Abstract
Background
Substantial variability in response to lifestyle interventions has been recognized for many years, and researchers have begun to disentangle sources of error from inherent differences in individual responsiveness. The objective of this secondary analysis of an intensive lifestyle intervention (diet and exercise) for metabolic syndrome (MetS) was to identify potentially important differences among study completers grouped by treatment response as measured by change in a continuous metabolic syndrome score (Gurka/MetS).
Methods
All study completers from a 12-month primary care study were categorized into one of five groups according to change in the Gurka/MetS score. A change of 0.4 in z-score defined clinically relevant change in line with results of previous studies. Repeated measures analysis of variance was used to examine cardiovascular disease risk and individual clinical indicators of MetS over 12 months, looking for differences in response over time by the five groups.
Results
Of 176 participants, 50% (n = 88) had stable scores, 10% (n = 18) had relevant change scores in the first 3 months only and reverted toward baseline, 20% (n = 35) achieved meaningful change over the whole study, 11% (n = 20) had a delayed response at 3–12 months, and 9% (n = 15) demonstrated worsening scores. Significant differential patterns were noted for groups over the duration of the intervention (p < .001). Improvement in diet quality and fitness scores were similar across all groups. Other available variables were tested and did not account for the differences.
Conclusion
Work is needed to identify key factors that account for differences in responses to lifestyle interventions that can be used to guide treatment decisions for intensive lifestyle interventions for this common condition.
Trial Registration
ClinicalTrials.gov Identifier: NCT01616563; first registered June 12, 2012.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12875-024-02608-w.
Keywords: Physical fitness, Diet quality, Healthy eating index, Metabolic syndrome, Cardiometabolic health, Primary care
Background
Cardiometabolic risk (CMR) conditions and diseases are a major and growing health burden in many countries, as obesity continues to increase worldwide [1]. A substantial subset of adults develop adverse metabolic profiles with weight gain as they age, marked by increased visceral truncal fat deposition and insulin resistance, with an estimated global prevalence of about 25% of adults [2]. These individuals are at higher risk of several chronic conditions, including type 2 diabetes, dyslipidemia, hypertension, cardiovascular disease (CVD), some cancers and viral diseases like COVID19 [3]. Since the early 1990s, the term metabolic syndrome (MetS) has been used to describe those identified clinically as having three or more components: higher waist circumference (WC), higher blood pressure (SBP/DBP), dyslipidemia characterized by low high-density lipoprotein (HDL-C) and elevated triglycerides (TG) and/or elevated glucose levels [4, 5]. They are known to be at 1.25-2 times higher risk of total mortality and CVD, compared to those without MetS [6, 7]. A 2016 prevalence estimate for MetS in the United States among adults ≥ 20 years was 34.7% [8], with comparable prevalence in Canada (combined 2012-15 data with measured glucose = 32.3%) [9]. Overall, the current epidemiological evidence confirms high prevalence and adverse health consequences of MetS. MetS is mostly addressed in the publicly funded primary care system in Canada; where family physicians provide first contact care under several organizational models. According to a recent Commonwealth survey, 16% of family physicians work in health centres or community clinics, with 59% in physician group practices, 15% in solo practice, and 10% in other settings [10]. Access to lifestyle services was not documented but is known to be limited in the Canadian system [11].
Beyond access issues, key challenges for development and spread of behavioural interventions to manage CMR conditions generally, and MetS specifically, include: (1) diversity of patient interest, capability and skills, (2) potential unknown physiological or genetic differences between individuals [12]; (3) day-to-day variability within individuals [13–16], (4) efficacy of different diets and physical activity interventions [17, 18], (5) lack of clarity on key aspects of program content so they can be compared [19, 20], (6) measurement and analysis challenges in assessing lifestyle and CMR risk changes.
Focusing on measurement and analysis, the wide variability in response to lifestyle (and other) interventions has been recognized for many years as well as the need for multiple repeated measurements and analysis that accounts for serial correlation [21, 22]. Researchers have also begun to disentangle measurement error from inherent differences in responsiveness among study participants who appear to be adherent to lifestyle interventions [23]. We became interested in the possibility of inherent differences among participants of a pre-post lifestyle program in primary care to treat MetS. A 19% reversal of MetS was seen in the study overall, with improved diet quality, aerobic fitness and CVD risk scores overall, yet unpublished data showed that individual scores for change in diet quality and aerobic capacity did not correlate with changes in individual CVD risk scores [24]. In addition, linear modelling of the data using a 12-month continuous MetS score [25] had identified 3-month MetS change as important, but otherwise did not identify any diet or exercise variables as predictive [12-month cMetS score = − 9.217 + 0.538 (baseline fasting glucose) + 0.447 (baseline triglycerides) + 0.052 (baseline waist circumference) + 0.012 (baseline systolic blood pressure) – 0.487 (3-month Δ_cMetS score)] [26]. Were there responders and non-responders in this data set? If there were demonstrable groups, did they differ from each other in ways that might provide additional insight on the reasons for the lack of association between interventions and outcomes in the original study?
To address these questions, we used data from the same pre-post one arm 12-month feasibility study conducted from 2012 to 2015 at three Canadian primary care clinics in Edmonton, Alberta, Toronto, Ontario, and Quebec City, Quebec [24] (see Additional File 1 for study description). We first examined measurement error of the short-term outputs of diet quality (assessed using a Canadian version of the Healthy Eating Index (HEI-C)) and physical fitness (assessed using V02 max, curl-ups, push-ups and treadmill speed) using structural equation modelling (SEM) and found that a reduced HEI-C score and a composite fitness score had better measurement properties than the original measures [27].
Next, possible outcome variables were considered. Continuous outcome scores usually provide more information than categorical definitions [4] and various continuous MetS scores have been developed, most in European cohorts [25, 28]. The Gurka continuous MetS severity score (Gurka/MetS) was chosen because it has been linked to clinical outcomes as described below and was developed based on factor analysis of cross-sectional NHANES data (1999–2010, aged 20–64) with sex-ethnicity specific equations. Their Caucasian nationally representative source population for development (n = 3318 men and women) had a MetS prevalence of 25.8%. The score is a z-score, so a mean = 0 suggests average risk, while a score = 1 is one SD above the population mean [29]. Of note, body mass index (BMI) did not add to the utility of the score [30].
Defining “responders” to interventions is currently highly controversial, with both statistical and clinical aspects to be considered [31]. Gurka and colleagues have been exploring predictive utility of their score against development of type 2 diabetes and CVD, with more success in adding to current prediction models for diabetes than CVD [32, 33]. For example, they re-analysed the Diabetes Prevention Program (DPP) study, a randomized trial of lifestyle, metformin and placebo and noted that intervention declines in the score were associated with reduced development of diabetes in years 1–5 and to a lesser extent CVD [34]. The lifestyle arm of DPP achieved a change in the Gurka/MetS score (mean change ± SD) of -0.40 ± 0.50 over one year, with metformin (-0.18 ± 0.44) and placebo (-0.08 ± 0.44) achieving more modest changes (see supplement of paper) [34].
Among the largest treatment studies to date reporting the Gurka/MetS score, was a 2-year primary care intensive lifestyle intervention (ILI) cluster randomized trial focused on weight loss in 803 (351 usual care, 452 ILI) adults (67% Black, 84% female) in Louisiana [35]. The ILI was similar in intensity to our intervention. Among 393 participants in the ILI program with complete measures, mean baseline Gurka/MetS score was 0.87 ± 0.96. Change in the ILI group at 12 months (mean ± se) was − 0.35 ± 0.06, whereas the score did not change in the usual care group at either 12- or 24- months, comparable to the changes seen in the DPP analysis. Thus, there is evidence to support defining response by the degree of change in the Gurka/MetS score.
The objective of this exploratory descriptive analysis was to identify possible change experience subgroups by differences in the Gurka/MetS z-score over time. These subgroups were then compared on baseline characteristics, changes in diet quality and physical fitness and the individual clinical indicators of the MetS to identify possible differences among the groups that could help explain the original study results.
Methods
Data from primary study
The study data were obtained from the patients’ medical charts and entered into a secure online data capture system using REDCap electronic data capture tools hosted at Queens University, Kingston, ON [36, 37]. The original sample was comprised of 305 adults at baseline, aged 18–81 years old (mean 59 years) and 52% female, who were recruited to a pre-post family physician led 12-month lifestyle (diet and exercise) program in three primary care organizations across Canada [24]. To accurately categorize those who finished the entire one-year intervention, the current sample comprises the 176 (60%) with complete data (baseline, 3 months, and 12 months). Laboratory measurement methods are described in the main paper [24]. The effects of 17 candidate single nucleotide polymorphisms (SNPs) were also assessed in a subgroup (n = 147, 50%) of all participants [38]. The data are available from the first author on request. Analyses were conducted using IBM SPSS Statistics (v. 28).
Measures
Equations for the reduced HEI-C diet quality score, composite fitness score and Gurka/MetS score are shown in Additional File 2 [27].
Based on the DPP [34] and Hochsmann et al. [35], a change of > 0.40 unit in the Gurka/MetS scores was considered as clinically meaningful for the purposes of this analysis. This value was slightly less than a standard deviation of our Gurka/MetS scores (SD range 0.50-0.72). Five mutually exclusive response groups accounted for all patterns of observed change over time. The five groups were defined as:
Stable – those showing no significant improvement or decline in Gurka/MetS score over 12 months (change ≤ 0.40 unit);
Early Change – participants who showed significant improvement in their Gurka/MetS score baseline to 3 months, which was not sustained 3–12 months (i.e., participants showed initial improvement, then reverted back toward baseline);
Maintained Change from 0 to 3 and 3–12 months – Scores showed improvement in first three months, and maintained change of > 0.40 from baseline through 3–12 months;
Delayed Change 3–12 months only - participants remained stable from 0 to 3 months, then Gurka/MetS score decreased > 0.40 from 3 to 12 months;
Worse – participants showed increased Gurka/MetS scores of at least > 0.40 baseline to 3 months and/or to 12 months.
Analytic approach
Descriptive analyses by Gurka/MetS response group were completed for all available variables. Repeated measures analysis of variance (RMANOVA) was the main analytic technique used, which accounts for serial correlation at the three time points [39]. To gain insight on patterns of change among relevant individual indicators, a series of analyses were done with diet quality, fitness score, blood pressure, WC, lipids, glucose, BMI and the continuous Gurka/MetS score as dependent variables. Models were not adjusted for any covariates.
Results
Baseline characteristics
As a group, study completers compared to non-completers and those with missing data were older, had lower baseline Gurka/MetS scores, lower BMI, WC, hemoglobin A1c, and FBG, but similar baseline TG, HDL-C, LDL-C, SBP and DBP (data not shown). Of the 176 participants with complete data (except for SNPs), 50% (n = 88/176) had Stable Gurka/MetS scores, 10% (n = 18) had Early change scores in the first 3 months only, 20% (n = 35) Maintained meaningful change over the whole study, 11% (n = 20) had a Delayed response at 3–12 months, and 9% (n = 15) demonstrated Worse scores over the 12 months (see Table 1).
Table 1.
Baseline characteristics by Gurka/MetS score change category (± SD)
| Stable (n = 88) |
Early Change (n = 18) |
Maintained Change (n = 35) |
Delayed Change (n = 20) |
Worse (n = 15) |
Total (n = 176) |
Tests of Association1,2,3 | |
|---|---|---|---|---|---|---|---|
| Baseline Gurka/MetS score ± SD | 0.90 ± 0.48ab | 1.23 ± 0.54bc | 1.34 ± 0.68c | 1.22 ± 0.62abc | 0.78 ± 0.54a | 1.02 ± 0.59 |
F = 7.07 (4,171) P < .0011 |
| Age (y) ± SD | 60.0 ± 9.7 | 59.2 ± 9.4 | 61.4 ± 7.2 | 62.7 ± 8.0 | 61.2 ± 9.0 | 60.6 ± 8.9 | NS 1 |
| Sex (F) % | 51 | 33 | 54 | 70 | 67 | 53 | NS2 |
| BMI (kg/m2) ± SD | 31.5 ± 3.2 | 31.2 ± 3.2 | 30.3 ± 3.8 | 30.8 ± 3.8 | 32.8 ± 3.1 | 31.3 ± 3.4 | NS1 |
| WC (cm) ± SD | 107 ± 8 | 106 ± 9 | 106 ± 9 | 106 ± 12 | 107 ± 8 | 107 ± 9 | NS1 |
| Hemoglobin A1c (%) ± SD | 6.12 ± 0.70 | 6.34 ± 0.84 | 6.66 ± 1.45 | 6.26 ± 0.95 | 6.60 ± 0.63 | 6.31 ± 0.94 | NS1,4,5 |
| Fasting glucose (mmol/L) ± SD | 6.0 ± 1.0a | 6.8 ± 1.6ab | 7.0 ± 1.5b | 6.6 ± 1.4ab | 6.7 ± 1.4ab | 6.4 ± 1.3 |
Welch test F = 4.79 (4,43) P = .0031,4,5 |
| TG (mmol/L) ± SD | 2.0 ± 0.8a | 2.5 ± 1.4a | 2.6 ± 1.6a | 2.2 ± 0.8a | 1.3 ± 0.4b | 2.1 ± 1.1 |
Welch test F = 8.71 (4, 49) P < .0011,4,5 |
| HDL-C (mmol/L) ± SD | 1.2 ± 0.3ab | 1.1 ± 0.2a | 1.2 ± 0.3ab | 1.2 ± 0.2ab | 1.4 ± 0.3b | 1.2 ± 0.3 |
F = 2.52 (4,171) P = .0431 |
| LDL-C (mmol/L) ± SD | 2.8 ± 1.2 | 2.5 ± 1.1 | 2.4 ± 1.2 | 2.8 ± 0.9 | 2.3 ± 0.8 | 2.7 ± 1.1 | NS1 |
| SBP (mm Hg) ± SD | 132 ± 13ab | 138 ± 16ab | 137 ± 15ab | 140 ± 19b | 128 ± 13a | 134 ± 15 |
F = 2.56 (4,171) P = .041 |
| DBP (mm Hg) ± SD | 81 ± 8 | 80 ± 10 | 80 ± 8 | 82 ± 11 | 76 ± 6 | 80 ± 9 | NS1 |
|
HEI-C ± SD Scale 1-100 |
58.9 ± 13.4 | 61.9 ± 13.4 | 59.7 ± 15.6 | 59.7 ± 17.7 | 56.2 ± 15.5 | 59.2 ± 14.4 | NS1 |
| Reduced HEI-C | 15.9 ± 5.0 | 16.3 ± 4.6 | 16.1 ± 5.0 | 15.0 ± 4.3 | 14.0 ± 4.9 | 15.2 ± 4.9 | NS1 |
| Age/sex %ile of VO2max ± SD | 47.0 ± 25.4 | 49.9 ± 26.1 | 42.8 ± 25.9 | 40.9 ± 19.3 | 41.4 ± 22.6 | 45.3 ± 24.7 | NS1 |
| Composite Fitness score | 50.2 ± 26.6 | 53.3 ± 27.6 | 47.4 ± 27.5 | 44.7 ± 20.7 | 43.4 ± 23.7 | 50.7 ± 25.4 | NS1 |
| Charlson Co-morbidity Score ± SD | 0.78 ± 0.81 | 0.67 ± 0.77 | 1.03 ± 0.92 | 0.75 ± 0.85 | 1.27 ± 1.03 | 0.88 ± 0.86 | NS1 |
| Glucose lowering medications (%) | 36 | 44 | 49 | 40 | 73 | 43 | NS2 |
| Anti-hypertensives (%) | 65 | 94 | 80 | 60 | 80 | 72 | P = .039 (two-sided)3 |
| Diuretics (%) | 32 | 39 | 49 | 25 | 40 | 36 | NS2 |
|
Lipid lowering Medications (%) |
57 | 72 | 74 | 45 | 87 | 63 | Χ2 = 10.40; 4df P = .0342 |
| Other medication (%)6 | 1 | 0 | 0 | 0 | 0 | 1 | |
| Reversion at any point (MetS category) % | 32 | 17 | 40 | 45 | 13 | 32 | NS3 |
| Mild liver disease (%) | 10 | 5 | 9 | 10 | 13 | 10 | NS3 |
| Type 2 diabetes (%) | 46 | 44 | 66 | 45 | 67 | 51 | NS2 |
| Myocardial infarction (%) | 2 | 6 | 6 | 10 | 7 | 4 | NS3 |
| SNP analysis (n) | 72 | 15 | 27 | 18 | 15 | 147 | |
|
ADIPOQ rs1501299 (GG)(%) |
57ab | 73ab | 33b | 72ab | 80a | 58 | P = .022 (two-sided)3 |
| GT or TT (%) | 43 | 27 | 67 | 28 | 20 | 42 |
1 Assessed by ANOVA after check for homogeneity of variances by Levene test, using Hochberg GT2 for post-hoc comparisons, except as noted. Means that bear different superscripts in each column are significantly different at p < .05
2 Pearson chi-square; then comparison of proportions with Bonferroni adjustment. Each superscript letter denotes a subset of Gurka/MetS change categories whose column proportions do not differ significantly from each other at the 0.05 level
3 Fisher-Freeman-Halton Exact test, where one or more cells have expected cell counts of < 5
4 Games-Howell post hoc test when homogeneity of variance is rejected by Levene test
5 Welch test for equality of means when normality cannot be assumed. Groups have unequal variances
6 None of the participants took appetite suppression medications
The Charlson comorbidity scores did not differ among the groups. Type 2 diabetes was prominent in the overall sample (51%) and did not differ by subgroup. History of mild liver disease (10%) or myocardial infarction (4%) was much lower and did not differ among the groups. Baseline fasting glucose differed by group, whereas hemoglobin A1c did not. Other lab values and use of medications differed across the groups, in line with differences in the Gurka/MetS score. The group who worsened had the lowest TG values, highest HDL-C, and lowest SBP values. The majority took anti-hypertensives and lipid lowering medications, but prevalence did not differ among response groups.
Age, sex, BMI and WC did not differ among the five groups. Notably, both the original HEI-C and the reduced HEI-C score and the original age/sex % for VO2max and the composite fitness score were also similar across the five groups at baseline.
Analysis of SNPs comparing major to minor alleles by chi-square revealed only ADIPOQ rrs1501299, one of the SNPs for adiponectin, differed by response group, such that the Maintained group was less likely to carry the major GG allele, compared to the Worse group, with all other groups being intermediate.
RMANOVA of diet quality and fitness scores
To assess whether differences in diet quality or fitness score changes by response groups could have accounted for the changes in the Gurka/MetS scores over time, RMANOVA analysis was completed on the reduced HEI-C score and composite fitness scores from our previous SEM analysis [27]. Equality of covariance matrices as examined by Box’s M was significant for both HEI-C and fitness scores, however, Levene’s tests were not significant. Appropriate adjustments were made. Mauchly’s test of sphericity was significant for both HEI-C and Fitness, and Greenhouse-Geisser adjustments were applied.
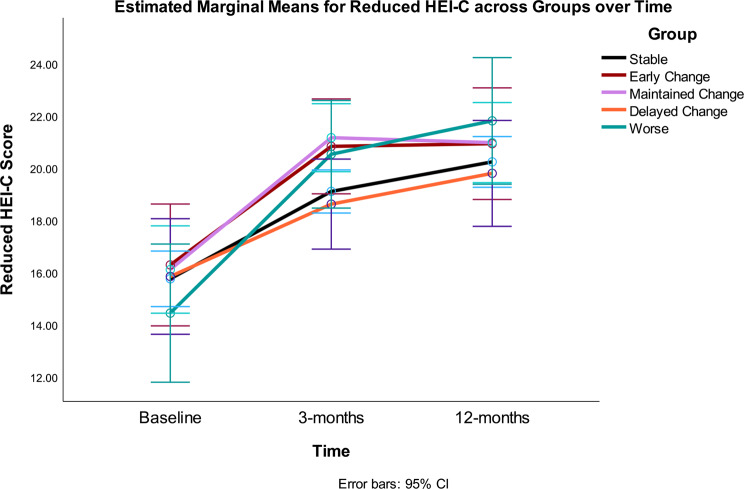
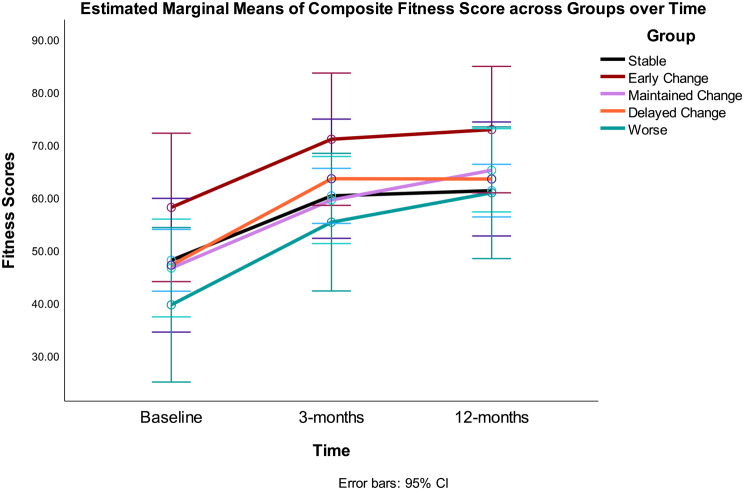
The overall RMANOVA model and the time effect were significant (i.e., there was improvement or increase in the scores over time), but the time*group interaction was not significant (Table 2) (i.e., there were no differences between Gurka/MetS groups on diet quality (Fig. 1) or fitness scores over time (Fig. 2) by the five response groups. Significant quadratic trends were found for the main effect of time for both HEI-C and fitness scores. The between effect for Gurka/MetS groups was not statistically significant for either model. Omega-squared for change over the intervention accounted for 45% variance in HEI-C scores and 39% of variance in fitness scores over 12 months.
Table 2.
RMANOVA results for diet quality and fitness scores
| Within Subjects Effects | ||||||
|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F | p | ω² | |
| Reduced HEI-C | ||||||
| Time | 1731.68 | 1.92 | 865.84 | 75.54 | < 0.001 | 0.45 |
| Time ✻ Gurka/MetS group | 115.34 | 7.69 | 14.42 | 1.26 | n.s. | - |
| Residual | 3874.03 | 324.95 | 11.92 | |||
| Fitness Score | ||||||
| Time | 15076.71 | 1.73 | 8737.15 | 66.30 | < 0.001 | 0.39 |
| Time ✻ Gurka/MetS group | 694.69 | 6.90 | 100.65 | 0.764 | n.s. | - |
| Residual | 32065.84 | 243.31 | 131.79 | |||
Note Type 3 Sums of Squares. Main effects not shown except Time. Greenhouse-Geisser adjusted degrees of freedom. Omega-squared included for significant effects
Fig. 1.
Diet quality as measured by reduced HEI-C (0–65) at baseline, 3- and 12-months by Gurka/MetS response categories. Stable = those showing no significant improvement or decline over 12 months (change < = 0.40 unit); Early Change = improvement baseline to 3 months, but not sustained 3–12 months; Maintained Change = maintained change over months; Delayed Change = change 3–12 months; Worse = increased scores
Fig. 2.
Composite fitness scores at baseline, 3- and 12-months by Gurka/MetS response categories. Stable = those showing no significant improvement or decline over 12 months (change < = 0.40 unit); Early Change = improvement baseline to 3 months, but not sustained 3–12 months; Maintained Change = maintained change over months; Delayed Change = change 3–12 months; Worse = increased scores
RMANOVA of individual variables and Gurka/MetS score at baseline, 3 and 12 months
Next, we examined individual indicators of MetS (i.e., abdominal obesity measured by BMI and WC; blood pressure measured by diastolic and systolic indicators; fasting glucose; TG and HDL-C levels), across the one-year intervention (i.e., time), and response groups. We also examined the continuous Gurka/MetS score to examine patterns and differences among the separate indicators versus the combined score. As shown in Table 3, a significant time effect was noted for all indicators, showing improvement (BMI, WC, SBP, DBP, TG, Gurka/MetS score all lower at 12 months). FBG decreased between baseline and 3-months, however, was not significantly different than baseline at 12-months. Significant interactions were noted for BMI, WC, SBP, FBG, TG, HDL-C, and the Gurka/MetS score (i.e., all outcome variables except DBP). Results focus on the significant within subjects effects (i.e., Time and Time x Group interaction). The between effect based on Response Groups was only significant for BMI and FBG as described below. Equality of covariance matrices as examined by Box’s M and Levene’s tests were significant for the following analyses: WC, FBG, TG, HDL-C, and Gurka/MetS score. Appropriate adjustments were made. Mauchly’s test of sphericity was significant for the following variables: BMI, WC, SBP, FBG, TG, HDL-C, and Gurka/MetS Score, and Greenhouse-Geisser adjustments were applied.
Table 3.
RMANOVA results for MetS indicators and Gurka/MetS score
| Within Subjects Effects | ||||||
|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F | p | ω² | |
| BMI | ||||||
| Time | 51.77 | 1.66 | 31.12 | 27.20 | < 0.001 | 0.20 |
| Time ✻ Gurka/MetS group | 33.14 | 6.66 | 4.98 | 4.35 | < 0.001 | 0.11 |
| Residual | 323.65 | 282.82 | 1.14 | |||
| WC | ||||||
| Time | 994.01 | 1.60 | 497.01 | 70.87 | < 0.001 | 0.39 |
| Time ✻ Gurka/MetS group | 617.84 | 6.41 | 96.34 | 11.01 | < 0.001 | 0.27 |
| Residual | 2398.44 | 274.17 | 8.75 | |||
| SBP | ||||||
| Time | 3527.58 | 1.88 | 1872.72 | 22.01 | < 0.001 | 0.18 |
| Time ✻ Gurka/MetS group | 1439.05 | 7.54 | 184.63 | 2.25 | 0.03 | 0.05 |
| Residual | 27401.45 | 332.11 | 85.07 | |||
| DBP | ||||||
| Time | 772.31 | 2 | 386.16 | 11.74 | < 0.001 | 0.11 |
| Time ✻ Gurka/MetS group | 276.14 | 8 | 34.52 | 1.05 | n.s. | - |
| Residual | 11252.33 | 342 | 32.90 | |||
| FBG | ||||||
| Time | 3.14 | 1.89 | 1.57 | 4.23 | 0.02 | 0.03 |
| Time ✻ Gurka/MetS group | 33.43 | 7.55 | 4.43 | 11.26 | <0.001 | 0.12 |
| Residual | 126.13 | 320.82 | 0.393 | |||
| TG | ||||||
| Time | 12.84 | 1.73 | 7.44 | 27.60 | < 0.001 | 0.21 |
| Time ✻ Gurka/MetS group | 28.60 | 6.90 | 4.14 | 15.38 | <0.001 | 0.36 |
| Residual | 79.52 | 295.04 | 0.270 | |||
| HDL-C | ||||||
| Time | 0.32 | 1.93 | 0.16 | 13.48 | < 0.001 | 0.12 |
| Time ✻ Gurka/MetS group | 0.53 | 7.98 | 0.07 | 5.58 | <0.001 | 0.17 |
| Residual | 4.05 | 329.76 | 0.01 | |||
| Gurka/MetS score | ||||||
| Time | 5.74 | 1.64 | 3.50 | 60.05 | < 0.001 | 0.36 |
| Time ✻ Gurka/MetS group | 20.21 | 6.56 | 3.08 | 52.11 | < 0.001 | 0.66 |
| Residual | 16.24 | 278.93 | 0.058 | |||
Note Type 3 Sums of Squares. Greenhouse-Geisser adjusted degrees of freedom (except for DBP). Omega-squared included for significant effects
The marginal means (se) from RMANOVA analyses are shown in Table 4 followed by consideration of differences among the five response groups.
Table 4.
Marginal means for metabolic syndrome indicators (± se)
| Stable (n = 88) |
Early Change (n = 18) |
Maintained Change (n = 35) |
Delayed Change (n = 20) |
Worse (n = 15) |
Total (n = 176) |
|
|---|---|---|---|---|---|---|
| Column1 | A | B | C | D | E | |
| Body Mass Index (kg/m 2 ) | ||||||
| Baseline | 31.5 ± 0.36a | 31.2 ± 0.80a | 30.3 ± 0.57a | 30.8 ± 0.76a | 32.8 ± 0.88 | 31.3 ± 0.26a |
| E | ||||||
| 3 mo | 30.7 ± 0.37b | 30.4 ± 0.80b | 29.0 ± 0.58b | 30.5 ± 0.76a | 33.0 ± 0.88 | 30.5 ± 0.26b |
| E | ||||||
| 12 mo | 30.7 ± 0.38b | 30.5 ± 0.84b | 28.3 ± 0.60c | 29.8 ± 0.80b | 32.8 ± 0.92 | 30.3 ± 0.28c |
| A, E | ||||||
| Waist circumference (cm) | ||||||
| Baseline | 107 ± 0.97a | 106 ± 2.15a | 106 ± 1.59a | 106 ± 2.04a | 107 ± 2.35 | 107 ± 0.68a |
| 3 mo | 105 ± 0.98b | 103 ± 2.17b | 102 ± 1.55b | 104 ± 2.06b | 105 ± 2.37 | 104 ± 0.69b |
| 12 mo | 104 ± 1.56b | 103 ± 2.34b | 98 ± 1.68c | 100 ± 2.22c | 106 ± 2.56 | 103 ± 0.77c |
| A, E | ||||||
| Systolic Blood Pressure (mm Hg) | ||||||
| Baseline | 132 ± 1.54a | 138 ± 3.40a | 137 ± 2.44a | 140 ± 3.23a | 128 ± 3.73 | 134 ± 1.13a |
| 3 mo | 127 ± 1.42b | 128 ± 3.13b | 127 ± 2.25b | 130 ± 2.97b | 125 ± 3.43 | 127 ± 0.98b |
| 12 mo | 130 ± 1.35a | 132 ± 2.99a | 129 ± 2.14b | 130 ± 2.83b | 132 ± 3.23 | 130 ± 0.98b |
| Diastolic Blood Pressure (mm Hg) | ||||||
| Baseline | 81 ± 0.92 | 80 ± 2.04 | 80 ± 1.46 | 82 ± 1.94 | 76 ± 2.24 | 80 ± 0.68a |
| 3 mo | 78 ± 0.93 | 78 ± 2.06 | 76 ± 1.47 | 77 ± 1.95 | 73 ± 2.25 | 77 ± 0.75b |
| 12 mo | 78 ± 0.87 | 76 ± 1.93 | 77 ± 1.38 | 77 ± 1.83 | 77 ± 2.11 | 77 ± 0.68b |
| Fasting blood glucose (mmol/L) | ||||||
| Baseline | 6.00 ± 0.14a | 6.78 ± 0.30a | 7.03 ± 0.21a | 6.62 ± 0.28a | 6.75 ± 0.33a | 6.42 ± 0.10 |
| 3 mo | 6.11 ± 0.12a | 6.28 ± 0.26b | 5.93 ± 0.19b | 6.76 ± 0.25a | 7.01 ± 0.29a | 6.24 ± 0.09 |
| 12 mo | 6.23 ± 0.15b | 6.94 ± 0.32a | 6.21 ± 0.23b | 6.15 ± 0.35b | 7.39 ± 0.35b | 6.39 ± 0.10 |
| E | ||||||
| Triglycerides (mmol/L) | ||||||
| Baseline | 1.95 ± 0.11 | 2.50 ± 0.24a | 2.60 ± 0.18a | 2.21 ± 0.23a | 1.35 ± 0.27 | 2.11 ± 0.08a |
| 3 mo | 1.87 ± 0.07 | 1.50 ± 0.15b | 1.43 ± 0.11b | 2.05 ± 0.15a | 1.48 ± 0.17 | 1.73 ± 0.05b |
| 12 mo | 1.93 ± 0.08 | 2.15 ± 0.18a | 1.57 ± 0.13b | 1.68 ± 0.17b | 1.71 ± 0.20 | 1.84 ± 0.06b |
| High density lipoprotein cholesterol (mmol/L) | ||||||
| Baseline | 1.22 ± 0.03a | 1.05 ± 0.07 | 1.20 ± 0.05a | 1.16 ± 0.06a | 1.36 ± 0.07a | 1.20 ± 0.08 |
| 3 mo | 1.18 ± 0.03b | 1.13 ± 0.07 | 1.22 ± 0.05a | 1.17 ± 0.06a | 1.24 ± 0.07b | 1.19 ± 0.05 |
| 12 mo | 1.24 ± 0.03a | 1.13 ± 0.07 | 1.37 ± 0.03b | 1.25 ± 0.07b | 1.28 ± 0.08b | 1.26 ± 0.06 |
| Gurka/MetS score | ||||||
| Baseline | 0.85 ± 0.06 | 1.23 ± 0.13a | 1.34 ± 0.09a | 1.21 ± 0.12a | 0.78 ± 0.14a | 1.02 ± 0.04 |
| 3 mo | 0.80 ± 0.06 | 0.56 ± 0.13b | 0.49 ± 0.09b | 1.13 ± 0.12a | 0.96 ± 0.14b | 0.77 ± 0.04 |
| A, B, C, E | ||||||
| 12 mo | 0.82 ± 0.06 | 1.04 ± 0.14a | 0.47 ± 0.10b | 0.65 ± 0.13b | 1.25 ± 0.15c | 0.79 ± 0.05 |
| A, B, E | A, B, E | |||||
a, b,c Variables with different superscripts differ significantly (p < .05) within response groups over time by RMANOVA analysis
1 Column names A, B, C, D, E for significant differences between response groups (p < .05) at each time point by RMANOVA analysis
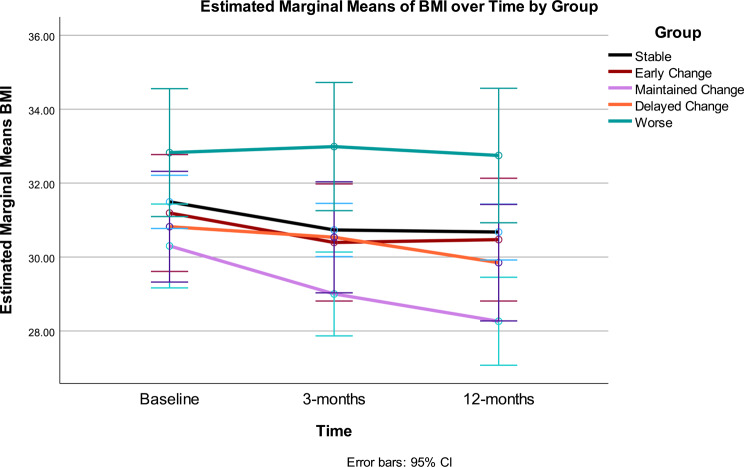
Body Mass Index: Change in BMI over time (p < .001), response group (p = .009) and the interaction between time and Gurka/MetS response group were statistically significant (p < .001) (see Table 3).
The Maintained group had significantly lower BMI than the Worse group (p = .004) overall. BMI showed significant improvement across the intervention. The time*response group interaction demonstrated differential patterns of change in BMI, which accounted for approximately 10% variance. A significant linear effect was noted for main effect of time, whereas a quadratic trend was noted for the time and group interaction. Change in BMI was consistent with changes seen in the different Gurka/MetS response groups: in Early Change, BMI improved between 0 and 3 months; while Maintained Change saw a reduction from baseline to 3 months and 3 months to 12 months. Delayed Change had no BMI change 0–3 months, but 3–12 months showed improvement. However, the Stable Gurka/MetS response group experienced significant BMI decrease at 0–3 months with no change 3–12 months. Participants with worsening Gurka/Mets scores did not experience significant change in BMI across the 12-month intervention. Statistically significant group differences included higher BMI in the Worse group versus the Maintained group at 3 months and 12 months, whereas the Stable group had a higher BMI than the Maintained group at 12 months (Table 4; Fig. 3).
Fig. 3.
BMI [kg/height(m2)] at baseline, 3- and 12-months by Gurka/MetS response categories. Stable = those showing no significant improvement or decline over 12 months (change < = 0.40 unit); Early Change = improvement baseline to 3 months, but not sustained 3–12 months; Maintained Change = maintained change over months; Delayed Change = change 3–12 months; Worse = increased scores
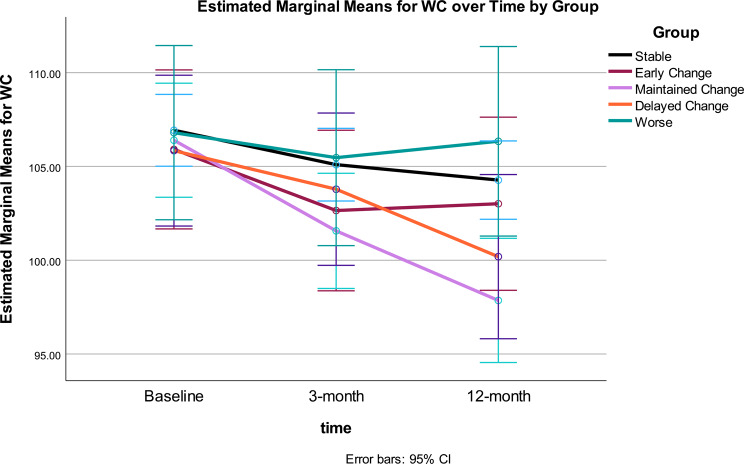
Waist Circumference: Change in WC over time (p < .001), and the interaction between time and Gurka/MetS response group were statistically significant (p < .001) (see Table 3). As with BMI, WC showed significant improvement across the intervention. The time*response group interaction demonstrated differential patterns of change in WC, accounting for 27% variance. Significant quadratic trends were found for the main effect of time and the interaction of time and group. Significant change in WC across Gurka/MetS response groups included: Early Change saw WC decrease between 0 and 3 months; Maintained Change and Delayed Change groups had significant decreases in WC from baseline to 3 months, and 3 months to 12 months (Table 4; Fig. 4). Reflecting the BMI changes noted earlier, the Stable Gurka/MetS response group experienced significant WC decrease 0–3 months with no change 3–12 months. Those classified as Worse did not experience any significant changes in WC across the 12-month intervention. After intervention, significant differences were noted between the Worse and Stable groups and the Maintained Change group, with the greatest improvement in the latter group, however, Worse and Stable groups did not differ at 12 months.
Fig. 4.
WC at baseline, 3- and 12-months by Gurka/MetS response categories. Stable = those showing no significant improvement or decline over 12 months (change < = 0.40 unit); Early Change = improvement baseline to 3 months, but not sustained 3–12 months; Maintained Change = maintained change over months; Delayed Change = change 3–12 months; Worse = increased scores
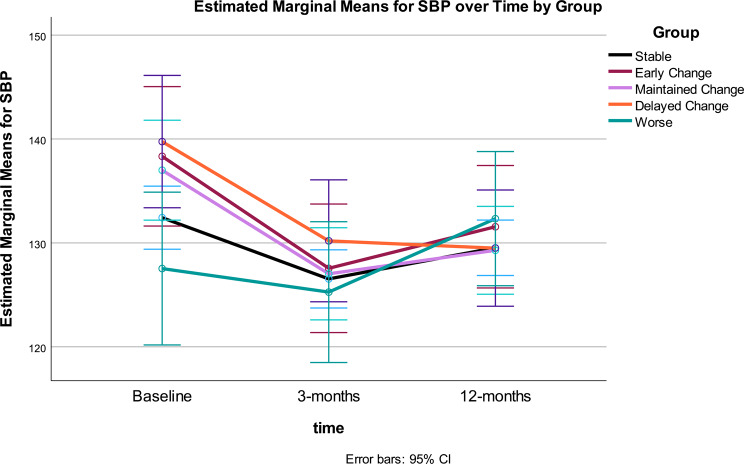
Blood pressure - systolic: Systolic blood pressure (SBP) showed a weaker pattern of change over time by Gurka/MetS group, only accounting for about 5% variance, whereas change in SBP over time accounted for 18%. Change in SBP over time was statistically significant (p < .001), whereas the interaction between time and Gurka/MetS response group was weaker (p = .03) (see Table 3).
Change in SBP showed significant improvement across the intervention, however, the largest improvement was during the first three months. The time*response group interaction demonstrated differential patterns of change in SBP. A significant linear trend was noted for the time effect, whereas a quadratic trend was found for the interaction of time and group. The between effect for MetS groups for SBP was not significant.
Change in SBP across Gurka/MetS response groups: both the Stable and Early Change Groups: showed change between 0 and 3 months (p < .001), however, their SBP at 12 months was not significantly different from baseline. Maintained Change: decreased SBP from baseline to 3 months, and 3 months to 12 months (p < .001), with improved SBP compared to baseline; Delayed Change showed significant improvement in SBP 0–3 months, but not from 3 to 12 months (n.s.). Despite increasing SBP, the Worse Gurka/MetS response group did not experience any significant change in SBP across the 12-month intervention. Group differences were noted at baseline, however, SBP converged at 3-months and continued to become more similar by 12-months, with no significant differences noted between groups at the end of the intervention (Table 4; Fig. 5).
Fig. 5.
SBP at baseline, 3- and 12-months by Gurka/MetS response categories. Stable = those showing no significant improvement or decline over 12 months (change < = 0.40 unit); Early Change = improvement baseline to 3 months, but not sustained 3–12 months; Maintained Change = maintained change over months; Delayed Change = change 3–12 months; Worse = increased scores
Blood pressure - diastolic: Diastolic blood pressure (DBP) over time accounted for 11% variance, however, the interaction of time x MetS group was not significant (see Table 3). Change in DBP showed significant improvement between baseline and 3-months, but no significant change from 3-months to 12-months. The between effect for Gurka/MetS response groups was not significant.
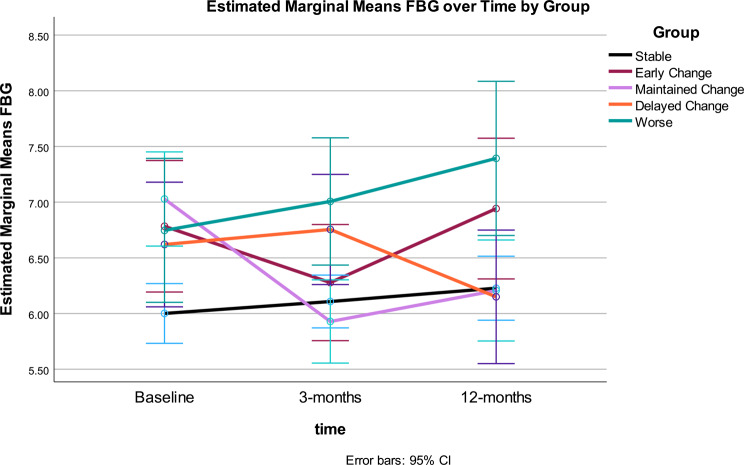
Fasting blood glucose: Fasting blood glucose (FBG) was statistically significant over time (p < .01) and for the interaction of time by MetS Groups (p < .001) (see Tables 3 and 4). Change in FBG showed significant improvement from baseline to 3-months, no change from 3–12-months, and no significant difference between baseline and 12-months. The time*response group interaction demonstrated differential patterns of change in FBG, which accounted for 12% variance. Significant quadratic trends were found for the main effect of time and the interaction of time and group. The between effect for Gurka/MetS response groups was also statistically significant for FBG (p < .03). Differences were noted between the FBG for Stable and Worse groups, with higher FBG for the Worse group (Fig. 6).
Fig. 6.
FBG at baseline, 3- and 12-months by Gurka/MetS response categories. Stable = those showing no significant improvement or decline over 12 months (change < = 0.40 unit); Early Change = improvement baseline to 3 months, but not sustained 3–12 months; Maintained Change = maintained change over months; Delayed Change = change 3–12 months; Worse = increased scores
Change in FBG across Gurka/MetS response groups differed. The Stable group showed a significant increase between baseline and 12-months, the Early Change group showed decrease between 0 and 3 months, however, their FBG at 12 months increased again but was not significantly different from baseline. The Maintained Change group saw a decrease in FBG from baseline to 3 months, no change between 3- and 12-months, and ended with an overall lower FBG compared to baseline; while the Delayed Change showed significant decrease in FBG from 3 to 12 months. The Worse Gurka/MetS response group showed significantly higher FBG at 12-months compared to baseline. After the 12-month intervention, significant differences in FBG were noted between the Stable and Worse groups, with higher FBG in the latter group.
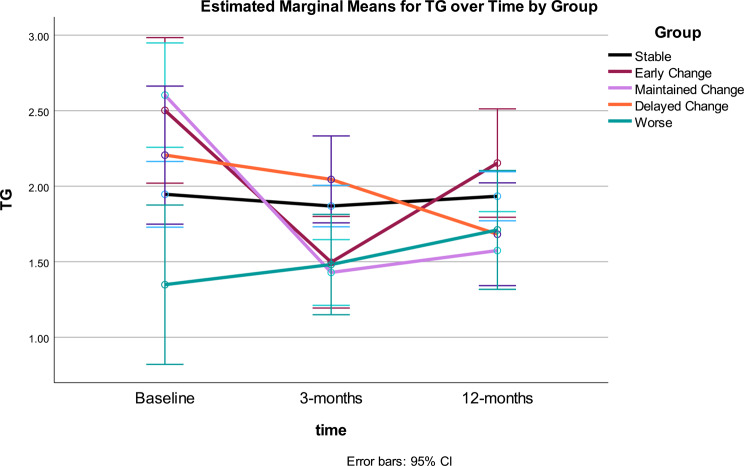
Triglycerides: Triglycerides (TG) were statistically significant over time and for the interaction of time by Gurka/MetS Groups (both p < .001) (see Table 3). Change in TG showed significant improvement across the intervention. The between effect for Gurka/MetS response groups was not statistically significant for TG. The time*response group interaction demonstrated differential patterns of change in TG, which accounted for 36% variance. Significant quadratic trends were found for the main effect of time and the interaction of time and group.
Change in TG across Gurka/MetS response groups: the Stable group showed no significant change, both the Early and Maintained Change Groups significantly decreased between 0 and 3 months (p = .001) only, with TG at 12 months significantly different from baseline. The Delayed Change showed significant decrease in TG from 3 to 12 months only. The Worse Gurka/MetS group showed a non-significant increase in TG throughout the intervention. After 12-months, no significant differences were noted in TG across groups (Table 4; Fig. 7).
Fig. 7.
TG at baseline, 3- and 12-months by Gurka/MetS response categories. Stable = those showing no significant improvement or decline over 12 months (change < = 0.40 unit); Early Change = improvement baseline to 3 months, but not sustained 3–12 months; Maintained Change = maintained change over months; Delayed Change = change 3–12 months; Worse = increased scores
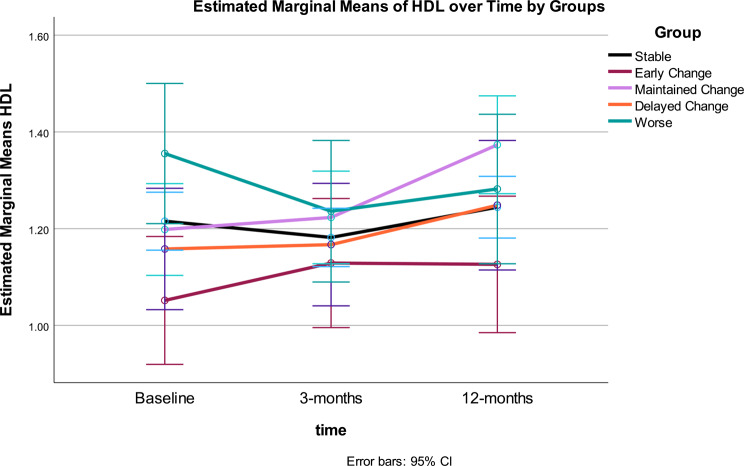
High density Lipoprotein Cholesterol: High density lipoprotein cholesterol (HDL-C) was statistically significant over time and for the interaction of time by MetS response groups (both p < .001) (see Table 3). The between effect for MetS groups was not statistically significant for HDL-C. The time*response group interaction demonstrated differential patterns of change in HDL-C, which accounted for 17% variance. Significant quadratic trends were found for the main effect of time and the interaction of time and group.
Overall, HDL-C was stable from baseline to 3-months, then increased significantly by 12-months. Change in HDL-C across Gurka/MetS response groups: the Stable group showed significant increase between 3- and 12-months (p < .001) but did not differ from baseline at 12 months. The Early Change group showed no significant change in HDL-C; the Maintained Change group had significant increase in HDL-C between 3- and 12-months (p < 001), Delayed Change showed significant change in HDL-C from 3 to 12 months only (p = .05). The Worse group had significant decrease in HDL-C from baseline to 3-months (p < .004). After 12-months, no significant differences were noted in HDL-C across groups (Table 4; Fig. 8).
Fig. 8.
HDL-C at baseline, 3- and 12-months by Gurka/MetS response categories. Stable = those showing no significant improvement or decline over 12 months (change < = 0.40 unit); Early Change = improvement baseline to 3 months, but not sustained 3–12 months; Maintained Change = maintained change over months; Delayed Change = change 3–12 months; Worse = increased scores
Analysis of Gurka/MetS score: The summary Gurka/MetS score has the advantage of combining the information from multiple clinical indicators and the disadvantage that it is based on the same variables that were used to define the response groups, which reflect participants’ experience over 12 months. Given there is no current alternative indicator of overall health risk that would be independent of the clinical indicators, RMANOVA was conducted. A significant main effect of time and a significant interaction between time and response group were noted. The between-groups effect based on Gurka/MetS response group was not significant, whereas the time*group interaction demonstrated differential patterns of change, accounting for approximately 66% of variance (Table 3).
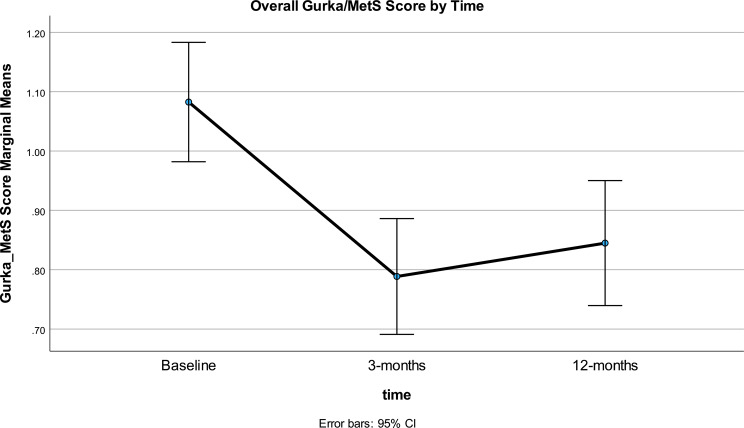
A significant quadratic trend was found for the interaction of time and response group. The overall trajectory (Fig. 9) for time ignoring Gurka/MetS groups showed the improvement (lowering) in scores, followed by scores reverting toward baseline. However, the difference in Gurka/MetS scores showed overall significant improvement from baseline in the range of 0.23 over 12 months or about half of the 0.4 unit considered clinically meaningful in our analysis.
Fig. 9.
Overall Gurka/MetS score at baseline, 3- and 12-months
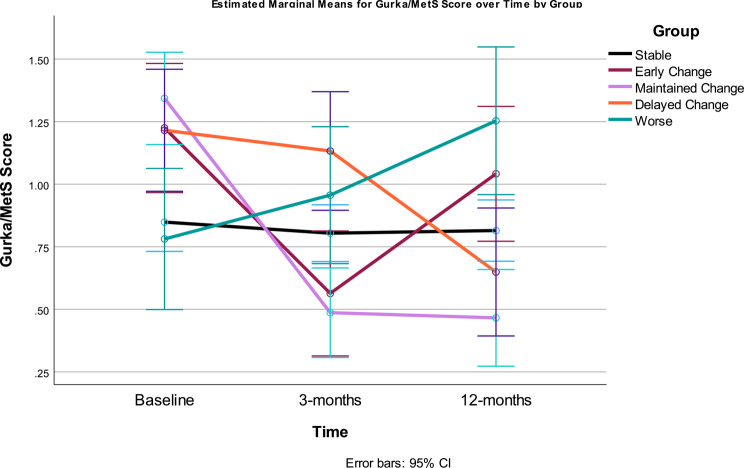
The separate Gurka/MetS trajectories for the five groups are shown in Fig. 10. Clear differences are seen in the five Gurka/MetS groups. The large Stable group (50% of participants) showed a flat trajectory across the duration of the study with no significant variation. This group started the study with a relatively lower Gurka/MetS score and were only marginally higher on the Gurka/MetS score than the Worse group that demonstrated no improvement or a negative outcome from the intervention. It should be noted that this latter group showed significant worsening (increase) in their Gurka/MetS scores from baseline to 3-months, and from 3-months to 12-months, resulting in the highest Gurka/MetS scores at 12 months.
Fig. 10.
Gurka/MetS scores at baseline, 3- and 12-months by response categories. Stable = those showing no significant improvement or decline over 12 months (change < = 0.40 unit); Early Change = improvement baseline to 3 months, but not sustained 3–12 months; Maintained Change = maintained change over months; Delayed Change = change 3–12 months; Worse = increased scores
The most dramatic improvement in Gurka/MetS was demonstrated between baseline and 3-months, with the Early Change group (baseline to 3-month group only) and the Maintained change group (change over the intervention) both showing significant improvement. Those categorized as only improving between 3- and 12-months (Delayed change) also reflected this pattern, with a non-significant improvement in Gurka/MetS in the first three months of the study, followed by significant improvement at 12 months.
At 3-months, participants in the Delayed change group showed the highest (worst) Gurka/MetS scores, significantly higher/worse than all other groups. By the end of the intervention (12 months), Gurka/MetS scores for the Delayed change group were significantly lower (better) than the Early Change, Stable, and Worse groups, and only marginally higher/worse than those participants in the Maintained Change group.
The Stable and Worse groups were not significantly different at 3-months and the two groups that showed early benefit from the intervention (i.e., Early and Maintained change groups) had the lowest/best Gurka/MetS scores but did not significantly differ from one another. At 12-months, participants in the Worse group had the highest Gurka/MetS score however, they were not significantly different than those who experienced Early Change (i.e., first three months of the study), as this group reverted back toward baseline scores, resulting in a non-significant difference between their baseline and 12-month scores. Therefore, if only measured at baseline and 12-month intervals, these participants would have been seen as “no change” or showing no benefit of the intervention.
Overall, the results suggest only about 30% saw results that could be considered clinically relevant for management of MetS among participants who completed the study. There was a high degree of interindividual variation among participants in terms of the response of the individual indicators, but not in the degree of diet quality and fitness change, which demonstrated similar improvements across the response groups. The pattern of change is highly consistent, whether considering individual clinical indicators or the Gurka/MetS score. Some participants demonstrated little to no impact of the intervention (Stable) or resulted in their Gurka/Mets scores increasing over the 12-month period (Worse). The Stable group’s Gurka/MetS scores were not only unchanging, but those participants were in the mid-range of scores, whereas the group that saw their metabolic scores rise (Worse group) had the lowest early Gurka/MetS scores at the beginning of the intervention. The groups which were most responsive regarding improvement in Gurka/MetS scores were those starting with the highest or most detrimental levels. Even so, two distinct beneficial patterns of change were noted in the Maintain change and the Delayed change groups. Both groups resulted in participants having the lowest/improved Gurka/MetS scores after the trial – therefore, demonstrating the most positive outcomes. Conversely, the Early change group, despite showing significant improvement from baseline to three-months, reverted to their initial baseline scores by twelve-months, negating any improvement gained.
Discussion
This novel exploratory descriptive analysis, whereby the overall clinical experience of participants over 12 months was used to create five mutually exclusive response groups, revealed several insights that are helpful in interpreting the original study results. Remission of MetS as a binary variable was demonstrated in only 19% of participants at 12 months. With 50% of participants demonstrating little to no impact of the intervention (Stable – 50%), 9% Worse and 10% only showing Early changes, the results are not surprising. Secondly, the analyses of diet quality and fitness, based on SEM analyses, showed expected overall improvement and lack of differences among response groups. We conclude that the results are more likely due to unknown or unmeasured factors affecting individual responsiveness, and less likely due to differences in adherence to the interventions.
Causal inference for lifestyle treatment is strengthened when the intervention can be linked to both improvement in diet and physical activity, and individual health risk indicators, such as biological markers and/or disease risk scores [40, 41]. We used a combination of techniques to reduce error among study completers and estimate effects on individual clinical indicators and a composite score for MetS, defining subgroups by response to the intervention using a cut-point approach.
The limited literature in MetS and associated conditions supports our use of the selected cut-point, but few have used the Gurka/MetS score in treatment studies to date. In addition to the two studies mentioned in the introduction [34, 35], a proof of concept weight loss study in MetS (n = 26), found that the Gurka/MetS score declined by 0.4 units over 6 months from baseline (0.8 ± 0.6) [42]. A small 12-month exercise intervention (n = 20) also showed very similar results [baseline Gurka/MetS score = 0.77 ± 0.77; 12 months = 0.35 ± 0.67] [43].
Only a few studies have considered differential responsiveness in MetS. Brennan et al. developed their own cardiometabolic risk z-score based on change in WC, TG and FBG in a clinical trial of health education, weight loss by diet or weight loss plus exercise intervention among 61 older, obese adults [44]. They defined high and low responders at the median for score change and demonstrated more “responders” in the weight loss plus exercise group, as would be expected for a multi-component intervention. Our results are consistent with theirs in that WC, TG and FBG were important contributors to differential response. In contrast to their approach we found that HDL and SBP also contributed.
Ramos et al. assessed responsiveness to three exercise interventions among 99 adults taking or not taking metformin for MetS [45]. They used a different MetS z-score calculation but did use the effect size estimates for clinically meaningful change from the DPP at one year [34]. They identified 44–49% as likely responders, 22 − 19% as uncertain and 33 − 32% as likely non-responders among those taking and not taking metformin, respectively. However, their baseline values for the MetS z-score were much higher than in our sample (no metformin 1.6 ± 2.3; metformin 4.2 ± 2.5), suggesting lack of comparability of the participants to those in our study. Conceptually, their approach was similar in defining a cut-point to assess responder status, but their study design had only two time points for measurement. Our multi-wave approach allowed for examining differential patterns of change over time, with three time points being a minimum [46–51].
Another 12-week community-based lifestyle change study, focused on weight loss, considered response variability in a group at high risk of diabetes (n = 257), but took a different approach to grouping responsiveness [52]. They used cluster analysis to define four groups who differed in the change in their glucose tolerance (area under the curve) after intervention. They identified high responders (7%), moderate responders (22%), non-responders (49%), and those whose glucose tolerance deteriorated (22%). Adherence to exercise was monitored only via gym attendance, and diet change was not assessed. Using principal component analysis, they were only able to account for 48% of the response variability, based on 19 clinical and physiological measures. The intervention was short-term, with only two data points, and behaviour change variability could have accounted for the response variability.
Therefore, the literature is currently very limited, and our results must be considered preliminary. This study is among the first to provide evidence that differences in responsiveness to intervention are unlikely to be solely due to measurement error and differences in adherence to lifestyle intervention among individuals who complete lifestyle programs. This is not to minimize the importance of lack of behaviour change in accounting for the failure of many lifestyle change interventions to influence clinical health risk, but suggests that differences in response may also be important.
Looking at our results in more detail for factors that might account for group differences, it was noted in the original study that greater response was seen among those with higher baseline PROCAM scores [24]. Results in this analysis, using the Gurka/MetS score also saw greater response among those with higher initial scores (Early, Maintain, Delayed groups), with the Worse group having distinctly lower scores at baseline. One could argue that some of the change between baseline to 3-months represented regression to the mean, and that cannot be ruled out, although the DPP showed little change in Gurka/MetS score in the placebo group over their 1-year intervention [34].
While the most change in diet and fitness was universally observed in the first three months, a delayed decline in Gurka/MetS score was seen in 10%. The SBP and WC declined in the first 3 months, while FBG, TG and HDL-C were stable to 3 months and then declined. There were no changes in glucose medications recorded at 3, 6, or 9 months that would account for these observations (data not shown), as participants were seen quarterly by physicians. Therefore reasons for the delayed response remain unclear.
Additionally, whereas early change or improvement in MetS was noted, at least 10% of those who saw improvement in their MetS risk in the first three months had risk scores revert to baseline, thereby eliminating the benefits gained by initial improvement from the intervention.
The results for the 9% of participants who increased their Gurka/MetS score over time (i.e. the Worse group) were unexpected. At baseline, this group of individuals had the lowest Gurka/MetS scores, as well as significantly lower TG levels than the other four groups. Gurka/MetS scores significantly increased to 12 months. This is not the first time that adverse effects to lifestyle programs have been observed. Gardner et al. have demonstrated weight gain among some weight loss trial participants [53] and Bouchard and others have also documented adverse clinical indicators with exercise [54]. Whether the adverse scores reflect measurement issues or real harm requires further study. The fact that this group showed positive changes in diet quality and fitness yet had adverse changes in their Gurka/MetS score is concerning.
Weight loss has been the primary focus of most studies in MetS, and our original study was unusual in its greater focus on improved diet quality, if weight loss was not feasible [55]. Rationale for greater focus on diet quality and not weight has been reviewed elsewhere [56]. Baseline BMI was lowest and changed most in the Maintained group and continued to decrease through the study (~ 2 BMI units or ~ 6 kg), whereas the Worse group was heaviest and weight stable, with the other three groups in between. The WC data are highly consistent with our understanding of the importance of the visceral abdominal fat depot in the pathophysiology of MetS and as an intervention target [57, 58]. At baseline, WC was similar across all groups, but diverged over time, such that Maintainers achieved substantially lower WC than either the Stable or Worse groups. Maintainers appeared to be more physiologically able to decrease WC and body weight, compared to others in our study.
The SNP analysis was suggestive of genetic differences as only the Maintained group showed a statistical difference in the minor T allele of the ADIPOQ SNP rs 1,501,299, one of many SNPs for the ADIPOQ gene on the chromosomal locus 3q27, in line with previous analysis of 17 SNPs published in the original study [38], using an older continuous MetS score based on a French cohort [25]. Adiponectin is secreted mainly by mature adipocytes, a protein with insulin-sensitizing and antiatherogenic effects. Polymorphisms of the ADIPOQ gene are an active area of research and the GG version this SNP has been associated with increased type 2 diabetes [59]. Thus, we have limited evidence for possible genetic differences in the five groups.
A key question is the extent to which combinations of genetic and physiological factors may be accounting for some of the observed variability in responses, relative to variable behaviour change and/or other environmental factors such as changes in sleep, stress, etc. This study contributes to the literature by finding distinct differences in response by logical subgroups of people completing the same intensive lifestyle intervention where similar changes in diet quality and physical fitness could be demonstrated. The results support arguments in the literature for the potential importance of inherent differences in responsiveness [60, 61].
Strengths of this analysis include use of multiple methods to reduce measurement error, assessment of overall diet and physical fitness, and detailed analysis of multi-wave data, using a more recently developed MetS z-score. Together, these and other descriptive methods may be helpful when predictive modelling does not yield expected results, in better assessing intervention effects and in comparing results across treatment studies, improving research efficiency over time [19].
Limitations include the lack of a control group in the original study and limits on the number of assessments done over one year. Assessment at more time points would provide greater confidence in assessing intervention trajectories in response subgroups, in line with emerging personalized medicine approaches [62]. Psychological, personality, sleep and other variables known to be relevant were not formally assessed [19]. Sample size was very limited to detect differences among some groups. In addition, participants were not specifically newly diagnosed with metabolic issues, since 50% had type 2 diabetes. For example, the large Stable group may have already achieved possible reductions in clinical indicators, even with additional improvements in their diet and fitness scores. The diet quality and fitness scores used in this study also may not be capturing the key elements of lifestyle that will be most contributory to improving MetS indicators, as they are based on public health guidance for general health. For example, the Canadian HEI-C is based on the 2007 Canada’s Food Guide. Legumes and olive oil are not tracked in detail as in scores for assessing Mediterranean diet. The selection of the cut-point was taken from a limited number of previous studies but is reasonable given the size and long term follow-up of the DPP, and definitely an advance over arbitrarily dividing responses by internal study quantiles.
The many challenges of research on lifestyle change were enumerated in the introduction. Attrition/incomplete data is common in such studies [63, 64] and the decision was made to focus on the 60% of participants with complete data, as these participants were mostly likely to have made changes to lifestyle, although program adherence was not formally measured. People who dropped out of the formal program may have a different pattern of response, even if they undertook the lifestyle changes. We addressed intervention process and efficacy by offering a detailed description of process for an intervention much more intensive than typical in the health system.
Whereas we were unable to identify factors that accounted for differential responsiveness, we believe that progress will be possible with use of newer, more accurate methods of study conduct and analysis. Longitudinal, repeated-measures designs lend themselves to newer analytic methods that are helpful in understanding sources of variation between groups and within individuals [65]. Further applications of multi-level models and/or latent growth models would help disentangle sources of variation from intervention effects. The basic factors we were able to assess provide few clues on relevant baseline differences that can guide treatment planning. Studies are now needed to examine the combined behavioural, genome, metabolomic, and other differences comparing lifestyle responders to non-responders, with those who experience adverse effects being a high priority group, to rule out possible risk of harm.
In the meantime, health professionals working with MetS patients in primary care need to be cognizant of the variability in response to lifestyle therapy, even among participants who will complete programs, as we have demonstrated in this exploratory analysis. We suggest programming should focus on those with higher risk scores at baseline who are most likely to participate in intensive programs. The analysis also shows that a weight loss focus is not essential for reduction in MetS. A shift to focus more on dietary pattern change and increased exercise may be more feasible, acceptable and sustainable in the long-term, while still yielding demonstrable health benefits, at least in a substantial proportion of patients. It will be important to advise patients that lifestyle may or may not affect clinical risk measures. Programs also need to be sufficiently intensive and sustained to allow for response beyond 3 months. Those with a delayed response can be accommodated by longer programs, and practitioners should encourage longer-term commitment to see if clinical indicators change.
Conclusion
Focusing on the overall pattern of change in groups can mask the high variability in subgroups among those with MetS. Determining what differentiates participants with MetS who successfully decrease their risk (31%), versus those who experience no significant improvement (50%), from those who experience negative outcomes (almost 9% had increased risk) despite lifestyle interventions is critically important for informing adoption of lifestyle change as a legitimate medical treatment. As with other complex and chronic health conditions, a toolbox of strategies, lifestyle, drugs, and surgical, are needed for best management of MetS. Studies now underway will further strengthen the evidence base for lifestyle treatment of this common condition [66]. Further methodological collaborations between implementation scientists and statisticians at the study design phase are needed to ensure relevant data for advanced repeated measures analyses are collected, considering both the measurement issues and need for tailoring to individual preferences and characteristics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Metabolic Syndrome Canada is a not-for-profit charitable organization that funded the original study. Daren Heyland, Rupinder Dhaliwal, Andrew Day and Roger Leung from Queen’s University, Kingston, ON were involved in the development and conduct of the original study. Dr. Lew Pliamm (Toronto) managed a site for patient enrolment and data collection, as did Caroline Rheaume and Douglas Klein (co-authors). Multiple dietitians, kinesiologists and nurses were also involved, as were numerous students. All are acknowledged for their contributions to the completion of the work. The study would not have been possible without the commitment of the participants in undertaking lifestyle change.
Abbreviations
- BMI
Body mass index
- CMR
Cardiometabolic risk
- CVD
Cardiovascular disease
- DPP
Diabetes Prevention Program
- FBG
Fasting blood glucose
- Gurka/MetS
Gurka continuous Metabolic Syndrome severity score
- HDL-C
High density lipoprotein cholesterol
- HEI-C
Canadian Healthy Eating Index
- MetS
Metabolic syndrome
- PROCAM
Prospective Cardiovascular Münster
- REDCap
Research Electronic Data Capture
- RMANCOVA
Repeated measures analysis of covariance
- SBP / DBP
Systolic / diastolic blood pressure
- SEM
Structural equation modelling
- SNPs
Single nucleotide polymorphisms
- TG
Triglycerides
- WC
Waist circumference
Author contributions
SBM and PB conceptualized the issues and jointly wrote the paper; SBM conducted the formal analyses. KJ and DK were principal investigators contributing to the conception, data analysis and interpretation of the main study on which this study is based, while DMM, PB, AT and CR were co-investigators contributing to data collection, data analysis and interpretation and DR was involved in overall project management. All authors contributed to the interpretation of data, critically reviewed and edited the manuscript for important intellectual content, and approved the final version.
Funding
Funding was provided by Metabolic Syndrome Canada (https://www.metabolicsyndromecanada.ca/). The funding source played no role in this secondary analysis.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted under the World Medical Association’s Declaration of Helsinki. Ethics approvals for the study were obtained from Health Research Ethics Board- Biomedical (University of Alberta), Comité d’éthique de la recherche des Centres de santé et de services sociaux de la Vieille-Capitale (Laval University), University of Guelph Research Ethics Board, and Institutional Review Board Services, a Chesapeake IRB Company (Aurora, Ont.). All the participants provided informed consent before participating.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of Disease Study 2021. Lancet. 2024;403(10440):2162–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lohia P, Kapur S, Benjaram S, Pandey A, Mir T, Seyoum B. Metabolic syndrome and clinical outcomes in patients infected with COVID-19: does age, sex and race of the patient with metabolic syndrome matter? J Diabetes. 2021;13(5):420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, Jr, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. [DOI] [PubMed]
- 5.Zimmet PZ. Kelly West lecture 1991. Challenges in diabetes epidemiology–from West to the rest. Diabetes Care. 1992;15(2):232–52. [DOI] [PubMed] [Google Scholar]
- 6.Ju SY, Lee JY, Kim DH. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: a meta-analysis of prospective cohort studies. Medicine. 2017;96(45):e8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–32. [DOI] [PubMed] [Google Scholar]
- 8.Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA. 2020;323(24):2526–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu LM, Karunanayake C, Aich P, Hecker M, Pahwa P. Association between liver enzymes and metabolic syndrome in Canadian adults: results from the Canadian health measures survey - cycles 3 &4. J Diabetes Metab Disord. 2022;21(2):1699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Commonwealth Fund S, Ottawa CIHI. 2023. https://www.cihi.ca/en/commonwealth-fund-survey-2022
- 11.Katz A, Lambert-Lanning A, Miller A, Kaminsky B, Enns J. Delivery of preventive care: the national Canadian Family Physician Cancer and Chronic Disease Prevention Survey. Can Fam Physician. 2012;58(1):e62–69. [PMC free article] [PubMed] [Google Scholar]
- 12.Fenwick PH, Jeejeebhoy K, Dhaliwal R, Royall D, Brauer P, Tremblay A, Klein D, Mutch DM. Lifestyle genomics and the metabolic syndrome: a review of genetic variants that influence response to diet and exercise interventions. Crit Rev Food Sci Nutr. 2019;59(13):2028–39. [DOI] [PubMed] [Google Scholar]
- 13.Almeida DM, Piazza JR, Stawski RS. Interindividual differences and intraindividual variability in the cortisol awakening response: an examination of age and gender. Psychol Paging. 2009;24(4):819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nesselroade JR. Interindividual differences in intraindividual change. In: Collins L, Horn J, editors. Best methods for the analysis of change: recent advances, unanswered questions, future directions. Washington: American Psychological Association; 1991. pp. 92–105. [Google Scholar]
- 15.Nesselroade JR, Molenaar P. In: Lerner R, Lamb M, Freund A, Hoboken A, editors. Emphasizing intraindividual variability in the study of development over the life span: concepts and issues. NJ: John Wiley; 2010. pp. 30–54. [Google Scholar]
- 16.Molenaar PC. The future of analysis of Intraindividual Variation. In: Diehl M, Hooker KS, Martin J, editors. Handbook of Intraindividual Variability across the Life Span. New York, NY: Routledge; 2014. pp. 343–56. [Google Scholar]
- 17.Kumanyika SK, Bowen D, Rolls BJ, Van Horn L, Perri MG, Czajkowski SM, Schron E. Maintenance of dietary behavior change. Health Psychol. 2000;19(1, Suppl):42–56. [DOI] [PubMed] [Google Scholar]
- 18.Bayerle P, Haufe S, Kück M, Protte G, Kerling A, Ewers S, Boeck HT, Sundermeier T, Ensslen R, Kahl KG, et al. The impact of Body Weight Changes versus Exercise Capacity Changes on Health-related factors following a lifestyle intervention in employees with metabolic syndrome. Nutrients. 2022;14(21):4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brauer P, Desroches S, Dhaliwal R, Li A, Wang Y, Conklin AI, Klein D, Drouin-Chartier J-P, Robitaille J, Keathley JR, et al. Modified Delphi Process To Identify Research Priorities and Measures for Adult Lifestyle Programs to address type 2 diabetes and other Cardiometabolic Risk conditions. Can J Diabetes. 2022;46(4):411–8. [DOI] [PubMed] [Google Scholar]
- 20.Michie S, West R, Finnerty A, Norris E, Wright A, Marques M, Johnston M, Kelly M, Thomas J, Hastings J. Representation of behaviour change interventions and their evaluation: development of the Upper Level of the Behaviour Change intervention ontology. Wellcome Open Res. 2021;5:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schober P, Vetter TR. Anesth Analgesia. 2018;127(2):569–75. Repeated Measures Designs and Analysis of Longitudinal Data: If at First You Do Not Succeed—Try, Try Again. [DOI] [PMC free article] [PubMed]
- 22.Schork NJ. Accommodating serial correlation and Sequential Design Elements in Personalized studies and aggregated personalized studies. Harv Data Sci Rev. 2022;2022Si3:10116299608f92f1eef6f4. [DOI] [PMC free article] [PubMed]
- 23.Li X, Perelman D, Leong AK, Fragiadakis G, Gardner CD, Snyder MP. Distinct factors associated with short-term and long-term weight loss induced by low-fat or low-carbohydrate diet intervention. Cell Rep Med. 2022;3(12):100870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeejeebhoy K, Dhaliwal R, Heyland DK, Leung R, Day AG, Brauer P, Royall D, Tremblay A, Mutch DM, Pliamm L, et al. Family physician-led, team-based, lifestyle intervention in patients with metabolic syndrome: results of a multicentre feasibility project. CMAJ Open. 2017;5(1):E229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillier TA, Rousseau A, Lange C, Lepinay P, Cailleau M, Novak M, Calliez E, Ducimetiere P, Balkau B. Practical way to assess metabolic syndrome using a continuous score obtained from principal components analysis. Diabetologia. 2006;49(7):1528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowry DE, Feng Z, Jeejeebhoy K, Dhaliwal R, Brauer P, Royall D, Tremblay A, Klein D, Mutch DM. Prediction modelling of 1-year outcomes to a personalized lifestyle intervention for canadians with metabolic syndrome. Appl Physiol Nutr Metab. 2020;45(6):621–7. [DOI] [PubMed] [Google Scholar]
- 27.Maitland SB, Brauer P, Mutch DM, Royall D, Klein D, Tremblay A, Rheaume C, Dhaliwal R, Jeejeebhoy K. Evaluation of latent models assessing physical fitness and the healthy eating index in Community studies: Time-, Sex-, and Diabetes-Status Invariance. Nutrients. 2021;13(12):4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assmann G, Schulte H, Seedorf U. Cardiovascular risk assessment in the metabolic syndrome: results from the prospective Cardiovascular Munster (PROCAM) Study. Int J Obes. 2008;32(Suppl 2):S11–16. [DOI] [PubMed] [Google Scholar]
- 29.Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism. 2014;63(2):218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurka MJ, Filipp SL, Musani SK, Sims M, Deboer MD. Use of BMI as the marker of adiposity in a metabolic syndrome severity score: derivation and validation in predicting long-term disease outcomes. Metabolism. 2018;83:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senn S. Three things that every medical writer should know about statistics. Write Stuff. 2009;18(3):159–62. [Google Scholar]
- 32.Guo Y, Musani SK, Sims M, Pearson TA, Deboer MD, Gurka MJ. Assessing the added predictive ability of a metabolic syndrome severity score in predicting incident cardiovascular disease and type 2 diabetes: the atherosclerosis risk in communities Study and Jackson Heart Study. Diabetol Metab Syndr. 2018;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeBoer MD, Gurka MJ, Golden SH, Musani SK, Sims M, Vishnu A, Guo Y, Pearson TA. Independent associations between Metabolic Syndrome Severity and Future Coronary Heart Disease by Sex and Race. J Am Coll Cardiol. 2017;69(9):1204–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeBoer MD, Filipp SL, Gurka MJ. Use of a metabolic syndrome severity Z score to Track Risk during Treatment of prediabetes: an analysis of the Diabetes Prevention Program. Diabetes Care. 2018;41(11):2421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Höchsmann C, Dorling JL, Martin CK, Newton RL, Apolzan JW, Myers CA, Denstel KD, Mire EF, Johnson WD, Zhang D, et al. Effects of a 2-Year primary care lifestyle intervention on cardiometabolic risk factors. Circulation. 2021;143(12):1202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowry DE, Fenwick PH, Roke K, Jeejeebhoy K, Dhaliwal R, Brauer P, Royall D, Tremblay A, Klein D, Mutch DM. Variants in APOA5 and ADIPOQ moderate improvements in metabolic syndrome during a one-year lifestyle intervention. Lifestyle Genom. 2018;11(2):80–9. [DOI] [PubMed] [Google Scholar]
- 39.Muhammad LN. Guidelines for repeated measures statistical analysis approaches with basic science research considerations. J Clin Invest. 2023;133(11):e171058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 41.Rothman KJ, Greenland S. Causation and causal inference in Epidemiology. Am J Public Health. 2005;95(S1):S144–50. [DOI] [PubMed] [Google Scholar]
- 42.Powell LH, Appelhans BM, Ventrelle J, Karavolos K, March ML, Ong JC, Fitzpatrick SL, Normand P, Dawar R, Kazlauskaite R. Development of a lifestyle intervention for the metabolic syndrome: Discovery through proof-of-concept. Health Psychol. 2018;37(10):929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinetti G, Mozzini C, Desenzani P, Boni E, Bulla L, Lorenzetti I, Romano C, Pasini A, Cominacini L, Assanelli D. Supervised exercise training reduces oxidative stress and cardiometabolic risk in adults with type 2 diabetes: a randomized controlled trial. Sci Rep. 2015;5(1):9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brennan AM, Standley RA, Yi F, Carnero EA, Sparks LM, Goodpaster BH. Individual Response Variation in the effects of Weight loss and Exercise on insulin sensitivity and cardiometabolic risk in older adults. Front Endocrinol. 2020;11:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos JS, Dalleck LC, Keith CE, Fennell M, Lee Z, Drummond C, Keating SE, Fassett RG, Coombes JS. Optimizing the Interaction of Exercise volume and metformin to induce a clinically significant reduction in metabolic syndrome severity: a Randomised Trial. Int J Environ Res Public Health. 2020;17(10):3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lord FM. A paradox in the interpretation of group comparisons. Psychol Bull. 1967;68(5):304–5. [DOI] [PubMed] [Google Scholar]
- 47.Oldham P. A note on the analysis of repeated measurements of the same subjects. J Chronic Dis. 1962;15(10):969–77. [DOI] [PubMed] [Google Scholar]
- 48.Rogosa D, Brandt D, Zimowski M. A growth curve approach to the measurement of change. Psychol Bull. 1982;92(3):726. [Google Scholar]
- 49.Tennant PW, Tomova GD, Murray EJ, Arnold KF, Fox MP, Gilthorpe MS. Lord’s’ paradox’explained: the 50-year warning on the use of ‘change scores’. J Epidemiol Comm Health. 2022;76:A5. [Google Scholar]
- 50.Tennant PWG, Arnold KF, Ellison GTH, Gilthorpe MS. Analyses of ‘change scores’ do not estimate causal effects in observational data. Int J Epidemiol. 2022;51(5):1604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearl J. Lord’s paradox revisited–(oh Lord! Kumbaya!). J Causal Inference. 2016;4(2):20160021. [Google Scholar]
- 52.O’Donoghue G, Kennedy A, Andersen GS, Carr B, Cleary S, Durkan E, Davis H, Færch K, Fitzpatrick P, Kenny H, et al. Phenotypic responses to a lifestyle intervention do not account for inter-individual variability in glucose tolerance for individuals at high risk of type 2 diabetes. Front Physiol. 2019;10:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gardner CD, Trepanowski JF, Del Gobbo LC, Hauser ME, Rigdon J, Ioannidis JPA, Desai M, King AC. Effect of Low-Fat vs Low-Carbohydrate Diet on 12-Month Weight loss in overweight adults and the Association with genotype pattern or insulin secretion: the DIETFITS Randomized Clinical Trial. JAMA. 2018;319(7):667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouchard C, Blair SN, Church TS, Earnest CP, Hagberg JM, Hakkinen K, Jenkins NT, Karavirta L, Kraus WE, Leon AS, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS ONE. 2012;7(5):e37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Royall D, Brauer P, Bjorklund L, O’Young O, Tremblay A, Jeejeebhoy K, Heyland D, Dhaliwal R, Klein D, Mutch DM. Development of a Dietary Management Care Map for metabolic syndrome. Can J DIet Pract Res. 2014;75(3):132–9. [DOI] [PubMed] [Google Scholar]
- 56.Brauer P, Royall D, Li A, Rodrigues A, Green J, Macklin S, Craig A, Chan M, Pasanen J, Brunelle L, et al. Key process features of personalized diet counselling in metabolic syndrome: secondary analysis of feasibility study in primary care. BMC Nutr. 2022;8(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leiter LA, Fitchett DH, Gilbert RE, Gupta M, Mancini GB, McFarlane PA, Ross R, Teoh H, Verma S, Anand S, et al. Cardiometabolic risk in Canada: a detailed analysis and position paper by the cardiometabolic risk working group. Can J Cardiol. 2011;27(2):e1–33. [DOI] [PubMed] [Google Scholar]
- 58.Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation. 2021;143(21):e984–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howlader M, Sultana MI, Akter F, Hossain MM. Adiponectin gene polymorphisms associated with diabetes mellitus: a descriptive review. Heliyon. 2021;7(8):e07851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonafiglia JT, Swinton PA, Ross R, Johannsen NM, Martin CK, Church TS, Slentz CA, Ross LM, Kraus WE, Walsh JJ, et al. Interindividual Differences in Trainability and Moderators of Cardiorespiratory Fitness, Waist circumference, and body Mass responses: a large-scale individual Participant Data Meta-analysis. Sports Med. 2022;52(12):2837–51. [DOI] [PubMed] [Google Scholar]
- 61.Sarzynski MA, Rice TK, Després JP, Pérusse L, Tremblay A, Stanforth PR, Tchernof A, Barber JL, Falciani F, Clish C, et al. The HERITAGE Family Study: a review of the effects of Exercise Training on Cardiometabolic Health, with insights into Molecular transducers. Med Sci Sports Exerc. 2022;54(5s):S1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Senn S. Mastering variation: variance components and personalised medicine. Stat Med. 2016;35(7):966–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cannon MJ, Masalovich S, Ng BP, Soler RE, Jabrah R, Ely EK, Smith BD. Retention among participants in the National Diabetes Prevention Program Lifestyle Change Program, 2012–2017. Diabetes Care. 2020;43(9):2042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller BM, Brennan L. Measuring and reporting attrition from obesity treatment programs: a call to action! Obes Res Clin Pract. 2015;9(3):187–202. [DOI] [PubMed] [Google Scholar]
- 65.Zoh RS, Esteves BH, Yu X, Fairchild AJ, Vazquez AI, Chapple AG, Brown AW, George B, Gordon D, Landsittel D, et al. Design, analysis, and interpretation of treatment response heterogeneity in personalized nutrition and obesity treatment research. Obes Rev. 2023;24(12):e13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ELM Trial research group. Enhancing lifestyles in the metabolic syndrome (ELM) multisite behavioral efficacy trial. Design and baseline cohort. Am Heart J. 2024;270:136–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.