Abstract
Background
Adolescent idiopathic scoliosis (AIS) corrective surgery may involve significant blood loss and blood transfusion requirements. Antifibrinolytic agents such as tranexamic acid (TXA) have been used to reduce blood loss, but its optimal dose is uncertain. The objective of this study is to determine the estimated blood loss and rate of blood transfusion between two groups of AIS patients receiving TXA as a single bolus versus bolus followed by infusion in scoliosis surgery.
Methods
This was a retrospective analysis of a single bolus versus bolus followed by infusion of TXA in AIS surgery. AIS patients undergoing posterior spinal fusion (PSF) from December 2018 to September 2019 at a tertiary university hospital were identified. Inclusion criteria were patients aged between 10 and 21 years who received either a single bolus of 30 mg/kg TXA (Group A) or a single bolus of 30 mg/kg followed by continuous infusion of 10 mg/kg/h of TXA (Group B). Patient demographics, operative data, estimated blood loss, blood transfusion rate, and complications were recorded.
Results
A total of 129 AIS patients were included. All operative surgeries were performed by two senior consultants. The mean age was 14.8 ± 3.4 years old, and 89.1% were female. The Cobb angle, number of fusion levels, number of screws, length of skin incision, and duration of surgery were comparable between the two groups. There was no difference in the total estimated blood loss between the two groups: 723.3 ± 279.4 mL (range: 175.0–1607.0 mL) in Group A and 819.4 ± 302.6 mL (range: 330.0–1556.0 mL) in Group B (p = 0.065). There were no complications, and none received blood transfusion.
Conclusion
TXA when administered as a single bolus or bolus followed by infusion in AIS patients undergoing PSF surgery was associated with similar estimated total surgical blood loss and blood transfusion requirement.
Keywords: Tranexamic acid, Blood loss, Spinal fusion, Scoliosis, Adolescent
Background
Adolescent idiopathic scoliosis (AIS) corrective surgery involves soft tissue dissection, bone decortication, osteotomies, and instrumentation which may result in a significant amount of blood loss and blood transfusion requirement (Lonstein 1994; Kesling and Reinker 1997). This exposes patients to the risk of blood-borne disease transmission, increased incidence of wound infections, and haemolytic and nonhaemolytic transfusion reactions (Parent et al. 2005; Lonstein and Carlson 1984).
Intraoperative administration of antifibrinolytic agents such as tranexamic acid (trans−4−aminomethyl−cyclohexane−1−carboxylic acid, TXA) has gained popularity (Nuttall et al. 2000; Sethna et al. 2005). TXA is a synthetic lysine analogue and exerts its antifibrinolytic effect through the reversible blockade of lysine binding sites on plasminogen molecules. Blood loss was significantly reduced in patients who were administered TXA during spine surgery (Jones et al. 2017). Additionally, the perioperative volume of blood transfusion decreased in patients who received TXA (Thompson et al. 2008). However, there is no universally accepted dosing protocol for its use in AIS corrective surgery (Yoo et al. 2019). Our group recently published a randomised trial in which AIS patients were randomised to receive either high−dose TXA (30 mg/kg loading dose followed by 10 mg/kg/h infusion) or low−dose TXA (10 mg/kg loading dose followed by 1 mg/kg/h infusion) (Hasan et al. 2021). In this study, we found that low−dose TXA was as efficacious as high−dose TXA in reducing blood loss and allogenic blood transfusion in AIS patients undergoing posterior spinal fusion (PSF) surgery (Hasan et al. 2021). A plausible explanation is that the median duration of surgery was 120 min, which was relatively short. Based on the plasma TXA versus time simulation curves from Goobie et al. (Goobie and Faraoni 2019), at 120 min, the plasma TXA levels were still within the therapeutic range for both high and low TXA doses (Goobie et al. 2018). Given this finding, we hypothesise that TXA given as a single intravenous bolus will be as efficacious as TXA given as a bolus followed by continuous infusion in reducing total surgical blood loss in scoliosis patients undergoing PSF surgery.
The primary objective of this study was to determine the estimated total surgical blood loss between two groups of AIS patients receiving a single bolus (30 mg/kg) TXA versus bolus (30 mg/kg) followed by continuous infusion (10 mg/kg/h) TXA in scoliosis surgery.
The secondary objectives were to compare the postoperative haemoglobin level, postoperative coagulation profile and fibrinogen level, rate of blood transfusion, and complications among the two groups.
Methods
This was a retrospective observational study. Ethics approval was obtained from the institution medical research ethics committee (MREC ID No.: 202057–8602), and waiver of informed consent was granted due to the retrospective study design. AIS patients who underwent an elective single-staged PSF surgery under general anaesthesia at a tertiary university hospital over a period of 18 months (2018 until 2019) were identified retrospectively. Patients who received a single bolus of 30 mg/kg TXA (Group A) and a bolus of 30 mg/kg followed by continuous infusion of 10 mg/kg/h of TXA (Group B) until the end of surgery were included in the analysis. Decision on TXA dosing regimen was at the discretion of the treating anaesthesiologist, and doses other than the two described above were excluded. Patients aged between 10 and 21 years diagnosed with idiopathic scoliosis, with American Society of Anaesthesiologists (ASA) physical status I–II, preoperative haemoglobin > 10 g/dL, platelet count > 150,000 µ/L, and normal coagulation profile were eligible for inclusion.
Exclusion criteria were patients with severe haematological disorder, severe cardiac disease, or severe restrictive pulmonary disease, received anticoagulants and antiplatelet within 14 days prior to operation, and those who had preoperative serum creatinine > 200 µmol/L or serum aspartate aminotransferase > 100 IU/L.
In addition, only patients who adhered to the anaesthetic and surgical protocol were included together with complete information on estimated total surgical blood loss, pre- and postoperative haemoglobin levels, international normalised ratio (INR), activated partial thromboplastin time (aPTT), and fibrinogen values.
Anaesthetic protocol
The patients received either total intravenous anaesthesia with target-controlled infusion (TCI) of propofol and remifentanil targeting a bispectral index of 40–60 or volatile anaesthetic desflurane with TCI remifentanil aiming a minimum alveolar concentration of 0.6 to 0.8. Intravenous TXA was administered either as a single bolus of 30 mg/kg at induction or as a bolus followed by continuous infusion of 10 mg/kg/h until the end of surgery. Patient monitoring includes invasive blood pressure, heart rate, oxygen saturation, and electrocardiogram. The lower limit of mean arterial pressure (MAP) during exposure was 60 mmHg, whilst during and after correction, the MAP was maintained above 75 mmHg. Cell salvage was used in all patients in which the harvested blood was reinfused when blood loss exceeded 20% of total blood volume or at the end of surgery. The transfusion trigger for allogenic blood transfusion was when haemoglobin level dropped below 8 g/dL.
Surgical protocol
All the surgeries were performed by the two senior surgeons (K. M. K., C. Y. W. C.) utilizing a dual attending surgeon strategy. Alternate-level pedicle screw instrumentation was performed for all patients. At the proximal and distal foundation levels, three to four pedicle screws were inserted. Posterior releases consisted of facetectomies with excision of the inferior articular processes. No posterior column osteotomies were performed. Fusion was augmented using local autogenous bone graft. All patients had subfascial drains inserted prior to closure which was initially clamped during the immediate postoperative period. On postoperative day 1, the drain was subsequently released to drain up to a maximum of 200 mL. The drain was then removed, and no further drainage was performed.
Data acquisition
Data of baseline characteristics including age, sex, height, weight, and body blood volume, as well as baseline operative details such as number of vertebral levels fused, Cobb’s angle, number of screws, duration of surgery, and skin incision length, were collected. Data on estimated total intraoperative and postoperative (from surgical drain) blood loss, blood volume harvested from cell salvage system, and number of allogenic packed cell transfusion were retrieved along with laboratory data (haemoglobin, INR, aPTT, fibrinogen levels) at following time points: T1, pre-operation; T2, post-operation 0 h; and T3, post-operation 48 h. Data on complication rates (seizure, thrombotic event) were gathered.
Statistical analysis
This was a retrospective exploratory analysis. Hence, a priori sample size calculation was not performed. All patients who fulfilled the eligibility criteria between December 2018 and September 2019 were included in the analysis.
Baseline characteristics, operative data, intraoperative blood loss, and laboratory values were compared between Groups A and B. For categorical variables, chi-square test was performed. For continuous variables, independent Student t-test was used for normally distributed data, whilst Mann–Whitney U-test was used for non-normally distributed data. All statistical analyses were performed using SPSS version 22 (IBM Corporation, NY, USA). A two-sided p < 0.05 was considered statistically significant. Multiplicity adjustment was not performed due to the exploratory nature of this study.
Results
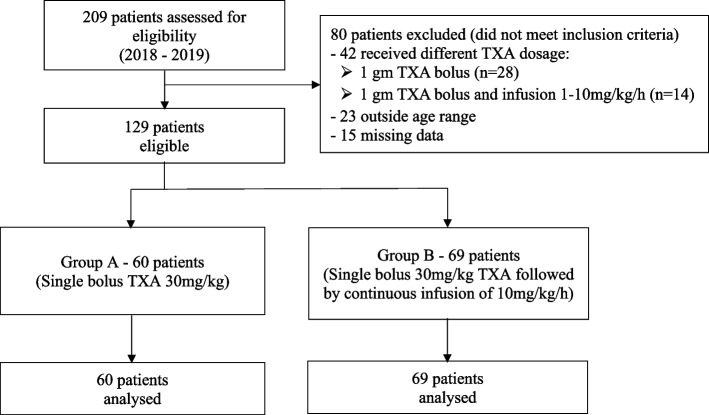
A total of 209 patients were reviewed for eligibility from the year 2018 to 2019. Eighty patients were excluded: 42 due to different TXA dosage regimens, 23 for being outside the age range of interest, and 15 for missing data. Finally, 129 elective AIS patients who fulfilled the inclusion criteria were included (Fig. 1). A total of 60 and 69 patients were in Group A and Group B, respectively. There were no significant differences in the demographic, baseline characteristics, and operative data between both groups. As demonstrated in Table 1, the mean age was 14.84 ± 3.36 years for all patients, and 89.1% were female. The mean weight and height for the overall population were 44.26 ± 7.72 kg and 155.65 ± 7.27 cm, respectively.
Fig. 1.
Study flow chart
Table 1.
Demographic and baseline characteristics
| Variable | All patients N = 129 |
Single bolus (Group A) N = 60 |
Bolus + infusion (Group B) N = 69 |
p-value |
|---|---|---|---|---|
| Age, years | 14.84 ± 3.36 | 14.98 ± 3.90 | 14.71 ± 2.84 | 0.647 |
| Sex | ||||
| Male | 14 (10.9) | 7 (11.7) | 7 (10.1) | 0.782 |
| Female | 115 (89.1) | 53 (88.3) | 62 (89.9) | |
| Weight, kg | 44.26 ± 7.72 | 44.83 ± 8.58 | 43.77 ± 6.91 | 0.441 |
| Height, cm | 155.65 ± 7.27 | 154.97 ± 8.08 | 156.24 ± 6.49 | 0.331 |
| Body blood volume, mLa | 3045.40 ± 448.64 | 3051.97 ± 501.69 | 3039.69 ± 400.53 | 0.879 |
| Haemoglobin, g/dL | 13.50 ± 1.05 | 13.27 ± 1.09 | 13.70 ± 0.98 | 0.021 |
| Haematocrit, % | 0.41 ± 0.03 | 0.40 ± 0.03 | 0.41 ± 0.03 | 0.015 |
| INR | 1.08 ± 0.08 | 1.09 ± 0.07 | 1.08 ± 0.08 | 0.602 |
| aPTT, s | 35.78 ± 3.56 | 35.41 ± 3.14 | 36.11 ± 3.88 | 0.262 |
| Fibrinogen, g/L | 2.69 ± 0.47 | 2.66 ± 0.44 | 2.71 ± 0.50 | 0.557 |
Data shown are mean ± standard deviation or frequency (percentage)
Abbreviations: aPTT activated partial thromboplastin time, INR International normalised ratio
aBody blood volume was calculated using Nadler equation
Table 2 shows the operative data. In the overall population, the mean Cobb angle was 66.57 ± 15.19°, median number of fusion levels was 11 (10–12), median number of screws was 14 (12–15), mean length of skin incision was 29.99 ± 5.17 cm, and mean duration of surgery was 110.64 ± 30.5 min.
Table 2.
Operative data
| Variable | All patients N = 129 |
Single bolus (Group A) N = 60 |
Bolus + infusion (Group B) N = 69 |
p-value |
|---|---|---|---|---|
| Vertebral level fused, n | 11.00 (10.00–12.00) | 11.00 (10.00–12.00) | 11.00 (9.50–13.00) | 0.635 |
| Cobb angle, ° | 66.57 ± 15.19 | 66.90 ± 14.79 | 66.28 ± 15.63 | 0.817 |
| Number of screws, n | 14.00 (12.00–15.00) | 14.00 (12.00–15.00) | 13.00 (12.00–15.50) | 0.541 |
| Duration of surgery, min | 110.64 ± 30.55 | 103.77 ± 21.98 | 116.61 ± 35.49 | 0.014 |
| Skin incision length, cm | 29.99 ± 5.17 | 29.85 ± 4.22 | 30.12 ± 5.90 | 0.767 |
Data shown are mean ± standard deviation or median (Q1–Q3)
The total TXA dose received in Group A was 1344.9 mg, whereas Group B received 2163.1 mg. There was no significant difference in the total estimated surgical blood loss between the two groups. The mean total estimated surgical blood loss was 723.30 ± 279.39 mL (range: 175.0–1607 mL) for Group A and 819.41 ± 302.60 mL (range: 330.0–1556.0 mL) for Group B (p = 0.065). However, the estimated blood loss per spinal fusion level was significantly higher in Group B compared to Group A (78.39 ± 34.33 vs 67.49 ± 22.31 mL/spinal level). The mean crystalloid volume infused was similar between the two groups (Table 3).
Table 3.
Intraoperative data on blood loss and blood transfusion
| Variable | All patients N = 129 |
Single bolus (Group A) N = 60 |
Bolus + infusion (Group B) N = 69 |
Mean difference (95% confidence interval) | p-value |
|---|---|---|---|---|---|
| Total TXA dose received, mg | 1782.53 ± 541.10 | 1344.85 ± 257.52 | 2163.12 ± 421.42 | − 818.27 (− 938.42 to − 698.12) | < 0.001 |
| Intraoperative blood loss | |||||
| mL | 696.88 ± 280.26 | 650.88 ± 267.31 | 736.87 ± 286.99 | − 85.99 (− 183.10 to 11.13) | 0.082 |
| mL/spinal level | 66.00 ± 28.17 | 60.67 ± 22.08 | 70.63 ± 31.99 | − 9.96 (− 19.45 to − 0.48) | 0.040 |
| mL/h | 388.73 ± 166.93 | 375.12 ± 149.84 | 400.57 ± 180.74 | − 25.45 (− 83.81 to 32.92) | 0.390 |
| Sponge blood volumea, mL | 23.36 ± 21.77 | 26.67 ± 26.30 | 20.48 ± 16.53 | 6.19 (− 1.62 to 14.00) | 0.119 |
| 24-h postoperative drain | 77.83 ± 48.30 | 72.42 ± 43.56 | 82.54 ± 51.92 | − 10.12 (− 26.76 to 6.52) | 0.231 |
| Total surgical blood loss (range)b | |||||
| mL | 774.71 ± 294.86 (175.00–1607.00) | 723.30 ± 279.39 (175.00–1607.00) | 819.41 ± 302.60 (330.00–1556.00) | − 96.11 (− 198.12 to 5.91) | 0.065 |
| mL/spinal level | 73.32 ± 29.76 (17.50–221.00) | 67.49 ± 22.31 (17.50–146.09) | 78.39 ± 34.33 (35.93–221.00) | − 10.90 (− 21.16 to − 0.64) | 0.037 |
| mL/h | 432.37 ± 175.39 (140.00–1115.00) | 418.09 ± 153.52 (140.00–1115.00) | 444.79 ± 192.67 (174.00–1037.33) | − 26.70 (− 88.03 to 34.63) | 0.391 |
| Cell salvage volume, mL | 321.12 ± 160.26 | 296.80 ± 147.06 | 342.26 ± 169.12 | − 45.46 (− 101.09 to 10.17) | 0.108 |
| Total crystalloid volume | |||||
| mL | 1151.94 ± 228.57 | 1132.50 ± 202.28 | 1168.84 ± 249.43 | − 36.34 (− 116.24 to 43.56) | 0.370 |
| mL/kg | 26.64 ± 6.36 | 26.04 ± 6.17 | 27.16 ± 6.53 | − 1.12 (− 3.34 to 1.11) | 0.322 |
| Allogenic blood transfusion | 0 (0) | 0 (0) | 0 (0) | ||
Data shown are mean ± standard deviation or frequency (percentage), unless specified otherwise
aEstimated sponge volume (mL) = weight of blood-soaked sponge − dry weight of sponge
bTotal surgical blood loss (mL) = total intraoperative blood loss + sponge blood volume + total blood collected in the surgical drain postoperatively. Abbreviation: TXA, tranexamic acid
There was a significant difference in the preoperative haemoglobin levels between the two groups, in which the mean preoperative haemoglobin was 13.27 ± 1.09 g/dL in Group A and 13.70 ± 0.98 g/dL in Group B (p = 0.021). However, the drop in haemoglobin levels at 48-h post-operative compared to preoperative value was not significant between groups (3.04 ± 1.10 g/dL vs 2.72 ± 0.99 g/dL, p = 0.081). Both groups showed an increase in fibrinogen levels after 48 h but were significantly higher in Group A (2.06 ± 0.65 vs 0.38 ± 1.44 g/L, p < 0.001; Table 4). There were no allogenic blood transfusion and no documented complications due to the use of TXA in both groups (Table 3).
Table 4.
Laboratory data
| Laboratory data | Single bolus (Group A) | Bolus + infusion (Group B) | p-value |
|---|---|---|---|
| Haemoglobin, g/dL | |||
| T1 | 13.27 ± 1.09 | 13.70 ± 0.98 | 0.021 |
| T2 | 11.49 ± 1.15 | 11.85 ± 1.17 | 0.082 |
| T3 | 10.56 ± 1.10 | 10.66 ± 1.13 | 0.609 |
| T3-T1 | − 2.72 ± 0.99 | − 3.04 ± 1.10 | 0.081 |
| Haematocrit, % | |||
| T1 | 0.40 ± 0.03 | 0.42 ± 0.03 | 0.015 |
| T2 | 0.34 ± 0.03 | 0.35 ± 0.03 | 0.093 |
| T3 | 0.32 ± 0.03 | 0.33 ± 0.04 | 0.331 |
| T3-T1 | − 0.08 ± 0.03 | − 0.09 ± 0.04 | 0.316 |
| International normalised ratio | |||
| T1 | 1.09 ± 0.07 | 1.08 ± 0.08 | 0.602 |
| T2 | 1.22 ± 0.07 | 1.22 ± 0.08 | 0.745 |
| T3 | 1.24 ± 0.10 | 1.22 ± 0.09 | 0.204 |
| T3-T1 | 0.15 ± 0.11 | 0.14 ± 0.09 | 0.429 |
| Activated partial thromboplastin time, s | |||
| T1 | 35.41 ± 3.14 | 36.11 ± 3.88 | 0.262 |
| T2 | 35.23 ± 3.13 | 35.34 ± 3.96 | 0.868 |
| T3 | 31.27 ± 2.07 | 33.90 ± 4.52 | < 0.001 |
| T3-T1 | − 4.14 ± 3.24 | − 2.21 ± 3.97 | 0.003 |
| Fibrinogen, g/L | |||
| T1 | 2.66 ± 0.44 | 2.71 ± 0.50 | 0.557 |
| T2 | 2.14 ± 0.53 | 2.14 ± 0.73 | 0.994 |
| T3 | 4.72 ± 0.60 | 3.09 ± 1.38 | < 0.001 |
| T3-T1 | 2.06 ± 0.65 | 0.38 ± 1.44 | < 0.001 |
Data shown are mean ± standard. T1, pre-operation; T2, 0-h post-operation; T3, 48-h post-operation. T3-T1, perioperative drift (pre-operation to 48-h post-operation)
Discussion
In this single-centre, retrospective study which compared single bolus of TXA (30 mg/kg, Group A) with bolus (30 mg/kg) followed by infusion (10 mg/kg/h, Group B) in AIS patients undergoing PSF surgery, we found no difference in the total estimated blood loss and blood transfusion requirement between the two groups. However, the estimated blood loss per spinal fusion level was significantly higher in the bolus followed by infusion compared to the bolus-only group. This could be explained by the duration of surgery which was significantly longer in the infusion group. Duration of surgery was one of the risk factors for increased blood loss in scoliosis surgery as demonstrated in our recently published study (Hasan et al. 2021).
The ultimate aim when administering TXA is to achieve an ideal plasma therapeutic concentration of TXA that provides maximum efficacy with lowest complication rates. Despite the general recommendation for using TXA to reduce blood loss in AIS corrective surgery, the optimum dosage of TXA is still questionable, as a wide range of dosing are currently being practised ranging from 10 to as high as 100 mg/kg loading dose followed by a maintenance infusion of between 1 and 10 mg/kg/h (Goobie et al. 2018; Farrokhi et al. 2011). A recent review by Goobie et al. suggested a loading dose of between 10 and 30 mg/kg/h followed by a maintenance infusion of 5 to 10 mg/kg/h to achieve a plasma TXA concentration of 20 µg/mL and 70 µg/mL, respectively (Goobie and Faraoni 2019). These optimal dosing regimens were based on population pharmacokinetic model and derived from computer simulations. In our recent prospective randomised trial adopting the higher dosing regimen (30 mg/kg loading dose followed by 10 mg/kg/h) as proposed by Goobie et al., we did not find significant difference in the estimated total blood loss when compared to a lower dosing regimen (Hasan et al. 2021). One of the probable reasons might be due to the relatively shorter duration of surgery in our study compared to a retrospective study by Mehta et al. (Mehta et al. 2022) and prospective study by Dupuis et al. (Dupuis et al. 2015) which was twice longer. The plasma TXA concentration could still be within the therapeutic range for both dosing regimens throughout surgery. In this current study, we did not find any significant difference in the total surgical blood loss between a group of patients who received only TXA loading dose of 30 mg/kg and a group which had a maintenance infusion (10 mg/kg/h) after receiving a similar loading dose. This could be explained by TXA having a short half-life of 2 h (Eriksson et al. 1974), which suggests that for a surgery lasting 2 h as in this study, the plasma concentration of TXA after the initial loading dose (without a continuous infusion) would still be well within the therapeutic range (Fig. 2) (Eriksson et al. 1974). In other words, to achieve the desired therapeutic plasma concentration, a maintenance infusion may not be necessary after an initial loading dose had been given provided the dose is high enough and the duration of surgery is about 2 h or less. However, in view of the retrospective nature of this study, future prospective randomised trial should be performed to confirm the findings. Goobie et al. also suggested in their review article that a lower-dose regimen may be adequate to inhibit fibrinolysis, but to maximise plasmin inhibition, a higher-dose regimen may be necessary (Goobie et al. 2018). This was demonstrated in the secondary analysis of Goobie et al.’s study, whereby to achieve maximum efficacy of TXA, its in vivo plasma concentration level should be in the range of 70 5 µg/mL (Goobie et al. 2018).
Fig. 2.
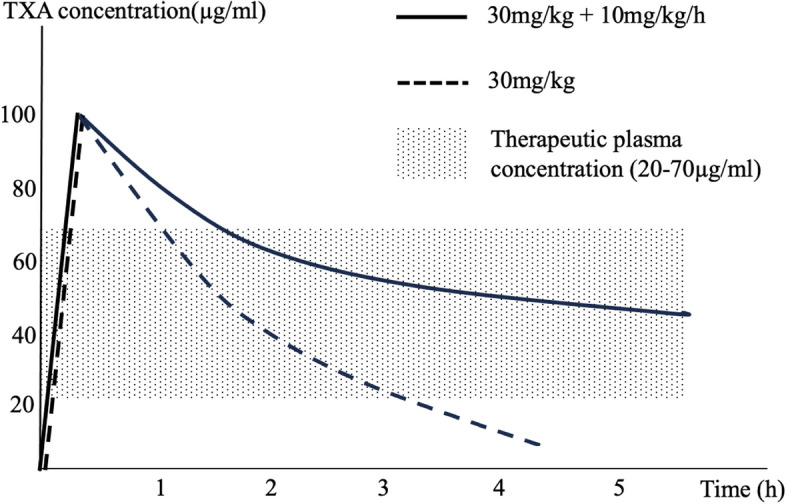
Simulated TXA concentration versus time graph. Simulated curves representing loading dose 30 mg/kg with infusion of 10 mg/kg/h (solid line) and loading dose 30 mg/kg only without infusion (dashed line). Modified from ‘Tranexamic acid and perioperative bleeding in children: what do we still need to know?’ by Goobie S. M. and Faraoni D. (2019), Curr Opin Anesthesiol, 32(3), Fig. 1 (Goobie and Faraoni 2019)
Presently, we are not certain if an even higher plasma TXA target would confer added advantage in terms of reducing blood loss. Haemorrhagic conditions lead to a huge release of tissue plasminogen activator (tPA) and a delayed release of urokinase plasminogen activator (uPA) which physiologically catalyse the conversion of plasminogen to active plasmin, inducing hyperfibrinolysis (Hijazi et al. 2015). To suppress plasmin-induced platelet activation in vitro, a concentration of 16 μg/mL TXA was required. TXA concentrations as high as 126–252 μg/mL may be necessary to increase thrombin formation (Stief 2009). This explains the TXA pharmacokinetic/pharmacodynamic relationship, whereby a lower TXA concentrations (~ 20 μg/mL) may be adequate to significantly inhibit tPA-induced fibrinolysis and a much higher concentrations (~ 150 μg/mL) may be required to inhibit uPA-induced fibrinolysis. This concept was supported by studies in cardiac surgery showing reduced blood loss and lower transfusion rates using dosing regimens that achieved the highest blood concentrations (~ 150 μg/mL) (Grassin-Delyle et al. 2013; Sigaut et al. 2014). Interestingly, a recent pharmacokinetic study to optimise TXA dosage in trauma patients suggested two dosing regimens (TXA plasma levels of 20 μg/mL or 150 μg/mL) based on the above explanation (Grassin-Delyle et al. 2018). For the former lower-dose regimen, a single bolus dose of 1-g TXA is enough to maintain a concentration above 20 μg/mL for a duration of 1.5 h, and, if necessary, a TXA infusion (250 mg/h) can be initiated 1.5 h later to maintain levels above 20 μg/mL. Alternatively, dosing based on body weight starting with a bolus of 6.9 mg/kg followed by maintenance infusion at 2.6–3.3 mg/kg/h can be considered to achieve similar TXA levels. For the higher-dose regimen targeting plasma concentration of 150 μg/mL, an initial loading dose of 54.6 mg/kg followed by a continuous infusion at 19–24 mg/kg/h was suggested. However, levels that are extremely high should be avoided to prevent thrombotic complications and development of seizures (Lin and Xiaoyi 2016). Nevertheless, such high concentrations had been shown to be safe in previous studies (Grassin-Delyle et al. 2013; Sigaut et al. 2014). Though spine surgery may not be akin to trauma, both situations involved significant blood loss, and extrapolating this observation in major spine surgery may potentially confer additional benefit. This postulation should be confirmed in future prospective trials comparing these different dosing regimens.
Another factor which could justify a higher dosing regime was due to the associated loss of TXA in cases of significant blood loss. TXA needs to be replenished either by administering a higher dose, or in the case of only loading dose has been given, an intraoperative redosing should be given in order to maintain the optimal plasma TXA concentration. The latter approach is currently being recommended for surgical antibiotic prophylaxis to prevent surgical site infection especially in long surgical procedures or after excessive intraoperative blood loss (Wolfhagen et al. 2022). One study suggests a redosing of antibiotic should be considered when the blood loss has exceeded 1500 mL (Jonge et al. 2021). Extrapolating this in the context of TXA where only an initial single bolus has been given, a redosing strategy that resulted in administration within two half-lives (about 4 h) after the initial TXA dose can be considered or after blood loss has exceeded about 30% of total blood volume (Jonge et al. 2021; Kasatpibal et al. 2017).
Our study has several strengths. To our knowledge, this is the first study that compared single bolus of TXA versus bolus followed by infusion in AIS surgery. The number of subjects was relatively large involving 129 adolescents who underwent major PSF surgery. There are several limitations in our study. The main limitation was the retrospective nature of this study which preclude any causal inferences. Thus, the results of the study should be interpreted with caution. We did not measure the plasma concentration of TXA in our patients which could give us a better insight of the relationship between the different dosing regimens and its plasma levels. On the other hand, our centre utilised the dual attending surgeon strategy which resulted in a shorter duration of surgery. Therefore, the results may not be applicable to other centres that have prolonged surgical duration.
Further, large, randomised, double-blinded trial which include a pharmacokinetic study should be performed to ascertain if this strategy of a single initial bolus of TXA without continuous infusion is non-inferior to the conventional bolus followed by infusion approach in reducing total surgical blood loss in AIS patients undergoing major PSF surgery.
Conclusions
TXA when administered as a single bolus or bolus followed by infusion in AIS patients undergoing PSF surgery is associated with similar estimated total surgical blood loss and blood transfusion requirement.
Acknowledgements
The authors express their appreciation to Ms. Carolyn Loh Tze Ing from the Department of Anaesthesiology, Faculty of Medicine, Universiti Malaya, for her valuable contribution.
Abbreviations
- AIS
Adolescent idiopathic scoliosis
- ASA
American Society of Anesthesiologists
- aPTT
Activated partial thromboplastin time
- INR
International normalised ratio
- MAP
Mean arterial pressure
- PSF
Posterior spinal fusion
- TCI
Target-controlled infusion
- tPA
Tissue plasminogen activator
- TXA
Tranexamic acid
- uPA
Urokinase plasminogen activator
Authors’ contribution
MSH, LMH, and SNY contributed to the conception and design of the study. MSH, NAR, CYWC, MKK, and CKC contributed to the acquisition of data. MSH, LMH, and LZY contributed to the analysis of data. MSH, LMH, SNY, and LZY contributed to the interpretation of data. MSH and LMH drafted the manuscript. All authors revised the manuscript critically for important intellectual content, approved the final version of the manuscript, and are accountable for all aspects of the work.
Funding
This study did not receive any funding.
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study received approval by the Medical Research Ethics Committee of University Malaya Medical Centre (MREC ID: 202057–8602) and waived the requirement for informed consent because of the retrospective observational nature of this study. All procedures were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- de Jonge SW, Boldingh QJJ, Koch AH, Daniels L, de Vries EN, Spijkerman IJB, Ankum WM, Kerkhoffs GMMJ, Dijkgraaf MG, Hollmann MW, Boermeester MA. Timing of preoperative antibiotic prophylaxis and surgical site infection: TAPAS, an observational cohort study. Ann Surg. 2021;274:e308–14. [DOI] [PubMed] [Google Scholar]
- Dupuis C, Michelet D, Hilly J, Diallo T, Vidal C, Delivet H, Nivoche Y, Mazda K, Dahmani S. Predictive factors for homologous transfusion during paediatric scoliosis surgery. Anaesth Crit Care Pain Med. 2015;34:327–32. [DOI] [PubMed] [Google Scholar]
- Eriksson O, Kjellman H, Pilbrant A, Schannong M. Pharmacokinetics of tranexamic acid after intravenous administration to normal volunteers. Eur J Clin Pharmacol. 1974;7:375–80. [DOI] [PubMed] [Google Scholar]
- Farrokhi MR, Kazemi AP, Eftekharian HR, Akbari K. Efficacy of prophylactic low dose of tranexamic acid in spinal fixation surgery: a randomized clinical trial. J Neurosurg Anesthesiol. 2011;23:290–6. [DOI] [PubMed] [Google Scholar]
- Goobie SM, Faraoni D. Tranexamic acid and perioperative bleeding in children: what do we still need to know? Curr Opin Anaesthesiol. 2019;32:343–52. [DOI] [PubMed] [Google Scholar]
- Goobie SM, Zurakowski D, Glotzbecker MP, McCann ME, Hedequist D, Brustowicz RM, Sethna NF, Karlin LI, Emans JB, Hresko MT. Tranexamic acid is efficacious at decreasing the rate of blood loss in adolescent scoliosis surgery: a randomized placebo-controlled trial. J Bone Joint Surg Am. 2018;100:2024–32. [DOI] [PubMed] [Google Scholar]
- Grassin-Delyle S, Tremey B, Abe E, Fischler M, Alvarez JC, Devillier P, Urien S. Population pharmacokinetics of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2013;111:916–24. [DOI] [PubMed] [Google Scholar]
- Grassin-Delyle S, Theusinger OM, Albrecht R, Mueller S, Spahn DR, Urien S, Stein P. Optimisation of the dosage of tranexamic acid in trauma patients with population pharmacokinetic analysis. Anaesthesia. 2018;73:719–29. [DOI] [PubMed] [Google Scholar]
- Hasan MS, Yunus SN, Ng CC, Chan CYW, Chiu CK, Kwan MK. Tranexamic acid in pediatric scoliosis surgery: a prospective randomized trial comparing high-dose and low-dose tranexamic acid in adolescent idiopathic scoliosis undergoing posterior spinal fusion surgery. Spine. 2021;46:E1170–7. [DOI] [PubMed] [Google Scholar]
- Hijazi N, Abu Fanne R, Abramovitch R, Yarovoi S, Higazi M, Abdeen S, Basheer M, Maraga E, Cines DB, Higazi AA. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood. 2015;125:2558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Butler EK, Barrack T, Ledonio CT, Forte ML, Cohn CS, Polly DW Jr. Tranexamic acid reduced the percent of total blood volume lost during adolescent idiopathic scoliosis surgery. Int J Spine Surg. 2017;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasatpibal N, Whitney JD, Dellinger EP, Nair BG, Pike KC. Failure to redose antibiotic prophylaxis in long surgery increases risk of surgical site infection. Surg Infect. 2017;18:474–84. [DOI] [PubMed] [Google Scholar]
- Kesling KL, Reinker KA. Scoliosis in twins. A meta-analysis of the literature and report of six cases. Spine. 1997;22:2009–14. [DOI] [PubMed] [Google Scholar]
- Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: a meta-analysis. Seizure. 2016;36:70–3. [DOI] [PubMed] [Google Scholar]
- Lonstein JE. Adolescent idiopathic scoliosis. Lancet. 1994;344:1407–12. [DOI] [PubMed] [Google Scholar]
- Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am. 1984;66:1061–71. [PubMed] [Google Scholar]
- Mehta N, Garg B, Bansal T, Aryal A, Arora N, Gupta V. Predictors of operative duration in posterior spinal fusion for adolescent idiopathic scoliosis: a retrospective cohort study. Int J Spine Surg. 2022;16:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall GA, Horlocker TT, Santrach PJ, Oliver WC Jr, Dekutoski MB, Bryant S. Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine. 2000;25:596–601. [DOI] [PubMed] [Google Scholar]
- Parent S, Newton PO, Wenger DR. Adolescent idiopathic scoliosis: etiology, anatomy, natural history, and bracing. Instr Course Lect. 2005;54:529–36. [PubMed] [Google Scholar]
- Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 2005;102:727–32. [DOI] [PubMed] [Google Scholar]
- Sigaut S, Tremey B, Ouattara A, Couturier R, Taberlet C, Grassin-Delyle S, Dreyfus JF, Schlumberger S, Fischler M. Comparison of two doses of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2014;120:590–600. [DOI] [PubMed] [Google Scholar]
- Stief T. Tranexamic acid triggers thrombin generation. Hemost Lab. 2009;2:73–82. [Google Scholar]
- Thompson GH, Florentino-Pineda I, Poe-Kochert C, Armstrong DG, Son-Hing JP. The role of Amicar in same-day anterior and posterior spinal fusion for idiopathic scoliosis. Spine. 2008;33:2237–42. [DOI] [PubMed] [Google Scholar]
- Wolfhagen N, Boldingh QJJ, de Lange M, Boermeester MA, de Jonge SW. Intraoperative redosing of surgical antibiotic prophylaxis in addition to preoperative prophylaxis versus single-dose prophylaxis for the prevention of surgical site infection: a meta-analysis and GRADE Recommendation. Ann Surg. 2022;275:1050–7. [DOI] [PubMed] [Google Scholar]
- Yoo JS, Ahn J, Karmarkar SS, Lamoutte EH, Singh K. The use of tranexamic acid in spine surgery. Ann Transl Med. 2019;7:S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
No datasets were generated or analysed during the current study.