Abstract
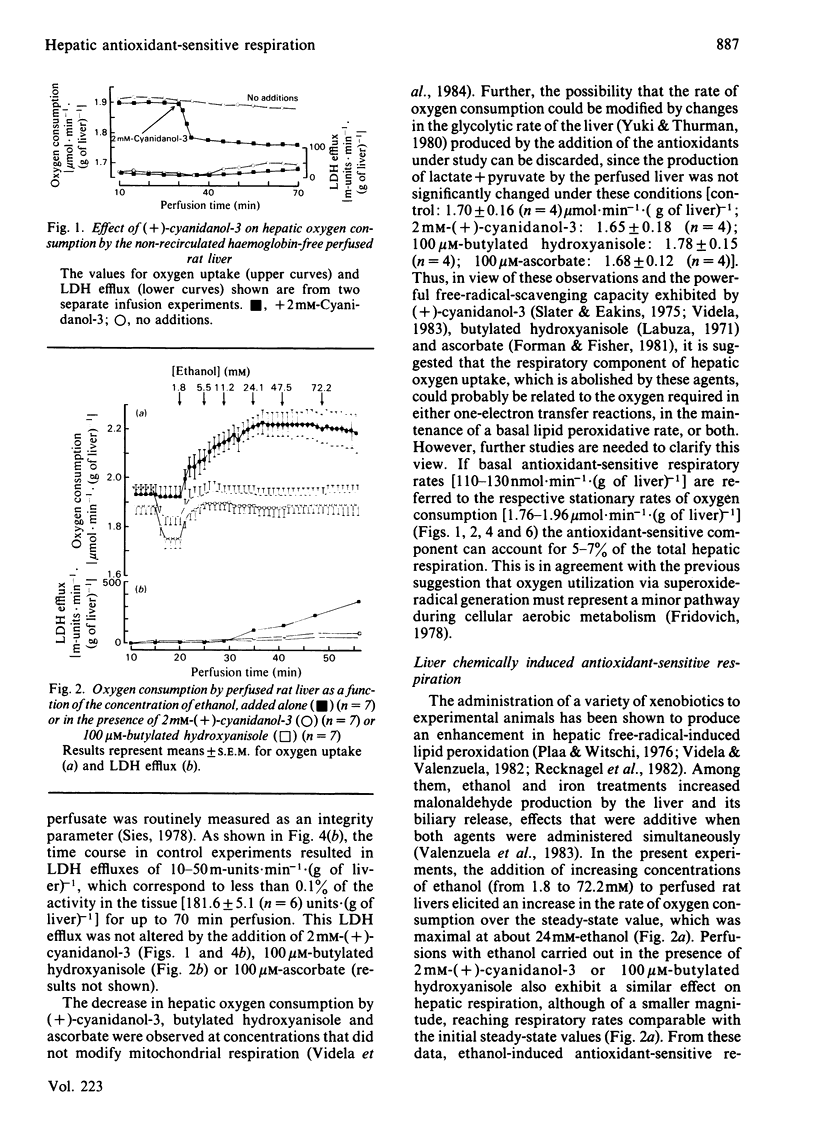
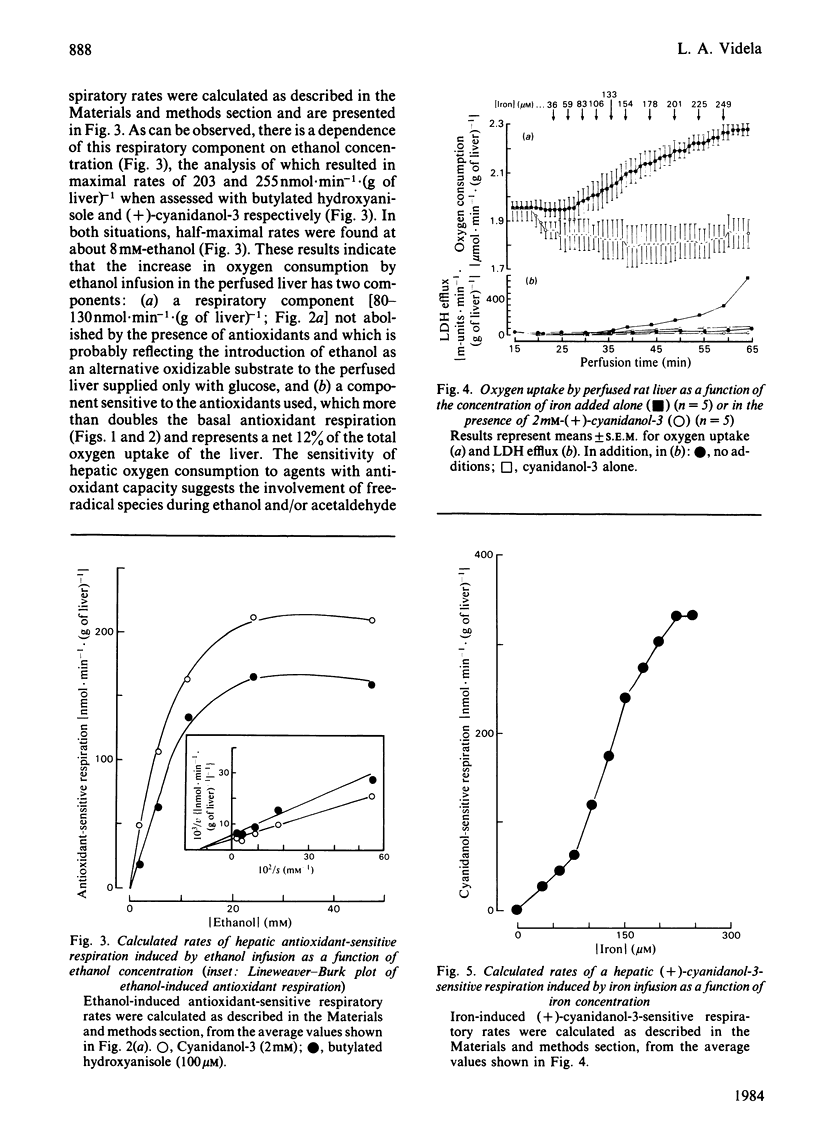
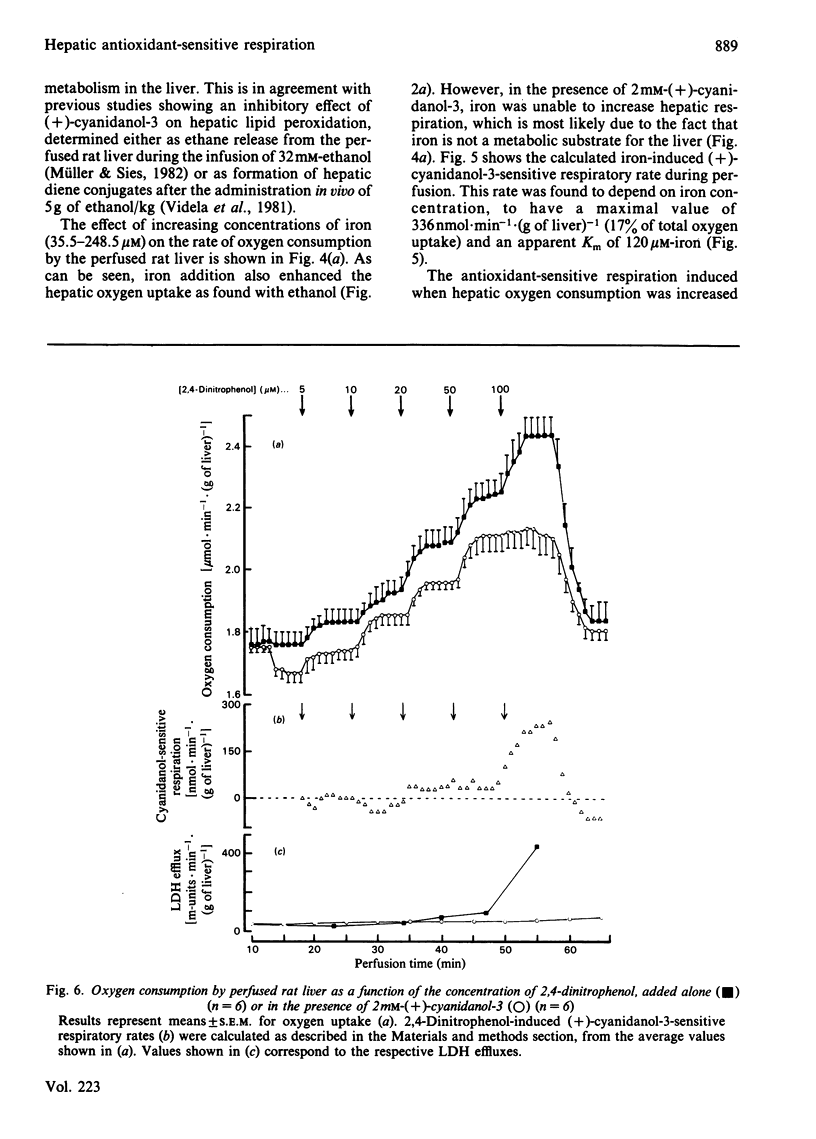
The addition of the antioxidants (+)-cyanidanol-3, butylated hydroxyanisole and ascorbate to the perfused rat liver resulted in a decrease in the rate of oxygen consumption. This basal antioxidant-sensitive respiration of 110-130nmol X min-1 X (g of liver)-1 represents 5-7% of total respiration. Increased antioxidant-sensitive respiratory rates are found after the infusion of increasing concentrations of ethanol (1.8-72.2mM) or iron (35.5-248.5 microM). This respiratory component exhibits a dependence on ethanol or iron concentration, with maximal rates of 200-255 and 330nmol X min-1 X (g of liver)-1 respectively. After the addition of 100 microM-2,4-dinitriphenol, an antioxidant-sensitive respiratory component of 230nmol X min-1 X (g of liver)-1 is found, which is not observed at lower concentrations of the uncoupler (5-50 microM). The lack of effect of the antioxidants used on mitochondrial respiration [the preceding paper, Videla, Villena, Donoso, Giulivi & Boveris (1984) Biochem. J. 223, 879-883] and on the glycolytic rate of the perfused liver suggests that the basal and chemically induced antioxidant-sensitive respiration observed are related to oxygen required for one-electron transfer reactions associated with the generation of active species of oxygen and lipid peroxidation in the liver cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979 Jul;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Dryer S. E., Dryer R. L., Autor A. P. Enhancement of mitochondrial, cyanide-resistant superoxide dismutase in the livers of rats treated with 2,4-dinitrophenol. J Biol Chem. 1980 Feb 10;255(3):1054–1057. [PubMed] [Google Scholar]
- Fridovich I. Superoxide radicals, superoxide dismutases and the aerobic lifestyle. Photochem Photobiol. 1978 Oct-Nov;28(4-5):733–741. doi: 10.1111/j.1751-1097.1978.tb07009.x. [DOI] [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Müller A., Sies H. Role of alcohol dehydrogenase activity and the acetaldehyde in ethanol- induced ethane and pentane production by isolated perfused rat liver. Biochem J. 1982 Jul 15;206(1):153–156. doi: 10.1042/bj2060153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaa G. L., Witschi H. Chemicals, drugs, and lipid peroxidation. Annu Rev Pharmacol Toxicol. 1976;16:125–141. doi: 10.1146/annurev.pa.16.040176.001013. [DOI] [PubMed] [Google Scholar]
- Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol. 1978;52:48–59. doi: 10.1016/s0076-6879(78)52005-3. [DOI] [PubMed] [Google Scholar]
- Singh A. Chemical and biochemical aspects of superoxide radicals and related species of activated oxygen. Can J Physiol Pharmacol. 1982 Nov;60(11):1330–1345. doi: 10.1139/y82-200. [DOI] [PubMed] [Google Scholar]
- Svingen B. A., Buege J. A., O'Neal F. O., Aust S. D. The mechanism of NADPH-dependent lipid peroxidation. The propagation of lipid peroxidation. J Biol Chem. 1979 Jul 10;254(13):5892–5899. [PubMed] [Google Scholar]
- Tappel A. L. Lipid peroxidation damage to cell components. Fed Proc. 1973 Aug;32(8):1870–1874. [PubMed] [Google Scholar]
- Turrens J. F., Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980 Nov 1;191(2):421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela A., Fernandez V., Videla L. A. Hepatic and biliary levels of glutathione and lipid peroxides following iron overload in the rat: effect of simultaneous ethanol administration. Toxicol Appl Pharmacol. 1983 Aug;70(1):87–95. doi: 10.1016/0041-008x(83)90181-3. [DOI] [PubMed] [Google Scholar]
- Videla L. A. Assessment of the scavenging action of reduced glutathione, (+)-cyanidanol-3 and ethanol by the chemiluminescent response of the xanthine oxidase reaction. Experientia. 1983 May 15;39(5):500–502. doi: 10.1007/BF01965175. [DOI] [PubMed] [Google Scholar]
- Videla L. A., Fernández V., Valenzuela A., Ugarte G. Effect of (+)-cyanidanol-3 on the changes in liver glutathione content and lipoperoxidation induced by acute ethanol administration in the rat. Pharmacology. 1981;22(6):343–348. doi: 10.1159/000137514. [DOI] [PubMed] [Google Scholar]
- Videla L. A., Valenzuela A. Alcohol ingestion, liver glutathione and lipoperoxidation: metabolic interrelations and pathological implications. Life Sci. 1982 Nov 29;31(22):2395–2407. doi: 10.1016/0024-3205(82)90743-3. [DOI] [PubMed] [Google Scholar]
- Videla L. A., Villena M. I., Donoso G., Giulivi C., Boveris A. Changes in oxygen consumption induced by t-butyl hydroperoxide in perfused rat liver. Effect of free-radical scavengers. Biochem J. 1984 Nov 1;223(3):879–883. doi: 10.1042/bj2230879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel A., Feuerstein S. Drug-induced lipid peroxidation in mice--I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol. 1981 Sep 15;30(18):2513–2520. doi: 10.1016/0006-2952(81)90576-1. [DOI] [PubMed] [Google Scholar]
- Yuki T., Thurman R. G. The swift increase in alcohol metabolism. Time course for the increase in hepatic oxygen uptake and the involvement of glycolysis. Biochem J. 1980 Jan 15;186(1):119–126. doi: 10.1042/bj1860119. [DOI] [PMC free article] [PubMed] [Google Scholar]


