Abstract
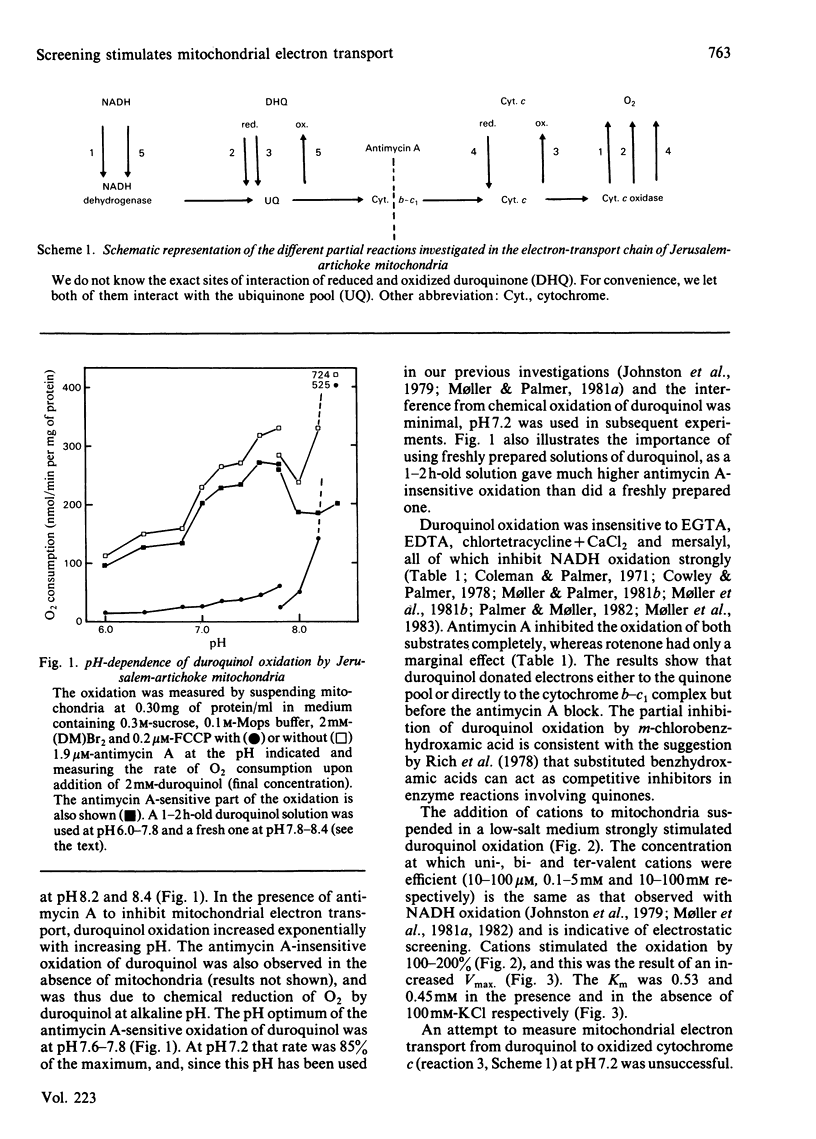
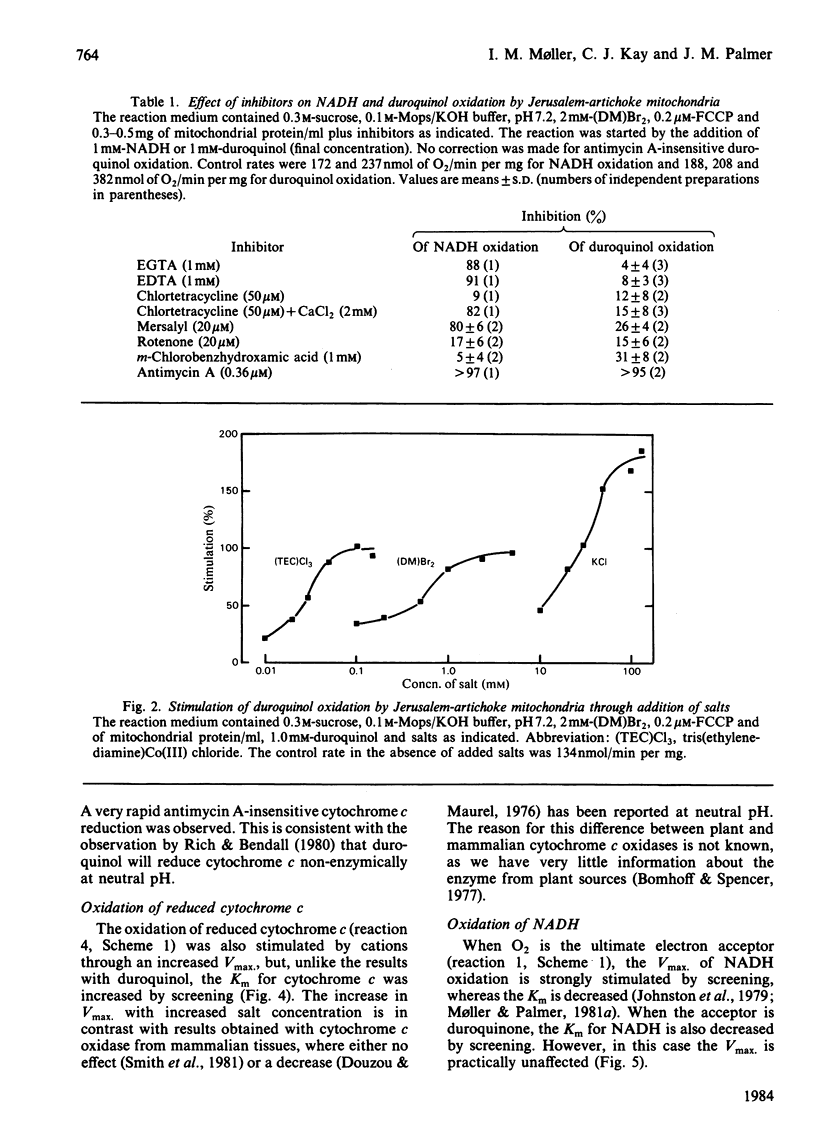
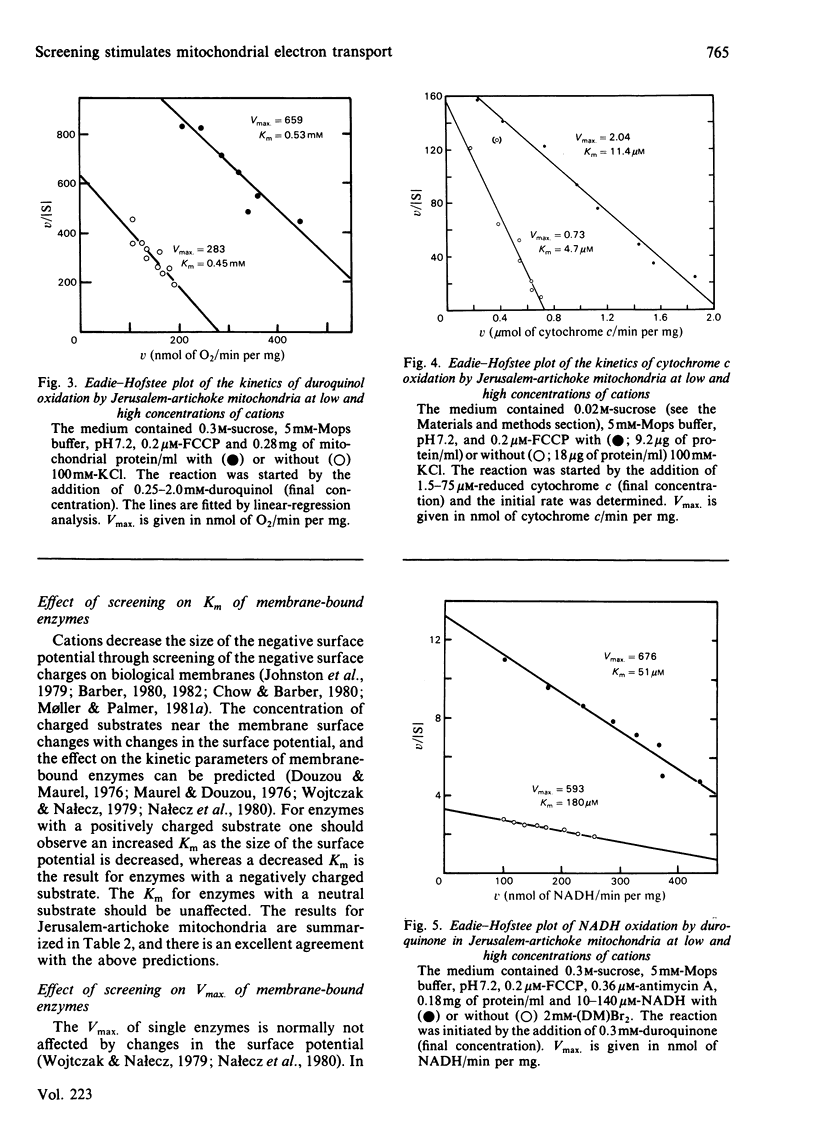
The effect of electrostatic screening of fixed negative charges on uncoupled mitochondrial electron transport was investigated with substrates with different charge and different sites of donation of electrons to the electron-transport chain of Jerusalem-artichoke (Helianthus tuberosus L.) mitochondria. Duroquinol (neutral substrate) was oxidized with a pH optimum of 7.6-7.8. The addition of cations caused a doubling of Vmax. (order of efficiency C3+ greater than C2+ greater than C+) through electrostatic screening, whereas the Km was unaffected. Screening stimulated (by 150%) the Vmax. for the oxidation of reduced cytochrome c (positive substrate; to O2), but in this case the Km doubled. The Vmax. of the oxidation of exogenous NADH (negative substrate) was also stimulated by screening when the acceptor was O2, but unaffected when duroquinone was the acceptor. In both cases, the Km for NADH was considerably decreased. The effect of screening on the Km for the different substrates can be explained by the changes in the effective concentration of substrate near the active site due to the lowering in the size of the surface potential. The effect of screening on the Vmax. of the different partial processes indicates that increasing the salt concentration of the medium enhances the maximal activity of cytochrome c oxidase. However, the results also point at the existence of other rate-limiting steps, which are affected by screening and may involve ubiquinone, in electron transport in plant mitochondria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber J. Membrane surface charges and potentials in relation to photosynthesis. Biochim Biophys Acta. 1980 Dec;594(4):253–308. doi: 10.1016/0304-4173(80)90003-8. [DOI] [PubMed] [Google Scholar]
- Bomhoff G. H., Spencer M. Optimum pH and ionic strength for the assay of cytochrome c oxidase from pea cotyledon mitochondria. Can J Biochem. 1977 Oct;55(10):1114–1117. doi: 10.1139/o77-165. [DOI] [PubMed] [Google Scholar]
- Chow W. S., Barber J. Salt-dependent changes of 9-aminoacridine fluorescence as a measure of charge densities of membrane surfaces. J Biochem Biophys Methods. 1980 Sep;3(3):173–185. doi: 10.1016/0165-022x(80)90016-0. [DOI] [PubMed] [Google Scholar]
- Coleman J. O.D., Palmer J. M. Role of Ca(2+) in the oxidation of exogenous NADH by plant mitochondria. FEBS Lett. 1971 Oct 1;17(2):203–208. doi: 10.1016/0014-5793(71)80148-5. [DOI] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- Douzou P., Maurel P. Le contrôle ionique des réactions biochimiques. C R Acad Sci Hebd Seances Acad Sci D. 1976 Jun 21;282(23):2107–2110. [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Hackett D. P. Effects of salts on DPNH oxidase activity & structure of sweet potato mitochondria. Plant Physiol. 1961 Jul;36(4):445–452. doi: 10.1104/pp.36.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Johnston S. P., Møller I. M., Palmer J. M. The stimulation of exogenous NADH oxidation in Jerusalem artichoke mitochondria by screening of charges on the membranes. FEBS Lett. 1979 Dec 1;108(1):28–32. doi: 10.1016/0014-5793(79)81171-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawford H. G., Garland P. B. Proton translocation coupled to quinone reduction by reduced nicotinamide--adenine dinucleotide in rat liver and ox heart mitochondria. Biochem J. 1972 Dec;130(4):1029–1044. doi: 10.1042/bj1301029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel P., Douzou P. Catalytic implications of electrostatic potentials: the lytic activity of lysozymes as a model. J Mol Biol. 1976 Apr 5;102(2):253–264. doi: 10.1016/s0022-2836(76)80052-6. [DOI] [PubMed] [Google Scholar]
- Møller I. M., Chow W. S., Palmer J. M., Barber J. 9-Aminoacridine as a fluorescent probe of the electrical diffuse layer associated with the membranes of plant mitochondria. Biochem J. 1981 Jan 1;193(1):37–46. doi: 10.1042/bj1930037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller I. M., Johnston S. P., Palmer J. M. A specific role for Ca2+ in the oxidation of exogenous NADH by Jerusalem-artichoke (Helianthus tuberosus) mitochondria. Biochem J. 1981 Feb 15;194(2):487–495. doi: 10.1042/bj1940487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller I. M., Palmer J. M. Charge screening by cations affects the conformation of the mitochondrial inner membrane. A study of exogenous MAD(P)H oxidation in plant mitochondria. Biochem J. 1981 Jun 1;195(3):583–588. doi: 10.1042/bj1950583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller I. M., Schwitzguébel J. P., Palmer J. M. Binding and screening by cations and the effect on exogenous NAD(P)H oxidation in Neurospora crassa mitochondria. Eur J Biochem. 1982 Mar;123(1):81–88. doi: 10.1111/j.1432-1033.1982.tb06501.x. [DOI] [PubMed] [Google Scholar]
- Nałecz M. J., Zborowski J., Famulski K. S., Wojtczak L. Effect of phospholipid composition on the surface potential of liposomes and the activity of enzymes incorporated. Eur J Biochem. 1980 Nov;112(1):75–80. doi: 10.1111/j.1432-1033.1980.tb04988.x. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Bendall D. S. The kinetics and thermodynamics of the reduction of cytochrome c by substituted p-benzoquinols in solution. Biochim Biophys Acta. 1980 Oct 3;592(3):506–518. doi: 10.1016/0005-2728(80)90095-x. [DOI] [PubMed] [Google Scholar]
- Rich P. R., Wiegand N. K., Blum H., Moore A. L., Bonner W. D., Jr Studies on the mechanism of inhibition of redox enzymes by substituted hydroxamic acids. Biochim Biophys Acta. 1978 Aug 7;525(2):325–337. doi: 10.1016/0005-2744(78)90227-9. [DOI] [PubMed] [Google Scholar]
- Schneider H., Lemasters J. J., Hackenbrock C. R. Lateral diffusion of ubiquinone during electron transfer in phospholipid- and ubiquinone-enriched mitochondrial membranes. J Biol Chem. 1982 Sep 25;257(18):10789–10793. [PubMed] [Google Scholar]
- Schneider H., Lemasters J. J., Höchli M., Hackenbrock C. R. Liposome-mitochondrial inner membrane fusion. Lateral diffusion of integral electron transfer components. J Biol Chem. 1980 Apr 25;255(8):3748–3756. [PubMed] [Google Scholar]
- Smith H. T., Ahmed A. J., Millett F. Electrostatic interaction of cytochrome c with cytochrome c1 and cytochrome oxidase. J Biol Chem. 1981 May 25;256(10):4984–4990. [PubMed] [Google Scholar]
- Storey B. T. Respiratory Chain of Plant Mitochondria: XVIII. Point of Interaction of the Alternate Oxidase with the Respiratory Chain. Plant Physiol. 1976 Oct;58(4):521–525. doi: 10.1104/pp.58.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak L., Famulski K. S., Nałecz M. J., Zborowski J. Influence of the surface potential on the Michaelis constant of membrane-bound enzymes: effect of membrane solubilization. FEBS Lett. 1982 Mar 22;139(2):221–224. doi: 10.1016/0014-5793(82)80856-9. [DOI] [PubMed] [Google Scholar]
- Wojtczak L., Nałecz M. J. Surface change of biological membranes as a possible regulator of membrane-bound enzymes. Eur J Biochem. 1979 Feb 15;94(1):99–107. doi: 10.1111/j.1432-1033.1979.tb12876.x. [DOI] [PubMed] [Google Scholar]


