cutaneous diphtheria in the acquisition of immunity. J Hyg (Lond). 1968;66:83–88. doi: 10.1017/s0022172400040961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peixoto RS, Hacker E, Antunes CA, et al. Pathogenic properties of a Corynebacterium diphtheriae strain isolated from a case of osteomyelitis. J Med Microbiol. 2016;65:1311–1321. doi: 10.1099/jmm.0.000362 [DOI] [PubMed] [Google Scholar]
- 23.Ott L, Möller J, Burkovski A.. Interactions between the Re-emerging pathogen Corynebacterium diphtheriae and host cells. IJMS. 2022;23:3298. doi: 10.3390/ijms23063298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alberto C, Osdoit S, Villani A-P, et al. Cutaneous ulcers revealing diphtheria: A re-emerging disease imported from Indian Ocean countries? Annales de Dermatologie et de Vénéréologie. 2021;148:34–39. doi: 10.1016/j.annder.2020.04.024 [DOI] [PubMed] [Google Scholar]
- 25.Bezjak V, Farsey SJ.. Corynebacterium diphtheriae in skin lesions in Ugandan children. Bull World Health Organ. 1970;43:643–650. [PMC free article] [PubMed] [Google Scholar]
- 26.Livingood CS, Perry DJ, Forrester JS.. Cutaneous diphtheria: a report of 140 Cases1. J Invest Dermatol. 1946;7:341–364. doi: 10.1038/jid.1946.42 [DOI] [PubMed] [Google Scholar]
- 27.Burkovski A. Cell envelope of Corynebacteria: structure and influence on pathogenicity. ISRN Microbiol. 2013;2013:1–11. doi: 10.1155/2013/935736 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.