Abstract
Bacteria that cause meningitis have been found to stimulate nerve fibres in the brain’s meninges to release a neuropeptide molecule that dampens the response of immune cells and aids bacterial invasion of the central nervous system.
Meningitis is a disease that occurs when bacteria enter the lining of the brain (the meninges) and trigger a potent inflammatory response. The disease is potentially life-threatening, with a high mortality rate if left untreated, and those who do survive often experience prolonged neurological complications1. Although much is known about the disease process associated with bacterial meningitis, there are key gaps in our understanding of how immune cells mount their defence against the bacteria and protect the underlying brain from damage. On page 472, Pinho-Ribeiro et al.2 identify a relationship between sensory neurons and immune cells in the meninges that facilitates bacterial invasion and the development of meningitis in mice. The authors demonstrate that bacteria can enhance their spread into the central nervous system (CNS) by stimulating meningeal sensory neurons to release a neuropeptide molecule that dampens the activity of immune cells that act as sentinels.
The meninges are a series of three over-lapping membranes called the dura mater, arachnoid mater and pia mater (Fig. 1) that surround the CNS and serve as a crucial barrier to protect it from various microbes3. The outermost layer, or dura mater, is a thick membrane that resides just beneath the skull bone. Below the dura are the arachnoid mater and pia mater, which together are referred to as the leptomeninges.
Figure 1 |. How bacteria dampen immune responses to meningitis.
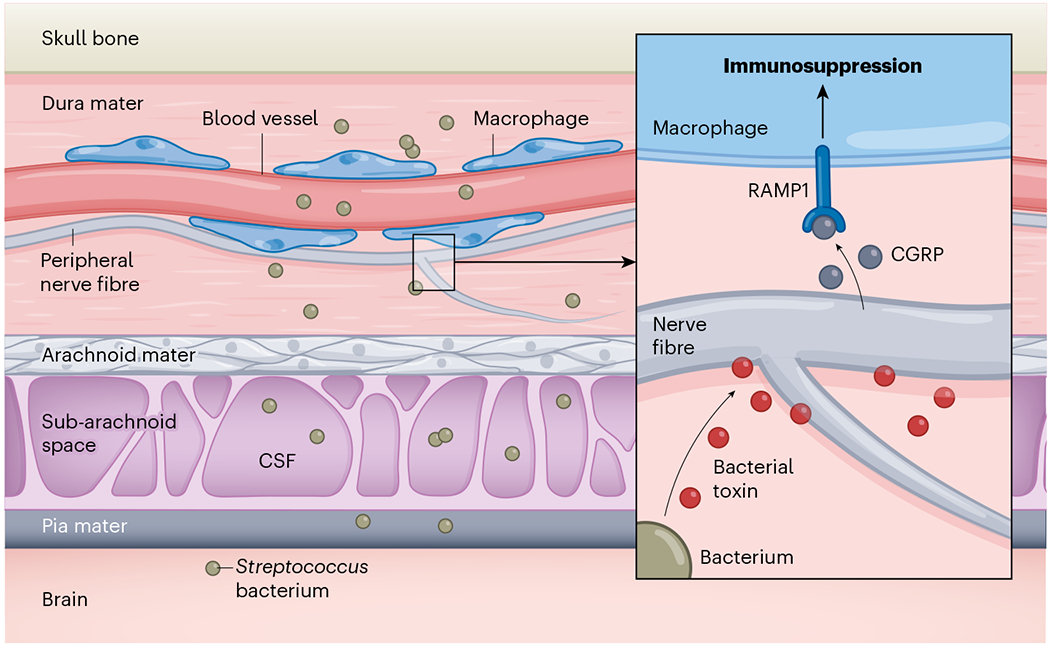
Pinho-Ribeiro et al.2 studied mice to examine how peripheral nerve fibres (structures that project from sensory neurons) and immune cells such as macrophages respond to infection by the bacteria that can invade the central nervous system and cause meningitis. Three layers beneath the skull – the dura mater, the arachnoid mater and the pia mater – form what are called the meninges. This region also contains a subarachnoid space that is filled with cerebrospinal fluid (CSF). If Streptococcus bacteria enter the dura mater through blood vessels, they will encounter immune cells capable of mounting an antibacterial response. The authors report that Streptococcus can dampen immune defences. The bacteria release toxins that activate peripheral nerve fibres, which then release the molecule CGRP. This neuropeptide binds to the RAMP1 receptor on macrophages and suppresses the immune-system defence response.
Blood vessels in the leptomeninges and brain are sealed by structures called tight junctions. These provide a barrier that helps to limit the entry of microbes into the CNS from the bloodstream. By contrast, blood vessels in the dura mater lack these tight junctions and the antimicrobial protection that they provide. In this way, the dura mater resembles tissues that reside outside the brain, referred to as peripheral tissues. Because of this susceptibility to microbial entry, the meninges are defended by a diverse assortment of immune cells that respond promptly to infections.
Another notable feature of the meninges is their innervation by peripheral sensory neurons called nociceptors that detect thermal, mechanical and chemical stimuli. These neurons can also respond to immune and microbial stimuli, inducing the sensation of pain. Previous studies have shown that nociceptors communicate with immune cells in peripheral tissues such as the skin and gut to regulate immunity against infection4. It has long been known that the dura mater is highly innervated by a variety of peripheral nerve fibres that project from clusters of neurons called ganglia5. There are various types of nerve fibre, called adrenergic, cholinergic and peptidergic depending on the type of molecule that they release6.
On activation, peptidergic nerve fibres can release a neuropeptide called CGRP that drives headaches and migraines6,7. Activation of meningeal nerves can cause headaches, but little is known about how or whether these sensory neurons regulate immune responses in the meninges. Interestingly, severe headache is an early symptom of bacterial meningitis1, suggesting that activation of meningeal nociceptors occurs after infection in humans.
Pinho-Ribeiro and colleagues sought to gain insight into the immunology of the meningeal barrier by exposing mice to the two bacterial species that commonly cause meningitis in humans: Streptococcus pneumoniae and Streptococcus agalactiae. After injecting bacteria into the bloodstream, the authors assessed the infection of the various meningeal layers. The results show that bacteria infected the dura mater first, then moved into the underlying leptomeninges and brain. In the dura mater, bacteria were found adjacent to nociceptors, and cell-culture studies revealed that toxins released by the bacteria activated these nerve fibres and promoted CGRP release.
To assess the role of nociceptors in meningitis, the authors used a combination of pharmacological and genetic approaches to deplete nerve fibres or to impede their signalling8. Importantly, local or systemic depletion of nociceptors reduced the level of bacteria in the meninges and brain after infection. This reduction was associated with a rise in the number of immune cells called macrophages and neutrophils in the dura mater, suggesting that nociceptors suppress a local antibacterial immune response.
Given that S. pneumoniae and S. agalactiae toxins stimulated CGRP production by nociceptors, the authors evaluated the role of this signalling pathway in the meningeal response to these bacteria. CGRP is known to induce signalling through the protein RAMP1 (ref. 9), and pharmacological blockade or genetic deletion of this receptor reduced the level of bacteria in the meninges and boosted immune responses. In addition, the therapeutic administration of a RAMP1 inhibitor, given to mice six hours after S. pneumoniae infection, delayed the onset of symptoms, although it did not prevent the animals from dying.
Pinho-Ribeiro and colleagues next sought to investigate how meningeal immune cells, tasked with mounting an early defence against invading bacteria, were influenced by CGRP signalling. The authors analysed meningeal immune cells from uninfected mice using single-cell RNA sequencing, and found that the gene encoding RAMP1 was highly expressed by a variety of immune cells, including monocytes, macrophages and neutrophils, which also express the enzyme lysozyme M (LyzM).
This finding led the authors to engineer mice in which the gene encoding RAMP1 was deleted from LyzM-expressing immune cells. In these mice, compared with non-engineered control animals, bacterial levels were reduced in the meninges and brain on a scale comparable to that observed in mice depleted of meningeal nociceptors. These results suggest that RAMP1 signalling in LyzM-expressing immune cells is responsible for suppressing antibacterial immune responses in the meninges.
Scavenger cells called phagocytes, which include macrophages, are crucial in the defence against bacterial infections and are the most abundant immune cells in the meninges. Pinho-Ribeiro et al. found that S. pneumoniae was associated with meningeal macrophages within a day of infection, and that, at the cellular level, these macrophages showed a rise in the expression of various immune-signalling molecules called chemokines. On the basis of these data, the authors propose that resident macrophages protect the meninges against infection by releasing chemokines that recruit immune cells from the periphery and that this function is dampened by RAMP1 signalling.
This theory is supported by several lines of investigation. Depletion of meningeal macrophages decreased the recruitment of monocytes and neutrophils to the meninges and enhanced bacterial invasion. By contrast, genetic deletion of RAMP1 from meningeal macrophages reduced bacterial invasion of the meninges to a level comparable to that observed in nociceptor-depleted mice. The authors also demonstrated in cell-culture experiments that S. pneumoniae promoted chemokine production by macrophages that could be suppressed by exposure to CGRP.
Together, these data support a mechanism by which bacterial invasion of the dura mater triggers the release of CGRP by nociceptors that suppresses chemokine expression in meningeal macrophages. This, in turn, dampens the recruitment of monocytes and neutrophils, and enhances bacterial invasion. Meningeal CGRP release after bacterial infection promotes the sensation of pain or headache, and this neuropeptide also seems to apply the brakes to resident macrophages, giving bacterial invaders a slight advantage.
It therefore seems reasonable to consider whether blockade of RAMP1 signalling in macrophages might offer a way to improve recovery during a case of bacterial meningitis. However, it is worth emphasizing that the immunological counterbalances that dampen an immune response are typically protective against excessive immune-system activity. The CNS is relatively intolerant of diseases that are mediated by immune cells, and recruited monocytes and neutrophils can cause extensive blood-vessel damage and swelling in the brain, especially after infection10.
The ability of nociceptors to sense bacteria provides the meninges with a potential mechanism for gauging the magnitude of an infection and tuning the resident macrophage response accordingly through CGRP release. This balancing act, however, comes with the risk of increasing bacterial invasion of the brain and meninges.
Footnotes
The authors declare no competing interests.
References
- 1.van de Beek D. et al. Nature Rev. Dis. Primers 2, 16074 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Pinho-Ribeiro FA et al. Nature 615, 472–481 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley MW & McGavern DB Immunol. Rev 306, 58–75 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udit S, Blake K & Chiu IM Nature Rev. Neurosci 23, 157–171 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper ER Acta Anat. 33, 298–318 (1958). [DOI] [PubMed] [Google Scholar]
- 6.Edvinsson L & Uddman R Cephalalgia 1, 175–179 (1981). [DOI] [PubMed] [Google Scholar]
- 7.Messoud AN Engl. J. Med 383, 1866–1876 (2020). [Google Scholar]
- 8.Pinho-Ribeiro FA et al. Cell 173, 1083–1097 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLatchie LM et al. Nature 393, 333–339 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Kim JV, Kang SS, Dustin ML & McGavern DB Nature 457, 191–195 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]


