Abstract
Background
Elevated mammographic density (MD) for a woman’s age and body mass index (BMI) is an established breast cancer risk factor. The relationship of parity, age at first birth, and breastfeeding with MD is less clear. We examined the associations of these factors with MD within the International Consortium of Mammographic Density (ICMD).
Methods
ICMD is a consortium of 27 studies with pooled individual-level epidemiological and MD data from 11,755 women without breast cancer aged 35–85 years from 22 countries, capturing 40 country-& ethnicity-specific population groups. MD was measured using the area-based tool Cumulus. Meta-analyses across population groups and pooled analyses were used to examine linear regression associations of square-root (√) transformed MD measures (percent MD (PMD), dense area (DA), and non-dense area (NDA)) with parity, age at first birth, ever/never breastfed and lifetime breastfeeding duration. Models were adjusted for age at mammogram, age at menarche, BMI, menopausal status, use of hormone replacement therapy, calibration method, mammogram view and reader, and parity and age at first birth when not the association of interest.
Results
Among 10,988 women included in these analyses, 90.1% (n = 9,895) were parous, of whom 13% (n = 1,286) had ≥ five births. The mean age at first birth was 24.3 years (Standard deviation = 5.1). Increasing parity (per birth) was inversely associated with √PMD (β: − 0.05, 95% confidence interval (CI): − 0.07, − 0.03) and √DA (β: − 0.08, 95% CI: − 0.12, − 0.05) with this trend evident until at least nine births. Women who were older at first birth (per five-year increase) had higher √PMD (β:0.06, 95% CI:0.03, 0.10) and √DA (β:0.06, 95% CI:0.02, 0.10), and lower √NDA (β: − 0.06, 95% CI: − 0.11, − 0.01). In stratified analyses, this association was only evident in women who were post-menopausal at MD assessment. Among parous women, no associations were found between ever/never breastfed or lifetime breastfeeding duration (per six-month increase) and √MD.
Conclusions
Associations with higher parity and older age at first birth with √MD were consistent with the direction of their respective associations with breast cancer risk. Further research is needed to understand reproductive factor-related differences in the composition of breast tissue and their associations with breast cancer risk.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01890-x.
Keywords: Mammographic density, Reproductive factors, Parity, Age at first birth, Breastfeeding
Introduction
Breast cancer accounts for the majority of incident cancer cases in women [1]. Reproductive factors which reduce a woman’s risk of breast cancer later in life include increased parity (i.e. number of births), younger age at first birth, ever having breastfed, and a longer lifetime breastfeeding duration [2]. The Oxford Collaborative Group Pooling studies reported reductions in breast cancer risk of 7% per birth, 3% per year younger for age at first birth, and 4.3% for every 12 months of breastfeeding [3]. Prior research suggests that the protective effect of increasing parity on breast cancer risk is greater among post-menopausal women and the risk reduction associated with breastfeeding does not differ by menopausal status [3–5]. Furthermore, the increase in breast cancer risk associated with increasing age at first birth is greater among pre-menopausal women [4–6]. A recent meta-analysis which examined these associations by breast cancer tumour subtypes found that higher parity was associated with reduced risk of hormone receptor-positive breast cancers [7]. Further, ever/longer breastfeeding duration was associated with reduced risk for all subtypes including hormone receptor-positive and -negative breast cancers, HER2-positive breast cancer, and triple-negative breast cancer [7], whilst older age at first birth was associated with an increased risk of hormone receptor-positive and HER2-positive breast cancers [7].
Mammographic density (MD) describes the fibroglandular or non-fatty tissue within the breast that radiologically appears white on mammograms [8]. Elevated MD for a woman’s age and body mass index (BMI) is associated with increased breast cancer risk independently of reproductive factors [9]. Furthermore, higher MD lowers the sensitivity of mammography to detect breast cancer [10]. As reproductive factors and MD can impact a woman’s risk of breast cancer independently, there is a need to understand the role of reproductive factors in the aetiology of MD throughout a woman’s lifetime.
Prior studies have reported inverse associations between increasing parity and the MD measures, percent MD (PMD) and dense area (DA) [11–19] but it is unclear if this relationship is linear, with each birth reducing MD regardless of the number of prior births, or if there is a threshold number of births where the influence of parity on MD is reached. Older age at first birth has been associated with higher PMD and DA in a number of studies conducted in the United States, Northern Greece, and Asia [12, 13, 20, 21]. Evidence of the association between breastfeeding and MD is not fully clear, despite its established protective effects on breast cancer. A study within the Nurses’ Health Study and Nurses’ Health Study II cohorts in the US reported positive associations between longer lifetime breastfeeding duration for all births and DA, among pre-menopausal women only [11]. In comparison, a study set in Northern Greece reported a decreasing linear trend between longer breastfeeding durations for all live births and a higher MD Wolfe-pattern (P2 or DY) among pre-menopausal women [13].
Examining the relationships between these reproductive factors with MD across populations with very diverse reproductive profiles may provide further insight into how variations in these reproductive factors influence a woman’s breast tissue composition. Therefore, this study aimed to investigate the associations between parity, age at first birth, ever/never breastfed and lifetime breastfeeding duration with MD within the International Consortium of Mammographic Density (ICMD).
Methods
Study design and population
This cross-sectional study used data from the ICMD, coordinated by the International Agency for Research on Cancer, and described in detail previously [22–24]. The consortium pooled individual-level epidemiological and MD data from 27 studies that spanned across 22 countries and included 40 country-, study- & ethnicity-specific population groups [22]. As many of the ICMD contributing studies included women from different ethnic groups within an individual study, the term population group was used to identify specific ethnic groups within a specific study. For example, the Malaysian study included women from three ethnic groups (Malaysian, Chinese, and Indian). Studies within the consortium included women, aged 35 years or older who were breast cancer-free and underwent organized, ad hoc, or opportunistic screening mammography. Within each population group approximately 200 pre- and post-menopausal women, selected at random, were included where possible [22]. To be eligible for inclusion within the consortium, one mammogram in electronic format and a core set of risk factor information were required for each woman [22]. Women with a previous breast cancer diagnosis were not eligible to be included.
Exposures
Exposure variables of interest in the present analysis included the following reproductive exposures including parity (defined as number of births), age at first birth, ever/never breastfed, and lifetime breastfeeding duration (defined as a woman’s cumulative lifetime breastfeeding duration in months). All variables were self-reported and collected close to or at the time of mammography for the majority of studies, with five studies collecting the information more than two years before or several years after the time of mammography [22]. Parity and age at first birth were available in all 27 studies within the ICMD. Parity was collected as a continuous variable in the 27 individual studies, while age at first birth was collected as a continuous variable in 24 studies and as a categorical variable in 3 studies. Ever/never breastfed was available in 25 studies and lifetime breastfeeding duration was collected as a continuous variable in 18 studies and as a categorical variable in 2 studies. At the study level, definitions for the exposure variables varied. Parity was defined as number of births (n = 5 studies), full-term births (n = 11 studies), live births (n = 2 studies), pregnancies (n = 2 studies), births as > 6.5 or 7 months gestation, live or not (n = 2 studies), children (n = 3 studies), or not defined (n = 2 studies) [22]. Similarly, age at first birth was defined as age at first live birth (n = 3 studies), age at first pregnancy (n = 2 studies), age at first full term pregnancy (n = 11 studies), age at first pregnancy lasting at least 6.5 or 7 months, live birth or not (n = 2 studies), age first child was born (n = 3 studies), age at first delivery (n = 1 study), or not defined (n = 5 studies) [22].
Mammographic density assessment
To assess MD, both previously digitised film and digital (raw and processed) mammograms were included spanning from 1986 to 2014 [22]. As previously described, three readers independently assessed one mammogram per woman using the semi-automated MD analysis software, Cumulus [25]. Where more than one mammogram per women was provided to the ICMD, the left mediolateral oblique (MLO) view was preferentially selected for MD assessment as it was more widely available in the contributing studies, followed by the right MLO view, the left craniocaudal (CC) view, or the right CC view [22]. Mammograms were grouped by mammogram type (i.e. digitised, digital raw, or digital processed), and within each group, they were randomly placed in batches of approximately 90 mammograms for assessment [22]. Protocols were developed to address variations between the three readers, between batches, and within batches by including approximately 15% of repeat images in each batch [22]. Within reader concordance was > 90% for each reader and between readers, the overall percent MD (PMD) distributions across women in the ICMD were right skewed to different degrees for each reader and therefore, the MD measures were transformed to improve normality of the data, as detailed previously by McCormack et al. [22]. Similar to prior studies conducted within the consortium, the outcome of interest was MD [23, 24]. MD was examined as PMD ((dense breast area (cm2)/total breast area (cm2)) × 100), dense area (DA) (cm2), and non-dense area (NDA) (cm2).
Statistical analysis
Descriptive analyses were performed to summarise the characteristics of the study sample and to describe the exposure variables, parity, age at first birth, ever/never breastfed, and lifetime breastfeeding duration, among the population groups. The MD measures were square-root transformed for analyses to normalize the skewed distribution and the beta-coefficient (β) from the regression analyses were reported for the √MD measures, √PMD, √DA, and √NDA [22]. For interpretation, the β from the regression analysis can be interpreted as the difference in the length of a side of a square within the DA [22]. Therefore, if the length of a side of a square within the DA (√DA) is 10 cm (corresponding to square of 10 cm × 10 cm with a DA of 100 cm2), and β = 0.10, this would represent an increase of 0.10 cm in the length of the side of the square within the DA, resulting in a √DA of 10.10 cm and a DA of 102.01 cm2, corresponding to a difference of 2.01 cm2 in the DA.
To investigate the associations between the reproductive factors and the √MD measures, two approaches were used including a population-specific meta-analysis and a pooled analysis. A meta-analysis approach was conducted for the 40 population groups using a random effects model. Forest plots were used to display the population group-specific effect estimates and the overall effect estimate. For this analysis, parity (per birth), age at first birth (per five-year increase in age at first birth), and lifetime breastfeeding duration (per six-month increase in lifetime breastfeeding duration) were defined as continuous variables. For continuous analyses, where exposures of interest were collected as categorical variables (e.g. age at first birth and lifetime breastfeeding duration) at the study level, the median of each category in those studies were pooled with the continuous data from the other studies [22]. Ever/never breastfed was defined as a binary variable (ever or never). Pooled analyses were conducted using a multi-level model allowing for grouping of √MD measures at the individual and population group level. For pooled analyses, parity was categorised as 0, 1, 2, 3, 4 ….10 and ≥ 11 births. Age at first birth was defined as a categorical variable (12–15, 16–17, 18–19…. 34–37, and ≥ 38 years). Lifetime breastfeeding duration was categorised as ≤ 1, > 1- ≤ 6, > 6- ≤ 12, > 12- ≤ 24, > 24- ≤ 48, > 48- ≤ 72, > 72- ≤ 200, and > 200 months. For analyses examining the associations between parity and the √MD measures, nulliparous and parous women were included, while the analyses examining the respective associations between age at first birth, ever/never breastfed, or lifetime breastfeeding duration and the √MD measures were only among parous women. Stratified analyses by menopausal status were also performed for the meta-analysis and pooled approaches with figures showing the findings for the √MD measure, √PMD, provided in Additional File 1.
In all analyses, models were adjusted for age at mammogram (continuous, years), BMI (continuous, measured in 18 studies and self-reported in 9 studies), menopausal status (pre/peri-menopausal and post-menopausal), use of hormone replacement therapy (ever, current, past, never, and unknown), age at menarche (continuous, years), calibration method (digitized, processed-Philips, processed-MediFuture, processed-Agfa, processed-Siemens, and calibrated raw), mammogram view (CC or MLO view), and mammogram reader (VM, IdSS, and NB). For analyses investigating the associations between parity and the √MD measures, age at first birth was adjusted for in these analyses by generating a categorical variable with the following categories, nulliparous, < 20, < 25…, ≥ 40 years. Similarly, parity (continuous, per birth) was adjusted for in the analysis examining the association between age at first birth and the √MD measures. Both parity (continuous, per birth) and age at first birth (continuous, years) were adjusted for in analyses examining the respective associations between the breastfeeding exposures and the √MD measures. To assess for bias in the population-specific meta-analyses, funnel plots of the effect size and standard error for each reproductive factor and √MD measure were generated and visual inspection indicated no strong evidence of bias (Additional File 1: Fig. S1(a-d)). All analyses were performed using Stata 17.0 software [26].
Sensitivity analysis
For each of the reproductive factors and the √MD measures, sensitivity analyses were performed excluding the five studies in which risk factor information was collected at least two years before or after the time of mammography. These sensitivity analyses were conducted to assess the impact of including these studies in the population-specific meta-analyses examining the associations between each of the reproductive factors and the √MD measures as there is a possibility that a woman’s parity or lifetime breastfeeding duration status may have changed between risk factor information collection and time of mammography. Additional sensitivity analyses were performed to examine the associations between the parity exposure and the √MD measures by restricting this analysis to parous women in order to assess the impact of including nulliparous women in the primary population-specific meta-analysis. For the lifetime breastfeeding duration exposure, sensitivity analyses excluding women who breastfed for shorter (< 1 month) or longer durations (> 200 months) were performed to examine the impact of including extreme lifetime breastfeeding durations on √MD associations in the main population-specific meta-analysis; while an additional sensitivity analysis examining the impact of including parous women that did not breastfeed in the pooled analysis examining the associations between lifetime breastfeeding duration categories and the √MD measures was also conducted. We also conducted sensitivity analyses to examine the associations between breastfeeding duration per birth, calculated by dividing lifetime breastfeeding duration by parity, and the √MD measures.
Results
Analytical sample
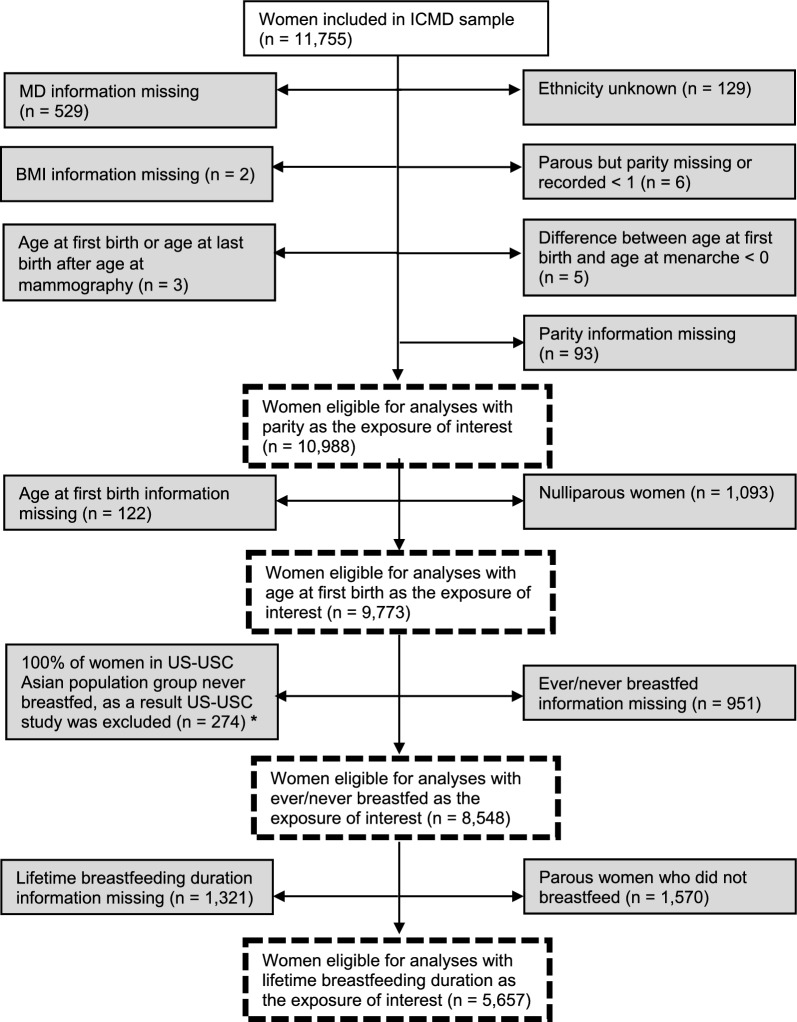
From the total ICMD sample (n = 11,755), for the analyses examining associations between the parity exposure and √MD measures, 10,988 (93.5%) women were eligible for inclusion. The included analytical sample are further described in Fig. 1. In analyses restricted to parous women, 9,773 women were included for the age at first birth analyses. For the breastfeeding analyses, 8,548 were eligible to be included in the ever/never breastfed analyses and among parous women who breastfed, 5,657 were eligible to be included in the lifetime breastfeeding duration analyses.
Fig. 1.
Flow chart of the study sample with reasons for exclusion in the grey boxes and the subsequent analytical samples in the dashed boxes for each exposure of interest – parity, age at first birth, ever/never breastfed, and lifetime breastfeeding duration. * This study was excluded from the ever/never breastfed analyses as 100% of women in the US-USC Asian population group reported never breastfeeding. This proportion may be as a result of the random sample selected from the study during the initial consortium development which may not reflect the true proportion of women who breastfed in this specific population group. Given this uncertainty, it was decided to exclude this study from the ever/never breastfed analyses
Study participant characteristics
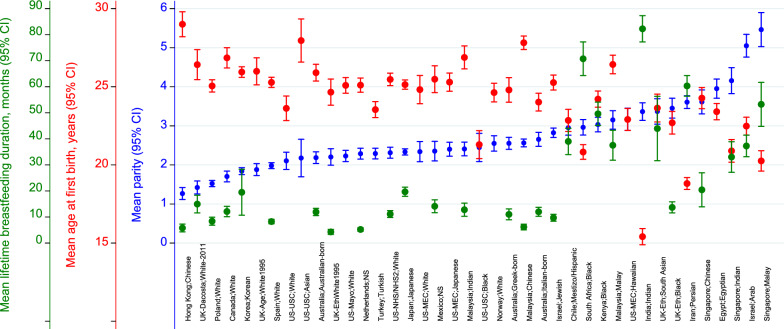
A summary of the reproductive exposures of the 40 country-ethnicity-specific population groups are described in Table 1 and the distribution of mean parity, age at first birth, and lifetime breastfeeding duration across the population groups are shown in Fig. 2. Among the total included study sample (n = 10,988), the mean age at mammography was 52.7 years (standard deviation (SD) = 8.2) and mean BMI was 27.0 kg/m2 (SD = 5.7). 90.1% (n = 9,895) of women were parous. The mean parity was 2.7 births (SD 1.8), ranging from 1.3 births (SD = 1.1) for Hong Kong Chinese women to 5.5 births (SD = 3.1) for Singapore-Malay women (Fig. 2). Overall, 13% (n = 1,286) of parous women had five births or more. Amongst parous women, the overall mean age at first birth was 24.3 years (SD = 5.1). Among the population groups, women in India had the lowest mean age at first birth (15.4 years, SD = 3.4) and the highest was among Chinese women in Hong Kong (29.0 years, SD = 4.7). Among the parous study sample, 71.8% (n = 7,143) breastfed and the median lifetime breastfeeding duration for women that breastfed was 10.0 months (interquartile range (IQR) = 28.0 months). Within this study sample, across the population groups, the group with the lowest median lifetime breastfeeding duration were white women in the UK (0.0 months, IQR = 6.0) and women in India had the highest median lifetime breastfeeding duration (72.0 months, IQR = 36.0).
Table 1.
Characteristics of study participants from 40 country-ethnicity specific population groups from the 27 ICMD contributing individual studies included in this analysis.
| Number of parous and nulliparous women | BMI* (kg/m2) (Mean ± SD) | Number of parous women | Age at First Birth$ (Years) (Mean ± SD) | Parity* (Number of births) (Mean ± SD) | Breastfeed ever$ (%) | Total breastfeeding duration$ (Months) (Mean ± SD) | Total breastfeeding duration$ (Months) (Median (IQR)) | ||
|---|---|---|---|---|---|---|---|---|---|
| Total study sample | 10,988 | 27.0 ± 5.7 | 9773 | 24.3 ± 5.1 | 2.7 ± 1.8 | 80.4 | 23.7 ± 34.8 | 10.0 (28.0) | |
| Individual study | Country-ethnicity specific population groups | ||||||||
| Australia | Australia-Australian | 393 | 25.7 ± 4.6 | 320 | 25.9 ± 4.9 | 2.2 ± 1.5 | 78.4 | 12.0 ± 12.8 | 8.5 (17.0) |
| Australia-Greek | 141 | 29.7 ± 4.4 | 138 | 24.8 ± 4.7 | 2.6 ± 0.9 | 84.8 | 11.0 ± 12.6 | 7.0 (13.0) | |
| Australia-Italian | 171 | 29.3 ± 5.0 | 164 | 24.0 ± 3.7 | 2.7 ± 1.2 | 85.9 | 12.0 ± 11.3 | 9.0 (14.0) | |
| Canada | Canada-White | 379 | 25.3 ± 5.7 | 276 | 26.9 ± 5.5 | 1.7 ± 1.4 | 77.3 | 12.1 ± 16.8 | 6 (16.5) |
| Chile | Chile-Mestizo/Hispanic | 186 | 29.1 ± 5.3 | 186 | 22.9 ± 4.9 | 3.0 ± 1.3 | 99.5 | 39.0 ± 35.3 | 28.5 (33.0) |
| Egypt | Egypt-Egyptian | 463 | 33.7 ± 6.7 | 433 | 23.4 ± 5.5 | 4.0 ± 2.7 | 93.8 | - | - |
| Hong Kong | Hong Kong-Chinese | 198 | 22.2 ± 2.9 | 130 | 29.0 ± 4.7 | 1.3 ± 1.1 | 71.4 | 5.8 ± 8.7 | 2.3 (7.0) |
| India | India-Indian | 181 | 22.2 ± 4.4 | 170 | 15.4 ± 3.4 | 3.4 ± 1.5 | 100.0 | 82.3 ± 33.2 | 72.0 (36.0) |
| Iran | Iran-Persian | 392 | 29.3 ± 4.2 | 392 | 18.8 ± 3.8 | 3.6 ± 1.7 | 97.2 | 60.3 ± 40.1 | 54.0 (43.0) |
| Israel | Israel-Arab | 345 | 30.6 ± 5.4 | 263 | 22.5 ± 4.7 | 5.0 ± 2.8 | 91.2 | 37.3 ± 31.7 | 30.0 (34.0) |
| Israel-Jewish | 374 | 26.0 ± 5.0 | 336 | 25.3 ± 4.5 | 2.8 ± 1.2 | 78.1 | 9.7 ± 10.1 | 6.0 (13.5) | |
| Japan | Japan-Japanese | 384 | 22.8 ± 3.1 | 375 | 25.1 ± 2.9 | 2.3 ± 0.8 | 91.9 | 19.7 ± 17.0 | 15.0 (22.0) |
| Kenya | Kenya-Black | 270 | 30.0 ± 5.0 | 261 | 24.2 ± 4.3 | 3.0 ± 1.6 | 100.0 | 49.6 ± 36.7 | 42.0 (48.0) |
| Korea | Korea-Korean | 389 | 23.1 ± 2.7 | 350 | 25.9 ± 3.2 | 1.8 ± 0.9 | 78.4 | 19.5 ± 9.0 | 24.0 (9.0) |
| Malaysia | Malaysia-Chinese | 394 | 23.5 ± 3.4 | 391 | 27.8 ± 4.3 | 2.6 ± 1.0 | 71.4 | 6.2 ± 10.5 | 3.0 (7.0) |
| Malaysia-Indian | 247 | 26.9 ± 4.7 | 219 | 26.9 ± 5.6 | 2.4 ± 1.4 | 82.8 | 12.8 ± 18.5 | 6.0 (14.0) | |
| Malaysia-Malay | 217 | 27.5 ± 4.6 | 192 | 26.4 ± 4.2 | 3.2 ± 1.8 | 94.6 | 37.6 ± 39.8 | 22.5 (46.5) | |
| Mexico | Mexico-NS | 147 | 28.2 ± 4.7 | 126 | 25.5 ± 4.9 | 2.4 ± 1.6 | 90.4 | 14.1 ± 14.1 | 12.0 (15.0) |
| Netherlands | Netherlands-NS | 361 | 25.7 ± 4.0 | 323 | 25.1 ± 4.2 | 2.3 ± 1.4 | 78.8 | 5.2 ± 6.3 | 3 (7.5) |
| Norway | Norway-White | 196 | 24.8 ± 3.8 | 181 | 24.6 ± 4.1 | 2.6 ± 1.4 | - | - | - |
| Poland | Poland-White | 396 | 27.3 ± 4.7 | 350 | 25.1 ± 3.8 | 1.5 ± 0.8 | 70.9 | 8.4 ± 14.6 | 3.5 (9.0) |
| Singapore | Singapore-Chinese | 196 | 24.0 ± 3.9 | 183 | 24.3 ± 4.7 | 3.6 ± 2.2 | 57.4 | 20.4 ± 45.0 | 2.0 (24.0) |
| Singapore-Indian | 199 | 26.4 ± 4.6 | 186 | 20.9 ± 5.1 | 4.2 ± 2.4 | 78.5 | 33.0 ± 40.8 | 16.5 (52.0) | |
| Singapore-Malay | 197 | 27.6 ± 4.5 | 186 | 20.3 ± 4.5 | 5.5 ± 3.1 | 82.3 | 53.3 ± 58.8 | 33.0 (77.0) | |
| South Africa | South Africa-Black | 371 | 32.2 ± 6.8 | 339 | 20.8 ± 4.4 | 3.0 ± 2.0 | 88.0 | 70.8 ± 56.2 | 54.0 (68.0) |
| Spain | Spain-White | 758 | 27.8 ± 4.8 | 683 | 25.3 ± 4.4 | 2.0 ± 1.1 | 76.4 | 8.3 ± 11.4 | 5.0 (9.0) |
| Turkey | Turkey-Turkish | 395 | 27.1 ± 4.9 | 378 | 23.5 ± 5.1 | 2.3 ± 1.4 | 92.6 | - | - |
| UK, London | UK-London-White | 159 | 26.0 ± 5.3 | 159 | 26.4 ± 6.2 | 1.4 ± 1.3 | 79.2 | 15.0 ± 19.2 | 9.5 (15.0) |
| UK-Age Trial | UK-Age Trial-White1995 | 159 | 27.4 ± 5.6 | 138 | 26.0 ± 5.2 | 1.9 ± 1.0 | 75.0 | - | - |
| UK Ethnicity | UK-Eth Black | 217 | 28.6 ± 5.7 | 198 | 22.7 ± 5.2 | 3.5 ± 1.9 | 77.5 | 13.7 ± 15.7 | 9.0 (19.0) |
| UK-Eth-South Asian | 133 | 26.9 ± 5.9 | 119 | 23.6 ± 5.0 | 3.4 ± 1.9 | 75.2 | 44.0 ± 60.1 | 21.0 (42.0) | |
| UK-Eth-White1995 | 245 | 25.8 ± 5.3 | 191 | 24.7 ± 5.9 | 2.2 ± 1.7 | 53.1 | 4.3 ± 7.7 | 0.0 (6.0) | |
| US-Mayo | US-Mayo-White | 397 | 28.2 ± 6.5 | 342 | 25.1 ± 4.7 | 2.2 ± 1.5 | 59.5 | - | - |
| US-MEC Hawaii | US-MEC-Hawaiian | 142 | 28.9 ± 6.3 | 133 | 22.9 ± 4.1 | 3.2 ± 1.7 | - | - | - |
| US-MEC-Japanese | 239 | 24.0 ± 4.3 | 210 | 25.3 ± 4.3 | 2.4 ± 1.4 | - | - | - | |
| US-MEC-White | 153 | 25.5 ± 5.5 | 127 | 24.8 ± 5.2 | 2.3 ± 1.6 | - | - | - | |
| US-NHS/NHS2 | US-NHS/NHS2-White | 397 | 26.3 ± 5.6 | 351 | 25.5 ± 3.8 | 2.3 ± 1.4 | 76.0 | 11.2 ± 12.7 | 9.0 (14.5) |
| US-USC € | US-USC-Asian | 51 | 23.2 ± 3.1 | 45 | 28.0 ± 4.7 | 2.2 ± 1.8 | 0.0 | - | - |
| US-USC-Black | 112 | 28.0 ± 6.1 | 97 | 21.3 ± 4.5 | 2.4 ± 2.0 | 42.3 | - | - | |
| US-USC-White | 162 | 25.6 ± 5.2 | 132 | 23.6 ± 4.6 | 2.1 ± 1.4 | 59.1 | - | - | |
Where (-) = Data not collected in individual study, (*) = Among parous and nulliparous women, ($) = Among parous women (Note: The number of parous women differed for ever/never breastfed and lifetime breastfeeding duration as this data was not available in each individual study), (€) = This study was excluded for ever/never breastfed analyses, SD = Standard deviation, BMI = Body mass index, IQR = Interquartile range
Fig. 2.
Graph showing mean parity (blue), mean age at first birth (red), and mean lifetime breastfeeding duration (green) for the 40 country-ethnicity specific population groups included in this study
Associations between parity and MD measures within ICMD
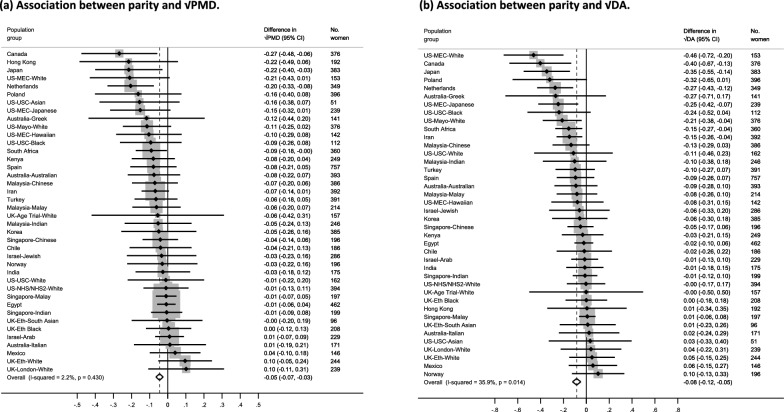
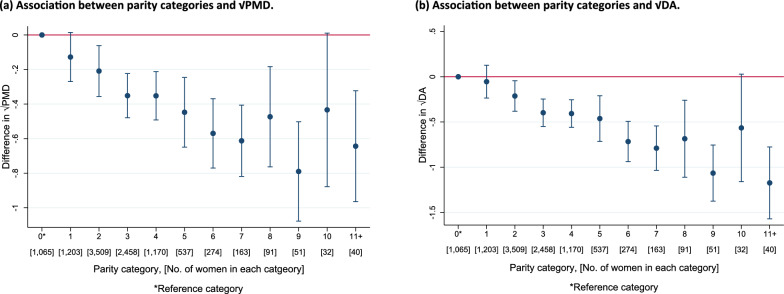
Population-specific meta-analyses showed inverse associations between increasing parity and √PMD (β = − 0.05 per birth, 95% CI = − 0.07, − 0.03) and √DA (β = − 0.08 per birth, 95% CI: − 0.12, − 0.05) (Figs. 3(a) and (b)). Findings were mostly consistent across population groups for √PMD (I2 = 2.2%, p = 0.430). Moderate heterogeneity was found for √DA (I2 = 35.9%, p = 0.014). Pooled categorical analyses were consistent with findings from the population-specific meta-analyses and demonstrated the linear inverse association between increasing parity and MD. This decreasing pattern was evident with √PMD and √DA among women with up to at least 9 births, though analysis of categories ≥ 8 births were limited by sample size (Fig. 4 (a) and (b)). For both analytic approaches, no clear pattern of association was observed between increasing parity and √NDA (Additional File 1: Fig. S2(a-b)).
Fig. 3.
a–b Forest plots showing the associations between parity and √MD measures (a: √PMD and b: √DA) for all women (parous and nulliparous) across the 40 country-ethnicity specific population groups in the ICMD sample. Analyses were adjusted for age at mammogram, BMI, menopausal status, use of hormone replacement therapy, age at menarche, age at first birth, and image parameters
Fig. 4.
a–b Pooled analysis plots showing the associations between parity (categorical) and √MD measures (a: √PMD and b: √DA) for all women (parous and nulliparous) in the pooled ICMD sample. Analyses were adjusted for age at mammogram, BMI, menopausal status, use of hormone replacement therapy, age at menarche, age at first birth, and image parameters
Compared to the main population-specific meta-analysis results, stratified meta-analysis findings did not differ by menopausal status (Additional File 1: Fig. S3(a-b), data not shown for √DA or √NDA). However, in the stratified meta-analyses among pre-menopausal women, moderate heterogeneity was observed across population groups for √PMD (I2 = 48.3%, p = 0.001), √DA (I2 = 38.8%, p = 0.010) and √NDA (I2 = 42.5%, p = 0.004) though the sample of pre-menopausal women was smaller (Additional File 1: Fig. S3a, data not shown for √DA or √NDA). Compared to the primary pooled analysis among all women, the patterns observed in the stratified pooled analysis among pre-menopausal women appeared slightly weaker for √PMD and were consistent for √DA and √NDA, respectively (Additional File 1: Fig. S4(a) and data not shown for √DA or √NDA). Findings from the stratified pooled analyses for post-menopausal women were consistent for √PMD and √DA to the findings observed in the main pooled analysis (Additional File 1: Fig. S4(b) and data not shown for √DA). Analysis of the higher parity categories were limited for the stratified pooled analyses due to the smaller sample sizes of pre- or post-menopausal women in those categories. Compared to the main population-specific meta-analysis findings, sensitivity analyses did not show any differences between increasing parity and the √MD measures when nulliparous women were excluded from the analysis (see Additional File 1: Fig. S5(a-c)).
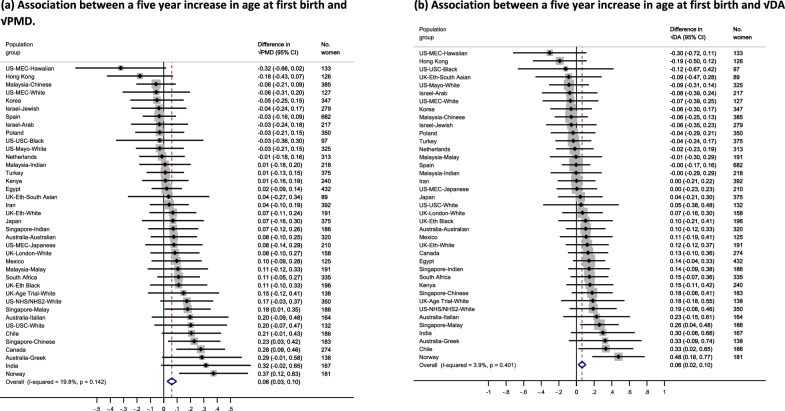
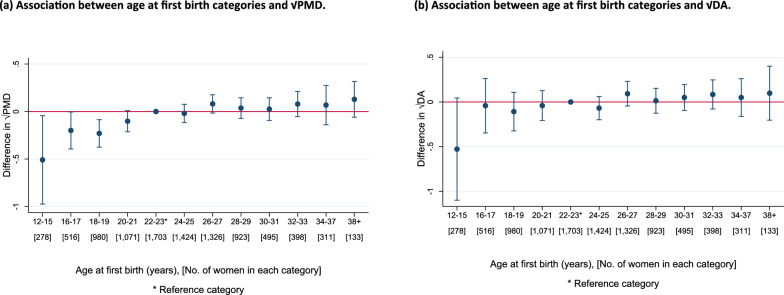
Associations between age at first birth and MD measures within ICMD
Among parous women, positive associations were observed between √PMD (β = 0.06, 95% CI = 0.03, 0.10) and √DA (β = 0.06, 95% CI = 0.02, 0.10) respectively per five-year increase in age at first birth (Fig. 5(a) and (b)). An inverse association was observed for √NDA per five-year increase in age at first birth (β = − 0.06, 95% CI = − 0.11, − 0.01) (Additional File 1 Fig. S6(a)). Heterogeneity was moderate across population groups for √PMD (I2 = 19.8%, p = 0.142) and √NDA (I2 = 22.2%, p = 0.112), and low for √DA (I2 = 3.9%, p = 0.401). In pooled categorical analyses, compared to the reference category (22–23 years), a consistently increasing pattern beginning at the youngest age categories (12–21 years) and slowing down at older age categories (≥ 24 years) was observed for √PMD (Fig. 6 (a)). No consistent pattern was observed with √DA (Fig. 6 (b)). For √NDA, a curvature pattern was observed, increasing initially among women who first gave birth between age 12–15 years and decreasing among those who gave birth for the first time aged 16–21 years, compared to the reference category (22–23 years) (Additional File 1: Fig. S6(b).
Fig. 5.
a–b Forest plot showing the associations between age at first birth and √MD measures (a: √PMD and b: √DA) among parous women across 39 country-ethnicity specific population groups in the ICMD sample. Analyses were adjusted for age at mammogram, BMI, menopausal status, use of hormone replacement therapy, age at menarche, parity, and image parameters
Fig. 6.
a–b Pooled analysis plots showing the associations between age at first birth (categorical) and √MD measures (a: √PMD and b: √DA) among parous women in the pooled ICMD sample. Analyses were adjusted for age at mammogram, BMI, menopausal status, use of hormone replacement therapy, age at menarche, parity, and image parameters
Population-specific meta-analyses stratified by menopausal status found consistent associations only amongst those who were post-menopausal women at MD assessment at the time of mammography compared to the primary meta-analysis findings (Additional File 1: Fig. S7(a-b)). For stratified pooled analyses, no consistent trends were observed for √PMD or √DA among pre-menopausal women, whilst similar patterns were seen for both √MD measures among post-menopausal women to those observed in the main pooled analysis among all parous women in the ICMD sample (Additional File 1: Fig. S8(a-b) and data not shown for √DA). Further, the patterns observed among pre- and post-menopausal women for √NDA in the stratified pooled analyses were consistent with those observed in the primary pooled analysis (Data not shown).
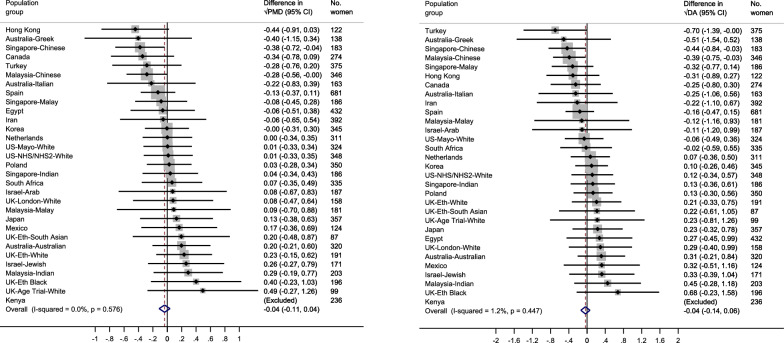
Associations between breastfeeding and MD measures within ICMD
Among parous women, no strong evidence of an association was found between ever/never breastfed and √PMD (β = − 0.04, 95% CI = − 0.11, 0.04), √DA (β = − 0.04, 95% CI = − 0.14, 0.06), or √NDA (β = − 0.00, 95% CI = − 0.12, 0.12) (Fig. 7 (a) and (b) and Additional File 1: Fig. S9). No heterogeneity was observed across population groups for √PMD (I2 = 0.0%, p = 0.576) or √DA (I2 = 1.2%, p = 0.447). Whilst heterogeneity between population groups was low for √NDA (I2 = 25.5%, p = 0.103). Findings from the meta-analyses stratified by menopausal status were similar to the findings from the primary population-specific meta-analysis (Additional File 1: Fig. S10(a-b)).
Fig. 7.
a–b Forest plot showing the associations between ever/never breastfed and √MD measures (a: √PMD and b: √DA) among parous women across 30 country-ethnicity specific population groups in the ICMD sample. Analyses were adjusted for age at mammogram, BMI, menopausal status, use of hormone replacement therapy, age at menarche, parity, age at first birth, and image parameters
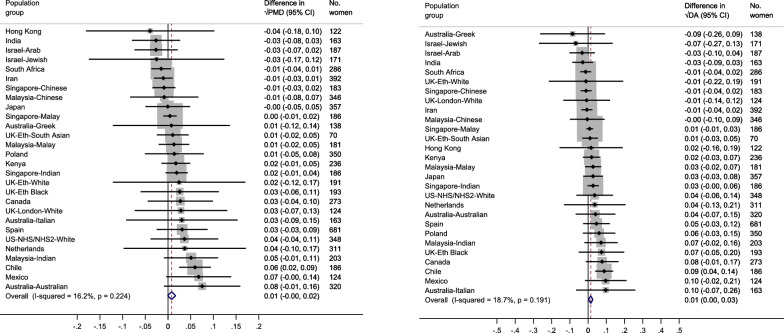
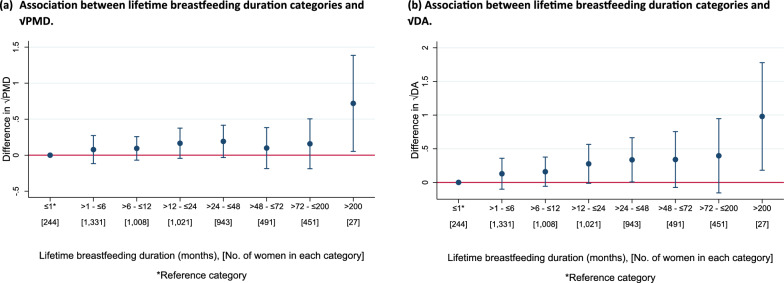
For lifetime breastfeeding duration, associations between each six-month increase in lifetime breastfeeding duration and √PMD were (β = 0.01, 95% CI = − 0.00, 0.02) and √DA (β = 0.01, 95% CI = 0.00, 0.03) (Fig. 8 (a) and (b)). No association was observed with √NDA per six-month increase in lifetime breastfeeding duration (Additional File 1: Fig. S11(a)). Low heterogeneity was found across population groups for both √MD measures, √PMD (I2 = 16.2%, p = 0.224) and √DA (I2 = 18.7%, p = 0.191). Results from the pooled categorical analyses showed no consistent pattern between increasing lifetime breastfeeding duration in categories and the √MD measures, compared to the reference category (≤ 1 month) (Figs. 9 (a) and (b) and Additional File 1: Fig. S11(b)).
Fig. 8.
a–b Forest plot showing the associations between lifetime breastfeeding duration and √MD measures (a: √PMD and b: √DA) among parous women that breastfed across 28 country-ethnicity specific population groups in the ICMD sample. Analyses were adjusted for age at mammogram, BMI, menopausal status, use of hormone replacement therapy, age at menarche, parity, age at first birth, and image parameters
Fig. 9.
a–b Pooled analysis plots showing the associations between lifetime breastfeeding duration (categorical) and √MD measures (a: √PMD and b: √DA) among parous women that breastfed in the pooled ICMD sample. Analyses were adjusted for age at mammogram, BMI, menopausal status, use of hormone replacement therapy, age at menarche, parity, age at first birth, and image parameters
Compared to findings from the main meta-analysis, stratified meta-analyses demonstrated similar associations with the √MD measures per six-month increase in lifetime breastfeeding duration among pre- and post-menopausal women (Additional File 1: Fig. S12(a-b) and data not shown for √DA and √NDA). For pooled categorical analyses stratified by menopausal status, the patterns observed were consistent with those observed in the main pooled analysis for lifetime breastfeeding duration and the √MD measures (Additional File 1: Fig. S13(a-b) and data not shown for √DA and √NDA).
Compared to the findings from the primary meta-analysis examining the association between a six-month increase in lifetime breastfeeding duration and the √MD measures, similar associations were observed in the sensitivity analysis when shorter (< 1 month) and longer (> 200 months) lifetime breastfeeding durations were excluded (Additional File 1: Fig. S14(a-c)). Further, pooled analysis findings did not differ between increasing lifetime breastfeeding duration categories and the √MD measures when parous women that did not breastfeed were included as the reference category, compared to the main pooled analyses which only included parous women who breastfed in the ICMD sample (data not shown). To further investigate the breastfeeding associations, we conducted additional analyses to investigate associations between an approximate per birth breastfeeding duration and the √MD measures. Meta-analysis findings examining the associations between a six-month increase in breastfeeding duration per birth (derived from dividing lifetime breastfeeding duration by parity) and the √MD measures were: √PMD (β = 0.03, 95% CI = − 0.01, 0.06), √DA (β = 0.05, 95% CI = 0.01, 0.09), and √NDA (β = 0.02, 95% CI = − 0.04, 0.07) (Additional File 1: Fig. S15(a-c)). These findings showed similar associations for √PMD and √NDA, and stronger for √DA compared to the primary meta-analysis examining the associations between the lifetime breastfeeding duration exposure and the √MD measures. While the patterns observed in the pooled categorical analyses for breastfeeding duration per birth and the √MD measures were consistent with patterns observed in the pooled analyses examining lifetime breastfeeding duration (Additional File 1: Fig. S16(a-c)).
Sensitivity analysis
Findings from the sensitivity analyses excluding the five studies that collected covariate data at least two years before or after time of mammography did not differ to the main population-specific meta-analyses examining the associations between each of the reproductive factors (parity, age at first birth, ever/never breastfed, or lifetime breastfeeding duration) and the √MD measures. In these sensitivity analyses, associations between increasing parity and √PMD were (β = − 0.05 per birth, 95% CI = − 0.07, − 0.02), √DA (β = − 0.08 per birth, 95% CI: − 0.12, − 0.04) and √NDA (β = 0.00 per birth, 95% CI: − 0.04, 0.04) (data not shown). Findings from the sensitivity analysis examining the associations between a five year increase in age at first birth and the √MD measures were: √PMD (β = 0.06, 95% CI = 0.02, 0.10), √DA (β = 0.07, 95% CI = 0.01, 0.12) and √NDA (β = − 0.05, 95% CI = − 0.11, 0.01) (data not shown). For the breastfeeding exposures, the associations between ever/never breastfed and √PMD were (β = − 0.07, 95% CI = − 0.15, 0.04), √DA (β = − 0.07, 95% CI = − 0.18, 0.04), and √NDA (β = 0.03, 95% CI = − 0.08, 0.15). Whilst findings from the sensitivity analysis examining the association between a six-month increase in lifetime breastfeeding duration and the √MD measures were: √PMD (β = 0.01, 95% CI = − 0.00, 0.02), √DA (β = 0.01, 95% CI = 0.00, 0.03), and √NDA (β = 0.01, 95% CI = − 0.00, 0.02) (data not shown).
Discussion
Amongst populations diverse in geography, ethnicity, and lifestyle included within the ICMD, this study found that increasing parity was inversely associated with the √MD measures, √PMD and √DA. Increasing age at first birth was positively associated with √PMD and √DA and inversely associated with √NDA in post-menopausal women. No consistent patterns of association were observed between breastfeeding measures including ever/never breastfed and lifetime breastfeeding duration and √MD. These findings suggest that these reproductive factors differently influence patterns of MD adjusted for age and BMI.
In the ICMD, consistent with prior literature, we observed an inverse association between increasing parity and √MD [11–14, 27]. This reduction in √PMD would appear to be driven by the subsequent reduction in √DA as no association was observed between √NDA and increasing parity. In terms of absolute differences in PMD and DA based on our findings, using an example of a woman with three children, 16% PMD and 25 cm2 DA (corresponding to 4% √PMD and 5 cm √DA), a woman with four children would have approximately 0.4% lower PMD and 0.8 cm2 lower DA. Consistent with our findings, a recent US study conducted by Alexeeff et al. also reported an inverse association between increasing parity and MD [12]. In their analysis comprised of non-Hispanic white women who were mostly post-menopausal, MD was lower for women with two children, compared to women with one child, with further reductions noted for women with three or more children [12]. Similar to results from our stratified meta-analysis which suggested lower √MD at higher parities did not differ by menopausal status, Yaghjyan et al. observed inverse associations between average √PMD and increasing parity amongst both pre- and post-menopausal women within the Nurses’ Health Study (NHS) and Nurses’ Health Study II (NHSII) cohorts based in the US [11]. Our study had a wider range of parities than these studies [11, 12] and we found the association was evident with increasing parity up to nine births and for both √MD measures, √PMD and √DA.
Our findings demonstrated a positive association between a five-year increase in age at first birth and √PMD and √DA, and an inverse association with √NDA among post-menopausal women at MD assessment at the time of mammography. In terms of absolute differences in PMD, DA, and NDA based on these findings for women were post-menopausal at MD assessment, using an example of a woman who first gave birth at age 25 years with 16% PMD, 25 cm2 DA, and 100 cm2 NDA (corresponding to 4% √PMD, 5 cm √DA, 10 cm √NDA), a woman who first gives birth aged 30 would have higher PMD and DA by approximately 0.6% and 1.0 cm2 and lower NDA by 1.2 cm2. When analyses were pooled, compared to the reference category (22–23 years), the positive association with √PMD was observed only among women who first gave birth between ages 12–21 years and the inverse association with √NDA was observed among those who first gave birth between ages 16–21 years. This would suggest that the linear associations between a five-year increase in age at first birth and √PMD and √NDA in the main meta-analysis are driven by associations observed among women who first give birth at younger ages as seen in the pooled analyses. Alternatively, this contrast in findings may be due to the different classification of age at first birth in the meta-analysis approach (per five-year increase in age at first birth) and in the pooled categorical analysis approach (12–15, 16–17, 18–19…. 34–37, and ≥ 38 years). Alexeeff et al. also observed a positive association between older age at first birth and MD reporting increases of 2.4% in PMD and 3.3 cm2 in DA for women aged 40 years and over compared to women less than 20 years giving birth for the first time [12]. The magnitude of these findings are larger than those reported in our study, however, this may be due to the use of a different reference group in their analysis. Our findings differed by menopausal status, with evidence of the association between increasing age at first birth and MD amongst post-menopausal women only and among post-menopausal women who first gave birth between ages 12–21 years in pooled analyses. In agreement with our stratified analysis, Yaghjyan et al. reported a positive association between older age at first birth and √PMD (β = 0.03, 95% CI = 0.01, 0.05) and an inverse association with √NDA (β = − 0.10, 95% CI = − 0.13, − 0.06) amongst post-menopausal women within the NHS and NHS II cohorts [11]. In comparison with our findings within ICMD, Rice et al. found no association between older age at first pregnancy and MD amongst pre- or post-menopausal parous women in the Mexican Teachers’ Cohort [28].
The association between breastfeeding and MD is less clear in the literature [11, 13, 15, 29]. Among parous women who breastfed in the ICMD, we found no strong evidence of an association between a six-month increase in lifetime breastfeeding duration and √MD. Similarly, when analyses were pooled among all the population groups, compared to the reference category (≤ 1 month), we observed no consistent pattern between longer lifetime breastfeeding durations and the √MD measures. In comparison with results from our stratified pooled analyses in which we observed similar trends as in the main analysis among pre- and post-menopausal women, Yaghjyan et al. reported significantly increasing trends with √DA and √NDA as lifetime breastfeeding duration increased among pre-menopausal women in the NHS and NHSII cohorts [11]. Rice et al. found a significant increase in PMD among pre-menopausal Mexican women who breastfed for 12 months or longer [28]. In both studies, Yaghjyan et al. and Rice et. al used different breastfeeding duration categories compared to our study and furthermore, Rice and colleagues used a different MD assessment method in their analysis compared to the MD assessment method used in this study [11, 28]. A recent US study conducted by Getz et al. which examined associations between breastfeeding and volumetric measures of MD, reported a reduction in volumetric PMD among parous women with a BMI less than 25 kg/m2 who breastfed up to six months [29]. Furthermore, they found an inverse association between dense volume and breastfeeding but neither of these findings differed by menopausal status [29]. Our findings from the sensitivity analyses examining the associations between breastfeeding duration per birth and the √MD measures were generally consistent with the findings from the primary analyses that examined lifetime breastfeeding duration. This analysis demonstrated a stronger positive association between a six-month increase in breastfeeding duration per birth and √DA. However a limitation of this analyses was that this information was not available within the ICMD and we derived this exposure by dividing lifetime breastfeeding duration by parity. Therefore, this variable assumes that women breastfed each child for the same duration and thus likely does not account for variability in breastfeeding length between different births and among women that breastfed in the ICMD sample. Variations in findings between studies continue to highlight the need for further research in this area to understand breastfeeding and MD associations.
The associations between reproductive factors and breast cancer risk have been well studied to date [3, 7, 30–32]. A potential mechanism underpinning these associations is the differentiation of breast tissue due to the hormonal, structural, and cellular changes in preparation for birth and lactation [33, 34]. It is hypothesised that the undifferentiated lobular structures in the breast tissue of a nulliparous woman are more susceptible to carcinogenesis through exposure to endogenous hormones or environmental exposures [33]. For women who first give birth at younger ages, these pregnancy-induced changes in the breast tissue occur as the breast tissue is still developing and reduces the period of time between menarche and pregnancy when the breast tissue may be more susceptible to carcinogenesis [33]. While breast cancer risk was not analysed in this study, a mediation analysis by Rice et al. among women in the NHS and NHSII cohorts found that the association between a five-year increase in age at first birth and breast cancer was partially mediated by PMD (13%, p = 0.05), and a 12-month increase in breastfeeding duration and breast cancer risk was not mediated by PMD among post-menopausal women [35]. Regarding parity, an analysis of four case–control studies estimated that MD explained less than 17% of the association between parity and breast cancer risk [36]. Therefore, while MD may partially mediate the associations between parity and age at first birth with breast cancer risk, further research is needed to elucidate the biological mechanisms driving the association between breastfeeding and breast cancer risk.
Strengths of conducting this study within the ICMD include the opportunity to examine these associations across diverse international and ethnic population groups that include women with highly varied reproductive patterns including high parity, young ages at first birth, and long lifetime breastfeeding durations. These childbearing patterns are not typical in more recent studies of Western populations due to the historical fertility transition that occurred over time with increased access to education, healthcare, and employment opportunities [37]. Limitations of this study include the differences in how exposure variables were defined at the study level within the ICMD which may have influenced the findings. In some studies, the exposure variable, parity, was defined as ‘births’, while in other studies, it was defined as ‘full term births’, ‘live births’ ‘pregnancies’, ‘ > 6.5/7 months, born live or dead’ or ‘children’ [22]. Similar variation was also present for the definition of age at first birth at the study level, as this is linked with the definition for parity. For multiparous women who breastfed each child, cumulative breastfeeding duration was self-reported which may be prone to bias. As the ICMD pooled data from existing studies, data on ever/never breastfeeding and lifetime breastfeeding duration were not available for all of the 40 population groups within the consortium.
In conclusion, we examined the associations between reproductive factors and MD adjusted for age and BMI among women within the ICMD. We observed an inverse association between increasing parity and √PMD and √DA and no strong evidence of an association between ever/never breastfeeding or duration of breastfeeding with √MD. We found a positive association between increasing age at first birth and √PMD and √DA and an inverse association with √NDA in women who were post-menopausal at MD assessment. Our findings highlight differing reproductive patterns across 40 country-ethnicity-specific population groups and the influence of these patterns on MD and breast tissue composition. Future research is needed to evaluate reproductive-related changes in the complex composition of the breast tissue and if these changes partially explain the association between reproductive factors and breast cancer risk. Improving our understanding of these reproductive factors may extend our knowledge of MD associated breast cancer risk and help further our understanding of differing breast cancer patterns among diverse population groups.
Supplementary Information
Additional file 1: Fig. S1(a-d): Funnel plots assessing bias in the population-specific meta-analyses for each of the reproductive factors and the √MD measures. Fig. S2(a-b): Population-specific meta-analysis and categorical pooled analysis for parity and √NDA. Figs. S3–S4(a-b): Stratified analyses (population-specific meta-analyses and pooled categorical analyses) by menopausal status for parity and √PMD. Fig. S5(a-c): Sensitivity analyses (population-specific meta-analyses) with parous women only for parity and √MD measures. Fig. S6(a-b): Population-specific meta-analysis and categorical pooled analysis for age at first birth and √NDA. Figs. S7–S8(a-b): Stratified analyses (population-specific meta-analyses and pooled categorical analyses) by menopausal status for age at first birth and √PMD. Fig. S9: Population-specific meta-analysis for ever/never breastfed and √NDA. Fig. S10(a-b): Stratified meta-analyses by menopausal status for ever/never breastfed and √PMD. Fig. S11(a-b): Population-specific meta-analyses and categorical pooled analysis for lifetime breastfeeding duration and √NDA. Figs. S12–S13(a-b): Stratified analyses (population-specific meta-analyses and pooled categorical analyses) by menopausal status for lifetime breastfeeding duration and √PMD. Fig. S14(a-c): Sensitivity analyses (population-specific meta-analyses) excluding shorter (<1 month) and longer (> 200 months) durations for lifetime breastfeeding duration and √MD measures. Figs. S15–S16(a-c): Sensitivity analyses (population-specific meta-analyses and pooled categorical analyses) for breastfeeding duration per birth and the √MD measures.
Acknowledgements
We acknowledge the support of Dr Caroline Dickens at the Department of Internal Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa and South Africa-Pink Drive Community Screening.
Abbreviations
- CC
Craniocaudal
- MLO
Mediolateral oblique
- BMI
Body mass index
- CI
Confidence interval
- DA
Dense area
- ICMD
International Consortium of Mammographic Density
- IQR
Interquartile range
- MD
Mammographic density
- NDA
Non-dense area
- OR
Odds ratio
- PMD
Percent mammographic density
- SD
Standard deviation
Author contributions
The study was conceptualized, designed, and supervised by VM and MM. Data were curated by GM, BPG, CV, HMi, ML, RLR, AP, MLG, RMT, KB, AK, GU, EL, HMa, SVinn, SMo, SA, RN, SVina, SHT, SMa, BP, AB-D, CN, JSt, JHo, GG, VO, MEA, JSc, CVG, JOPW, RS, MS, JHi, JK, JWL, CD, MH, KSC, CS, AMC, LL, MP, AAF, DS, RK, NB, IdSS and VM. Centralized breast density readings were performed by VM, IdSS and NB, and data harmonization was conducted by ABur. Primary data analyses were performed by JOD, with additional analyses and input from VM and MM. Results were interpreted by JOD, MM, VM, ABur, JSt, AAF, JHo, GM, SAQ, GU, JSch, RK, RT, AHE, AP, BP, EL, MLG, KB, JL, MP, NB, and IdSS. The manuscript was drafted by JOD, VM and MM. All authors read and approved the final manuscript.
Funding
This work was supported by the US National Cancer Institute at the National Institutes of Health [R03CA167771]; the International Agency for Research on Cancer, and the Health Research Board [EIA-2019-012]. Original studies were supported, according to country by: Australia VicHealth; Cancer Council Victoria; Australian National Health and Medical Research Council [209057, 251,553 and 504711]; Australian National Breast Cancer Foundation [to JSt]; Canada the National Cancer Institute of Canada [to NFB]; Chile Fondecyt [11100238 to MLG, 1120326, 1130277, 3130532]; World Cancer Research Fund [2010/245]; Ellison Medical Foundation Grant [to AP]; Iran Isfahan University of Medical Sciences; Israel The Israel Cancer Association; Republic of Korea Asan Medical Center [2010-0811]; Malaysia Sime Darby LPGA Tournament; Ministry of Education University Malaya [High Impact Research Grant UM.C/HIR/MOHE/06]; University Malaya [Research Grant UMRG RP046B- 15HTM]; Mexico National Council of Science and Technology (Mexico); the American Institute for Cancer Research [10A035]; Netherlands EPIC-NL-Europe against Cancer Programme of the European Commission (SANCO); Dutch Ministry of Health; Dutch Cancer Society; ZonMW the Netherlands Organisation for Health Research and Development; World Cancer Research Fund (WCRF); Poland Polish-Norwegian Research Programme [PNRF-243-AI-1/07]; Singapore National Medical Research Council [Clinician Scientist Award]; National University Cancer Institute Singapore (NCIS) Centre grant programme from National Medical Research Council; South Africa Pink Drive; Spain Spain’s Health Research Fund (Fondo de Investigacion Santiaria) [PI060386 and PS09/0790]; Spanish Federation of Breast Cancer Patients (FECMA) [EPY1169-10]; Turkey- Roche Mustahzarlari San. A.S., Istanbul, Turkey; UK Engineering and Physical Sciences Research Council [EP/K020439/1 to JHi]; Breast Cancer Campaign [2007MayPR23], Cancer Research UK [G186/11 and C405/A14565]; Da Costa Foundation UK; USA National Cancer Institute [R01CA85265, R37 CA54281, R01 CA97396, P50 CA116201, R01 CA177150, R01 CA140286, UM1 CA186107 and UM1 CA176726]; Cancer Center Support Grant [CA15083; CA131332, and CA124865]; the Susan G. Komen Foundation. MM/JOD are supported by funding from the Health Research Board. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policies or views of these organisations.
Availability of data and materials
The ICMD data cannot be deposited publicly as these data originate from 27 research institutions across 22 countries with different legal and ethical frameworks. Researchers seeking the analysis data set for this work can apply to the Environment and Lifestyle Epidemiology Branch at the International Agency for Research on Cancer for access (email: env@iarc.fr).
Declarations
Ethics approval and consent to participate
Ethics approvals for the ICMD were obtained from the International Agency for Research on Cancer (IEC 12 ± 34). Each individual participating study had received local ethical approval at the time of the original conduct of the study and again to contribute to the consortium.
Consent for publication
Not applicable.
Competing interests
ML has received a non-restricted investigator-initiated grant from AstraZeneca and minor support from Swiss Re. All other co-authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. 2021;71(3):209–49. [DOI] [PubMed]
- 2.Ursin G, Bernstein L, Wang Y, Lord SJ, Deapen D, Liff JM, et al. Reproductive factors and risk of breast carcinoma in a study of white and African-American women. Cancer. 2004;101(2):353–62. [DOI] [PubMed] [Google Scholar]
- 3.Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50 302 women with breast cancer and 96 973 women without the disease. The Lancet. 2002;360(9328):187–95. [DOI] [PubMed] [Google Scholar]
- 4.Clavel-Chapelon F, Gerber M. Reproductive factors and breast cancer risk. Do they differ according to age at diagnosis? Breast Cancer Res Treat. 2002;72(2):107–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clavel-Chapelon F. the ENEG, Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer. 2002;86(5):723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clavel-Chapelon F. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer. 2002;86(5):723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao X, Omeogu C, Karanth S, Joshi A, Meernik C, Wilson L, et al. Association of reproductive risk factors and breast cancer molecular subtypes: a systematic review and meta-analysis. BMC Cancer. 2023;23(1):644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaffe M, Boyd N. Mammographic breast density and cancer risk: the radiological view. Gynecol Endocrinol. 2005;21(sup1):6–11. [DOI] [PubMed] [Google Scholar]
- 9.McCormack VA, dos Santos SI. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15(6):1159. [DOI] [PubMed] [Google Scholar]
- 10.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. Cancer Epidemiol Biomark Prev. 2007;356(3):227–36. [DOI] [PubMed] [Google Scholar]
- 11.Yaghjyan L, Colditz GA, Rosner B, Bertrand KA, Tamimi RM. Reproductive factors related to childbearing and mammographic breast density. Breast Cancer Res Treat. 2016;158(2):351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexeeff SE, Odo NU, McBride R, McGuire V, Achacoso N, Rothstein JH, et al. Reproductive factors and mammographic density: associations among 24,840 women and comparison of studies using digitized film-screen mammography and full-field digital mammography. Am J Epidemiol. 2019;188(6):1144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riza E, dos Santos SI, De Stavola B, Perry N, Karadedou-Zafiriadou E, Linos D, et al. Correlates of high-density mammographic parenchymal patterns by menopausal status in a rural population in Northern Greece. Am J Epidemiol. 2005;41(4):590–600. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TL, Schmidt DF, Makalic E, Dite GS, Stone J, Apicella C, et al. Explaining variance in the cumulus mammographic measures that predict breast cancer risk: a twins and sisters study. Var Mammogr Den Measur Predict Breast Cancer. 2013;22(12):2395–403. [DOI] [PubMed] [Google Scholar]
- 15.Lope V, Pérez-Gómez B, Sánchez-Contador C, Santamariña MC, Moreo P, Vidal C, et al. Obstetric history and mammographic density: a population-based cross-sectional study in Spain (DDM-Spain). Science. 2012;132(3):1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochi T, Tsunoda H, Yamauchi H, Takahashi OJBWSH. Impact of childbirth history on dense breast in mammographic screening: a cross-sectional study. Science. 2022;22(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TAJCC. Control Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Science. 2000;11:653–62. [DOI] [PubMed] [Google Scholar]
- 18.Kang D, Kim J-Y, Kim J-Y, Mun HS, Yoon SJ, Lee J, et al. The relationship between breast density change during menopause and the risk of breast cancer in Korean women. Cohort Study Breast Den Breast Cancer Risk. 2021;14(12):1119–28. [DOI] [PubMed] [Google Scholar]
- 19.Butler LM, Gold EB, Greendale GA, Crandall CJ, Modugno F, Oestreicher N, et al. Menstrual and reproductive factors in relation to mammographic density: the Study of Women’s Health Across the Nation (SWAN). Science. 2008;112:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariapun S, Li J, Yip CH, Taib NA, Teo SH. Ethnic differences in mammographic densities: an Asian cross-sectional study. PLoS ONE. 2015;10(2):e0117568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim S-E, Ahn H, Lee ES, Kong S-Y, Jung S-Y, Lee S, et al. Interaction effect between breast density and reproductive factors on breast cancer risk in Korean population. Science. 2019;24(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormack VA, Burton A, dos Santos-Silva I, Hipwell JH, Dickens C, Salem D, et al. International consortium on mammographic density: methodology and population diversity captured across 22 countries. Cancer Epidemiol. 2016;40:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton A, Maskarinec G, Perez-Gomez B, Vachon C, Miao H, Lajous M, et al. Mammographic density and ageing: a collaborative pooled analysis of cross-sectional data from 22 countries worldwide. PLoS Med. 2017;14(6):e1002335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward SV, Burton A, Tamimi RM, Pereira A, Garmendia ML, Pollan M, et al. The association of age at menarche and adult height with mammographic density in the International Consortium of Mammographic Density. Science. 2022;24(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byng JW, Boyd N, Fishell E, Jong R, Yaffe M. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39(10):1629. [DOI] [PubMed] [Google Scholar]
- 26.StataCorp. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC; 2021.
- 27.Yan H, Ren W, Jia M, Xue P, Li Z, Zhang S, et al. Breast cancer risk factors and mammographic density among 12518 average-risk women in rural China. BMC Cancer. 2023;23(1):952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice MS, Bertrand KA, Lajous M, Tamimi RM, Torres G, López-Ridaura R, et al. Reproductive and lifestyle risk factors and mammographic density in Mexican women. Ann Epidemiol. 2015;25(11):868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Getz KR, Adedokun B, Xu S, Toriola AT. Breastfeeding and mammographic breast density: a cross-sectional study. Cancer Prev Res. 2023;16(6):353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res BCR. 2006;8(4):R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islami F, Liu Y, Jemal A, Zhou J, Weiderpass E, Colditz G, et al. Breastfeeding and breast cancer risk by receptor status–a systematic review and meta-analysis. Ann Oncol. 2015;26(12):2398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo J, Moral R, Balogh GA, Mailo D, Russo IH. The protective role of pregnancy in breast cancer. Breast Cancer Res BCR. 2005;7(3):131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russo J, Mailo D, Hu Y-F, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res. 2009;11(2):931s-s936. [PubMed] [Google Scholar]
- 35.Rice MS, Bertrand KA, VanderWeele TJ, Rosner BA, Liao X, Adami H-O, et al. Mammographic density and breast cancer risk: a mediation analysis. Breast Cancer Res. 2016;18(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolcott CG, Koga K, Conroy SM, Byrne C, Nagata C, Ursin G, et al. Mammographic density, parity and age at first birth, and risk of breast cancer: an analysis of four case-control studies. Breast Cancer Res Treat. 2012;132(3):1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belmin C, Hoffmann R, Pichler P-P, Weisz H. Fertility transition powered by women’s access to electricity and modern cooking fuels. Nat Sustain. 2022;5(3):245–53. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1(a-d): Funnel plots assessing bias in the population-specific meta-analyses for each of the reproductive factors and the √MD measures. Fig. S2(a-b): Population-specific meta-analysis and categorical pooled analysis for parity and √NDA. Figs. S3–S4(a-b): Stratified analyses (population-specific meta-analyses and pooled categorical analyses) by menopausal status for parity and √PMD. Fig. S5(a-c): Sensitivity analyses (population-specific meta-analyses) with parous women only for parity and √MD measures. Fig. S6(a-b): Population-specific meta-analysis and categorical pooled analysis for age at first birth and √NDA. Figs. S7–S8(a-b): Stratified analyses (population-specific meta-analyses and pooled categorical analyses) by menopausal status for age at first birth and √PMD. Fig. S9: Population-specific meta-analysis for ever/never breastfed and √NDA. Fig. S10(a-b): Stratified meta-analyses by menopausal status for ever/never breastfed and √PMD. Fig. S11(a-b): Population-specific meta-analyses and categorical pooled analysis for lifetime breastfeeding duration and √NDA. Figs. S12–S13(a-b): Stratified analyses (population-specific meta-analyses and pooled categorical analyses) by menopausal status for lifetime breastfeeding duration and √PMD. Fig. S14(a-c): Sensitivity analyses (population-specific meta-analyses) excluding shorter (<1 month) and longer (> 200 months) durations for lifetime breastfeeding duration and √MD measures. Figs. S15–S16(a-c): Sensitivity analyses (population-specific meta-analyses and pooled categorical analyses) for breastfeeding duration per birth and the √MD measures.
Data Availability Statement
The ICMD data cannot be deposited publicly as these data originate from 27 research institutions across 22 countries with different legal and ethical frameworks. Researchers seeking the analysis data set for this work can apply to the Environment and Lifestyle Epidemiology Branch at the International Agency for Research on Cancer for access (email: env@iarc.fr).