Abstract
Background
Autosomal dominant polycystic kidney disease (ADPKD) is the most prevalent hereditary kidney disease, characterized by enlarged kidneys with numerous cysts, high blood pressure, and a variety of extrarenal complications. This disease is a significant cause of renal failure and requires accurate differentiation from other cystic kidney diseases, especially when family history does not clearly indicate ADPKD. This is crucial due to differences in prognosis, treatment, and familial implications. Advanced molecular genetics and imaging techniques are employed to diagnose and assess the prognosis of patients and their families.
Case presentation
The case study revolves around three patients from the same family—two sisters and one daughter—referred to a nephrology department for ADPKD management. The initial proband, a 42-year-old woman, experienced abdominal discomfort leading to an ultrasound that suggested ADPKD. However, MRI findings indicated standard-sized kidneys with bilateral parapelvic cysts, and no genetic markers for ADPKD were found. Her sister, presenting with controlled hypertension and similar ultrasound findings, also had her initial ADPKD diagnosis refuted by MRI and genetic testing, which revealed no significant mutations. The daughter, however, exhibited a different scenario with enlarged kidneys and multiple cysts characteristic of early-stage ADPKD. Genetic testing confirmed a deleterious PKD1 mutation, suggesting a de novo mutation, as her father showed no signs of the disease.
Conclusion
This study highlights the complexity and necessity of thorough diagnostic processes in suspected ADPKD cases to prevent misdiagnosis. The initial symptoms and imaging might misleadingly suggest ADPKD, as seen in the cases of the two older patients. Still, further detailed imaging and genetic analyses revealed no ADPKD, preventing inappropriate treatment and stress. In contrast, the younger patient's distinctive clinical and genetic profile confirmed ADPKD, illustrating the variability within even closely related individuals. Such detailed assessments are crucial in guiding correct treatment decisions and providing accurate familial counseling, emphasizing the importance of considering a broader spectrum of renal cystic disorders before confirming a diagnosis of ADPKD.
Keywords: Kidney, Cysts, Polycystic kidney, Autosomal dominant, Mutation
Background
Autosomal dominant polycystic kidney disease (ADPKD) is the most prevalent hereditary kidney disease and remains one of the leading causes of renal failure. The condition presents various clinical features, including enlarged kidneys due to proliferating cysts, high blood pressure, and a range of extrarenal complications. Molecular genetics and imaging techniques are tools to diagnose and prognose individual patients and their families [1]. Understanding the differential diagnoses among cystic kidney diseases is essential, especially in cases where family history is unclear. This differentiation is necessary due to the disparities in prognosis, treatment, and familial implications [2]. The following case study details the clinical trajectories of three related patients referred to our specialized center for ADPKD management who turned out to have different diseases.
Case presentation
Three patients, namely the proband (II-2), her sister (II-3), and her daughter (III-2), were referred to the Nephrology Department of our university hospital by their shared primary care physician for the management of suspected ADPKD.
Patient II-2 (proband)
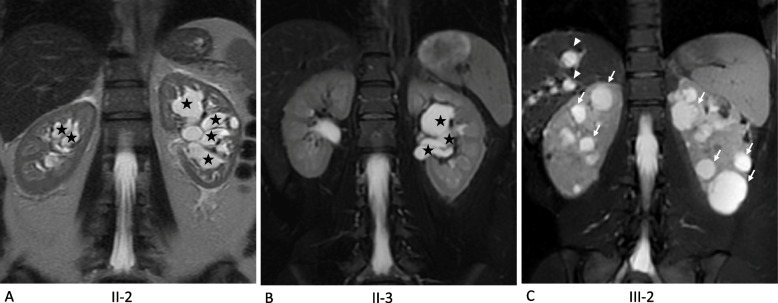
In the case of the 42-year-old woman (II-2), abdominal discomfort prompted an abdominal ultrasound (US) that revealed multiple bilateral hypoechoic structures suggestive of ADPKD. The patient had no family history of kidney disorders, prior medical history, or high blood pressure. No proteinuria or hematuria was noted, and her estimated glomerular filtration rate (eGFR) was normal (106 ml/min/1.73 m2). Subsequent MRI aimed at renal volumetry displayed standard-sized kidneys (10.7 × 5.7 cm on the right and 11.6 × 6 cm on the left). Our radiology center, genitourinary specialized, later suggested bilateral parapelvic cysts without cortical cysts rather than typical ADPKD features (Fig. 1). Furthermore, there were no liver cysts.
Fig. 1.
Comparative MRI Findings in Patients with Parapelvic (Patients II-2 & II-3) vs ADPKD (Patients III-2). Coronal T2-weighted fat-suppressed (fat sat) MRI images demonstrate the presence of bilateral parapelvic cysts (black stars) without any accompanying cortical cysts in Patients II-2 (A) and II-3 (B). In contrast, coronal T2-weighted fat-suppressed MRI images for Patient III-2 reveal bilaterally enlarged kidneys, multiple cortical cysts (white arrows), and associated liver cysts (white arrowheads) (C)
Patient II-3
The patient's sister, aged 38, had arterial hypertension diagnosed at age 35 and successfully managed with a calcium channel blocker. Her medical history remained unremarkable, without urological symptoms. The abdominal US, conducted after her sister's diagnosis, also revealed kidneys with hypoechoic lesions and a hepatic cyst. The attending physician drew parallels to ADPKD in her case as well. During her initial visit to our center, the physical examination yielded no anomalies and normal blood pressure (127/85 mmHg). Laboratory results indicated normal renal function (eGFR 107 ml/min/1.73 m2), a normal urine protein to creatinine (UPC) ratio of 0.1 g/g, and the absence of microscopic hematuria. Renal MRI corroborated normal-sized kidneys (10.2 × 5.3 cm on the right and 11.3 × 6 cm on the left) but characterized the cysts as parapelvic cysts, a banal solitary hepatic cyst measuring 10 mm was present in segment VII (Fig. 1). This MRI invalidated the diagnosis of ADPKD yet again.
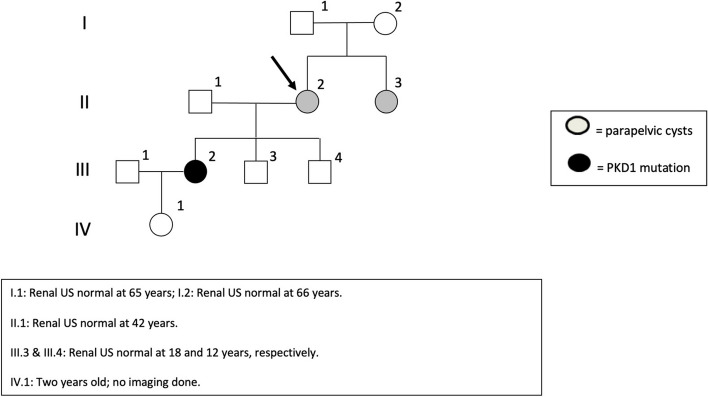
Both patients underwent genetic testing for a panel of cystic kidney diseases genes comprising PKD1, PKD2, GANAB, HNF1b, SEC63, PRKCSH, and UMOD. Nevertheless, no deleterious variants were identified. No signs of Fabry disease (FD) were identified, and no history of renal failure, cardiomyopathy, or stroke was recorded in the relatives. Since no clinical features suggesting FD were identified in the 2 sisters with parapelvic cysts or in their parents, no genetic testing of the GLA gene was performed. Of note, neither their father (I-1) or mother (I-2) had renal cysts at age 65 and 66 years, respectively.
Patient III-2
The 21-year-old proband ‘s daughter's medical history was marked only by managed hypothyroidism (Levothyroxine, 50 µg per day). Her blood pressure and renal function (eGFR 120 ml/min/1.73 m2) were within normal ranges, without microscopic hematuria or proteinuria. She was referred to our center for familial screening. A kidney US unveiled slightly enlarged kidneys, measuring 12.8 × 5.4 cm on the right and 12.2 × 6 cm on the left, each housing more than ten cysts. A subsequent MRI confirmed the mostly cortical cysts, while parapelvic cysts were absent. Her height-adjusted total kidney volume (htTKV) was 352 ml/m. Three small hepatic cysts were also identified (Fig. 1).
Her renal imaging pattern aligned with ADPKD's early stages. Despite the lack of PKD1 or PKD2 mutations in her mother (II-2) and maternal aunt (II-1), next-generation sequencing (NGS) genetic testing was performed owing to her distinctive renal phenotype. The evaluation uncovered a deleterious mutation in the PKD1 gene (c.11453dup in exon 41), confirming an ADPKD molecular diagnosis. In light of her mother's (II-2) absence of a renal ADPKD phenotype or PKD1 mutation, attention turned to the paternal lineage. Her 42-year-old father had no history of hypertension, urological symptoms, or chronic kidney disease. A renal ultrasound conducted at age 42 showed no renal cysts, effectively ruling out a PKD1 mutation. Patient II-2 thus had a PKD1 de novo mutation (Fig. 2).
Fig. 2.
Genealogical tree. Square: males; circles: females. Renal US normal at 65 years (I.1); renal US normal at 66 years (I.2); renal US normal at 42 years (II.1); renal US normal at 18 and 12 years (III.3 & III.4, respectively); 2 years old, ADPKD screening not performed (IV-1)
Discussion and conclusions
US findings are pivotal in ADPKD diagnosis, mainly when there is a positive family history [3, 4]. For individuals under 40, MRI is more accurate when a threshold of more than ten cysts for positive diagnosis is applied [5]; however, this diagnostic criteria is applicable only when another sibling has ADPKD. Standardized ADPKD diagnostic criteria for patients without a family history are currently lacking, placing genetic testing at the forefront for such cases, which reveals de novo mutations in half of these patients [6]. Most of these mutations are concentrated within the PKD1 gene, with truncating mutations constituting roughly two-thirds.
Accurate ADPKD diagnosis is crucial for treatment decisions, such as Tolvaptan eligibility, which is FDA and EMA-approved for adults with rapidly progressive ADPKD as per MAYO and ERA-EDTA classifications [7]. These classifications consider various factors like age, eGFR, htTKV, which is the primary predictor of rapid progression to ESKR, and PROPKD score, taking into account the molecular diagnosis of a truncating PKD1 mutation, linked to a more adverse renal prognosis [8]. Patient III-2 did not yet qualified for Tolvaptan despite her htTKV-based Mayo class IC and a PKD1 truncating mutation predicting rapid progression, because of her young age, but she will go on follow-up. The PROPKD score is inappropriate for patients under 35 without complications, for whom htTKV and PKD1 mutations primarily guide treatment.
Currently, octreotide long-acting release (octreotide-LAR), a somatostatin analog, is also approved in Italy for the treatment of ADPKD fast progressors. Somatostatin analogs have demonstrated efficacy not only in the treatment of ADPKD but also in polycystic liver disease [9].
Given the implications for prognosis and family, avoiding ADPKD misdiagnosis is equally crucial. This condition should not be confused with renal sinus cysts, benign simple renal cysts within the renal sinus, categorized as parapelvic or peripelvic cysts [10]. Parapelvic cysts originate from the renal parenchyma and protrude into the renal sinus [11], generally solitary or limited in number [12, 13]. Peripelvic cysts originate within the sinus, can be simple or multiloculated, and often manifest bilaterally and in multiple numbers. They stem from the cystic dilatation of lymph-filled cavities [14, 15]. Clinically, "renal sinus cyst" broadly describes any fluid-filled cyst within the renal sinus, as the specific type usually has minimal impact. They are frequently misdiagnosed as pyelocaliceal dilation on unenhanced CT scans or US due to their fluid-attenuated, hypoechoic nature [16], but interconnected calyces and a “cauliflower-like” renal pelvis can help to suggest hydronephrosis. Contrast-enhanced CT during the excretory phase distinguishes peripelvic cysts from hydronephrosis, as sinus cysts displace the enhanced collecting systems [12]. The prevalence of renal sinus cysts is between 1.5% and 6% of the older population [17, 18], less common than the 5.8% to 10% for simple cortical cysts [19].
Renal sinus cysts are usually asymptomatic and found incidentally, though larger cysts may cause obstruction by compressing the renal collecting system, negatively affecting renal function [20, 21]. Complications encompass cyst infection, chronic pyelonephritis, stone formation due to stasis [22], and intracystic bleeding. Renal pedicle compression from vascular sources might trigger renin-mediated hypertension [22]. Finally, renal sinus cysts could potentially obscure malignant lesions. Umemoto et al. demonstrated that three of 73 patients with renal sinus cysts had renal pelvic cancer, and another displayed ureteral cancer. Tumors could impair lymph flow, contributing to cyst development [23].
Importantly, FD patients face an elevated risk of developing renal sinus cysts [24]. In a cohort comprising affected carriers harboring classic and cardiac FD variants, the prevalence of renal parapelvic cysts was heightened (14.5% in males and 10% in female carriers), with earlier onset than in the general population [25]. FD patients may also experience limb-related lymphedema. Riccio et al. recently reported a case of a 30-year-old woman presenting with both renal cysts and parapelvic cysts; she was shown to carry both a PKD1 gene variant (c.10549G > T) and a GLA gene variant (c.868A > C) [26]. No signs of FD in our patients II-2 and II-3 were identified, and no history of renal failure, cardiomyopathy, or stroke was recorded in the relatives making it very unlikely that they had FD related renal sinus cysts.
In conclusion, this study illuminates the potential pitfalls of hastily attributing familial renal cysts solely to ADPKD. A misdiagnosis can lead to inappropriate treatment strategies, undue stress, and inaccurate familial counseling. Hence, a holistic approach, including precise imaging and genetic assessment, is essential to diagnose and manage patients with cystic kidney diseases accurately. Clinicians must remain vigilant and consider the broader spectrum of renal cystic disorders, including renal sinus cysts, before settling on a definitive diagnosis of ADPKD.
Acknowledgements
The authors thank Cecile Berberat for the editing service. Guarantor: Prof. Bertrand Knebelmann is the scientific guarantor of this publication.
Abbreviations
- ADPKD
Autosomal Dominant Polycystic Kidney Disease
- US
Ultrasound
- eGFR
Estimated Glomerular Filtration Rate
- MRI
Magnetic Resonance Imaging
- UPC
Urine Protein to Creatinine Ratio
- PKD1, PKD2
Polycystic Kidney Disease 1 and 2 genes
- GANAB
Neutral Glucosidase Alpha AB
- HNF1b
Hepatocyte Nuclear Factor 1beta
- SEC63
SEC63 Homolog, Protein Translocation Regulator
- PRKCSH
Protein Kinase C Substrate 80K-H
- UMOD
Uromodulin
- NGS
Next-Generation Sequencing
- htTKV
Height-Adjusted Total Kidney Volume
- FDA
Food and Drug Administration
- EMA
European Medicines Agency
- ERA-EDTA
European Renal Association-European Dialysis and Transplant Association
- ESKR
End Stage Kidney Disease
- PROPKD
Prediction of Polycystic Kidney Disease Progression
- PI3K/AKT/mTOR
Phosphoinositide 3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin Pathway
- FD
Fabry Disease
- GLA
Galactosidase Alpha
Authors’ contributions
SB and RN analyzed and interpreted the patient data. SB performed the bibliography. SB and OH performed the radiological analysis. BK performed the work supervision. All authors read and approved the final manuscript.
Funding
None. The authors state that this work has not received any funding.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Yes; consent obtained from patients and their families.
Consent for publication
Patients and their families were informed of this publication, and they gave their informed consent.
Competing interests
The authors declare no competing interests.
Footnotes
The original version of this article was revised: the authors identified an error in the name of author, Sylvain Bodard.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/11/2024
A Correction to this paper has been published: 10.1186/s12882-024-03900-8
References
- 1.Ong ACM, Devuyst O, Knebelmann B, Walz G. ERA-EDTA Working Group for Inherited Kidney Diseases. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385(9981):1993–2002. 10.1016/S0140-6736(15)60907-2. [DOI] [PubMed] [Google Scholar]
- 2.Sekine A, Hidaka S, Moriyama T, et al. Cystic Kidney Diseases That Require a Differential Diagnosis from Autosomal Dominant Polycystic Kidney Disease (ADPKD). J Clin Med. 2022;11(21):6528. 10.3390/jcm11216528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205–12. 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belibi FA, Edelstein CL. Unified ultrasonographic diagnostic criteria for polycystic kidney disease. J Am Soc Nephrol. 2009;20(1):6–8. 10.1681/ASN.2008111164. [DOI] [PubMed] [Google Scholar]
- 5.Pei Y, Hwang YH, Conklin J, et al. Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2015;26(3):746–53. 10.1681/ASN.2014030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iliuta IA, Kalatharan V, Wang K, et al. Polycystic Kidney Disease without an Apparent Family History. J Am Soc Nephrol. 2017;28(9):2768–76. 10.1681/ASN.2016090938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller RU, Messchendorp AL, Birn H, et al. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders, the European Rare Kidney Disease Reference Network and Polycystic Kidney Disease International. Nephrol Dial Transplant. 2022;37(5):825–39. 10.1093/ndt/gfab312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornec-Le Gall E, Audrézet MP, Chen JM, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24(6):1006–13. 10.1681/ASN.2012070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capuano I, Buonanno P, Riccio E, Rizzo M, Pisani A. Tolvaptan vs. somatostatin in the treatment of ADPKD: A review of the literature. Clin Nephrol. 2022;97(3):131–40. 10.5414/CN110510. [DOI] [PubMed] [Google Scholar]
- 10.Capuano I, Buonanno P, Riccio E, Crocetto F, Pisani A. Parapelvic Cysts: An Imaging Marker of Kidney Disease Potentially Leading to the Diagnosis of Treatable Rare Genetic Disorders? A Narrative Review of the Literature. J Nephrol. 2022;35(8):2035–46. 10.1007/s40620-022-01375-0. [DOI] [PubMed] [Google Scholar]
- 11.Nahm AM, Ritz E. The renal sinus cyst-the great imitator. Nephrol Dial Transplant. 2000;15(6):913–4. 10.1093/ndt/15.6.913. [DOI] [PubMed] [Google Scholar]
- 12.Rha SE, Byun JY, Jung SE, et al. The renal sinus: pathologic spectrum and multimodality imaging approach. Radiographics. 2004;24(Suppl 1):S117-131. 10.1148/rg.24si045503. [DOI] [PubMed] [Google Scholar]
- 13.Alves M, Fonseca T, de Almeida EAF. Differential Diagnosis of Autosomal Dominant Polycystic Kidney Disease. In: Li X, ed. Polycystic Kidney Disease. Codon Publications; 2015. Accessed August 14, 2023. http://www.ncbi.nlm.nih.gov/books/NBK373390/ [PubMed]
- 14.Eskildsen DE, Guccione J, Menias CO, et al. Perirenal lymphatics: anatomy, pathophysiology, and imaging spectrum of diseases. Abdom Radiol (NY). 2023;48(8):2615–27. 10.1007/s00261-023-03948-4. [DOI] [PubMed] [Google Scholar]
- 15.Cronan JJ, Amis ES, Yoder IC, Kopans DB, Simeone JF, Pfister RC. Peripelvic cysts: an impostor of sonographic hydronephrosis. J Ultrasound Med. 1982;1(6):229–36. 10.7863/jum.1982.1.6.229. [DOI] [PubMed] [Google Scholar]
- 16.Gopaluni S, Gibson M, Mohteshamzadeh M. An unusual case of familial cystic kidney disease. Clin Kidney J. 2014;7(5):484–5. 10.1093/ckj/sfu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amis ES, Cronan JJ. The renal sinus: an imaging review and proposed nomenclature for sinus cysts. J Urol. 1988;139(6):1151–9. 10.1016/s0022-5347(17)42845-x. [DOI] [PubMed] [Google Scholar]
- 18.Henthorne JC. Peripelvic Lymphatic Cysts of the Kidney: A Review of the Literature on Perinephric Cysts. Am J Clin Pathol. 1938;8(1):28–38. 10.1093/ajcp/8.1.28. [Google Scholar]
- 19.Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol. 2002;167(1):21–3. [PubMed] [Google Scholar]
- 20.Amis ES, Cronan JJ, Pfister RC. The spectrum of peripelvic cysts. Br J Urol. 1983;55(2):150–3. 10.1111/j.1464-410x.1983.tb06543.x. [DOI] [PubMed] [Google Scholar]
- 21.Hinman F. Obstructive renal cysts. J Urol. 1978;119(5):681–3. 10.1016/s0022-5347(17)57588-6. [DOI] [PubMed] [Google Scholar]
- 22.Shah JB, Whitman C, Lee M, Gupta M. Water under the bridge: 5-year outcomes after percutaneous ablation of obstructing parapelvic renal cysts. J Endourol. 2007;21(10):1167–70. 10.1089/end.2007.9914. [DOI] [PubMed] [Google Scholar]
- 23.Umemoto Y, Okamura T, Akita H, Yasui T, Kohri K. Clinical evaluation of parapelvic renal cysts: do these represent latent urological malignant disease? Asian Pac J Cancer Prev. 2009;10(6):1119–20. [PubMed] [Google Scholar]
- 24.Ries M, Bettis KEB, Choyke P, et al. Parapelvic kidney cysts: a distinguishing feature with high prevalence in Fabry disease. Kidney Int. 2004;66(3):978–82. 10.1111/j.1523-1755.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- 25.Glass RBJ, Astrin KH, Norton KI, et al. Fabry disease: renal sonographic and magnetic resonance imaging findings in affected males and carrier females with the classic and cardiac variant phenotypes. J Comput Assist Tomogr. 2004;28(2):158–68. 10.1097/00004728-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Riccio E, Imbriaco M, Daniele A, Iaccarino G, Pisani A. The Case | A patient with autosomal dominant polycystic kidney disease with an atypical kidney magnetic resonance image. Kidney Int. 2023;104(3):625–6. 10.1016/j.kint.2023.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.