Abstract
Background
To formulate effective strategies for antimicrobial stewardship (AMS) in primary care, it is crucial to gain a thorough understanding of factors influencing prescribers' behavior within the context. This qualitative study utilizes the Theoretical Domains Framework (TDF) to uncover these influential factors.
Methods
We conducted a qualitative study using in-depth interviews and focus group discussions with primary care workers in two provinces in rural Vietnam. Data analysis employed a combined inductive and deductive approach, with the deductive aspect grounded in the TDF.
Results
Thirty-eight doctors, doctor associates, and pharmacists participated in twenty-two interviews and two focus group discussions. We identified sixteen themes, directly mapping onto seven TDF domains: knowledge, skills, behavioral regulation, environmental context and resources, social influences, social/professional role and identity, and optimism. Factors driving unnecessary prescription of antibiotics include low awareness of antimicrobial resistance (AMR), diagnostic uncertainty, prescription-based reimbursement policy, inadequate medication supplies, insufficient financing, patients’ perception of health insurance medication as an entitlement, and maintaining doctor-patient relationships. Potential factors facilitating AMS activities include time availability for in-person patient consultation, experience in health communication, and willingness to take action against AMR.
Conclusion
Utilizing the TDF to systematically analyze and present behavioral determinants offers a structured foundation for designing impactful AMS interventions in primary care. The findings underscore the importance of not only enhancing knowledge and skills but also implementing environmental restructuring, regulation, and enablement measures to effectively tackle unnecessary antibiotic prescribing in this context.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-024-01471-9.
Keywords: Primary care, Antimicrobial resistance, Theoretical domains framework, Antibiotic prescribing, Antibiotic stewardship
Introduction
Antimicrobial resistance (AMR) is a major global health challenge, leading to elevated mortality and healthcare costs [1, 2]. While resistance to antimicrobials is an inherent biological process in pathogens, and can be intrinsic or acquired, human activity accelerates this trend [3, 4]. In Europe, higher bacterial resistance is evident in countries with higher outpatient consumption of antibiotics [5]. Misuse and overuse of antibiotics are widespread in low-middle income countries (LMICs). Antibiotic prescriptions were unnecessarily issued in 83% of outpatient pediatric consultations in Cambodia, 74% in Senegal, and 72% in Madagascar [6]. Two-thirds of patients on antimicrobial therapy in Pakistani hospitals received at least one inappropriate prescription following the British National Formulary guidelines [7].
Primary care is a key provider of antibiotics in the community with a profound effect on AMR [8]. Antimicrobial stewardship (AMS) interventions aimed at promoting the judicious use of antibiotics at the grassroots level of the health system are imperative, particularly in LMICs, where resistance is prevalent [1, 9]. Lam et al. (2021) reviewed eleven studies employing different community-based AMS strategies in LMICs such as education, peer review of guidelines, audit and feedback, point-of-care CRP testing, and an electronic algorithm for decision-making. These studies reported reductions in antibiotic prescription rates, ranging from 3 to 55%. Nevertheless, other pertinent outcomes such as sustainability of effect, impacts on AMR, clinical outcomes, and cost-effectiveness were either unfavorable or not measured [10].
To develop effective AMS intervention strategies, it is essential to understand barriers and enablers to appropriate prescription of antibiotics from the primary care workers’ perspective. However, existing research on this matter has predominantly focused on hospital settings, and when primary care is considered, the majority of evidence is derived from high-income contexts [11, 12]. Another challenge is the limited use of theories or frameworks for a comprehensive understanding, which could lead to important factors being overlooked and limited utility of findings for intervention development. These practical considerations necessitate a systematic approach to understand behavioral determinants involved in antibiotic prescribing [13].
Vietnam is a lower-middle income country in South-East Asia with a population of approximately 100 million and a GDP per capita of 4320 USD [14]. The Vietnam healthcare system has four levels: national, provincial, district, and commune, corresponding to the country's administrative structure. The extended network of more than 11,000 commune health centers (CHCs), approximately 90% are staffed by doctors, provides essential curative and preventive services to the population under the health insurance scheme [15, 16]. By 2021, national health insurance coverage surpassed 92% and has continued to increase [16, 17].
Having a high prevalence of infectious diseases, coupled with relatively unregulated access to antibiotics, Vietnam has become a global hotspot for AMR [18–21]. Antibiotics were prescribed for 80% of patient-visits at CHCs, and 54% of these prescriptions were for non-infectious conditions [22]. Approximately 97% of outpatient visits for acute respiratory infections (ARIs), the most common infectious condition in primary care, resulted in antibiotic prescriptions [23]. A mixed methods study on antibiotic prescribing by commune health workers in Vietnam revealed barriers such as lack of knowledge, improper facilities, and peer and community pressure. However, it was limited to one district and failed to address crucial aspects like organizational structure, physicians' perceptions of AMR, patient consultation skills, and the medication supply chain [22].
This qualitative study aimed to provide an all-inclusive investigation of factors that influence antibiotic prescribing behaviors among primary care workers in the limited-resource context of Vietnam.
Methods
Study design and setting
We conducted a qualitative study that included focus group discussions and in-depth interviews with healthcare workers, including doctors, doctor associates, and pharmacists in CHCs in two Red-River Delta provinces in northern Vietnam, Nam Dinh and Ha Nam (out of 63 provinces in the country). Substantial evidence of antibiotic misuse and resistance has been reported in this region [22].
Sampling and recruitment
In-depth interviews: We used purposive sampling, aimed at the inclusion of different geographical regions (in five different districts, two in Ha Nam and three in Nam Dinh), and commune population size (< 5000, between 5000 and 15,000, and > 15,000) [24]. We included both doctors and doctor associates, who were active prescribers at their CHCs, and excluded those who did not provide consent. All interviews took place privately, face-to-face, within the interviewees' consultation rooms.
Focus group discussions: We conducted two face-to-face focus group discussions in Nam Dinh—one involving CHC doctors and the other with CHC pharmacists. Each session comprised eight participants from three different districts. The district coordinators determined the selection of participants based on their availability.
Interview process
All participants provided written informed consent at the commencement of the interviews and focus group discussions. Interviews were conducted by four researchers (CNTH and TTH in Ha Nam in 2019, VMD and NTHY in Nam Dinh in 2023). In 2019, an interview guide was developed based on existing literature, covering open-ended questions about the diagnosis and treatment of ARIs, understanding of AMR, and factors influencing antibiotic prescribing. It was used in six interviews, which were supposed to inform the development of a whole system intervention study aiming at reducing antibiotic consumption in community. However, the study was delayed due to the Covid-19 pandemic. The interview guide used in 2023 was updated to address emerging issues in the health system, particularly in primary care settings post-Covid-19. Both guides were piloted before data collection began (by NHY, social science researcher and NTCT, public health researcher), and were continuously updated after initial interviews as we encountered new insights. The final versions of both interview guides are provided as supplementary materials (Additional file 1: IDI guide 2019 and Additional file 2: IDI guide 2023). Recorded interviews were transcribed verbatim by an external service.
The focus group discussions (conducted by NVM and DTTN) used a question guide focusing on three overarching themes: workflow in CHCs, determinants of AMR, and suggestions for reducing AMR (see Table 1). All interviewers/facilitators had received training on conducting semi-structured interviews and focus group discussions and none of them had established relationships with participants prior to data collection.
Table 1.
Interview topics
| In-depth interviews | Focus group discussions |
|---|---|
| 2019 interviews | Workflow in commune health centers |
| Diagnosis and treatment practice for common infectious illnesses | Determinants of AMR according to doctors and pharmacists in CHCs |
| Factors making doctors more or less likely to prescribe antibiotics | Suggestions to reduce AMR |
| Handling patients’ request for antibiotics | |
| Understanding of AMR and training received | |
| 2023 interviews (added topics) | |
| Antibiotic supply process and its impacts | |
| Effectiveness of launched antimicrobial stewardship initiatives & potential alternative measures | |
| Patient consultation and public communication |
Data analysis
This study utilized the Theoretical Domains Framework (TDF), an inclusive model integrating 83 behavioral theories/models, with 14 domains and 128 constructs, to guide data analysis [25]. The framework has been applied in a number of health promotion initiatives, serving various purposes such as behavioral diagnosis, intervention design, and process evaluation [26–29]. The TDF expands upon the COM-B model, which identifies the three pillars of behavior as "capability", "opportunity", and "motivation" emphasizing that an individual needs both physical and psychological capability, along with social and physical opportunity, coupled with automatic and reflective motivation, to engage in a behavior (Table 2) [30].
Table 2.
The Theoretical Domains Framework (TDF)
| COM-B components | TDF domains |
|---|---|
| Physical capability | Physical skills |
| Psychological capability | Cognitive and interpersonal skills |
| Knowledge | |
| Memory, attention, and decision processes | |
| Behavioral regulation | |
| Physical opportunity | Environmental context and resources |
| Social opportunity | Social influences |
| Automatic motivation | Reinforcement |
| Emotion | |
| Reflective motivation | Social/professional role and identity |
| Beliefs about capabilities | |
| Optimism | |
| Intentions | |
| Goals | |
| Beliefs about consequences |
Table reproduced from Atkins et al. (2017) [25]
Two researchers (VMD, NTHY) independently coded the data using the Nvivo12 software. We conducted a thematic content analysis, combining both inductive and deductive approaches, with the deductive aspect grounded in the TDF. The analysts initially coded transcripts to identify overarching inductive themes related to the facilitators and barriers to reducing antibiotic prescriptions for ARIs. Subsequently, these themes were categorized into the 14 TDF domains and further aligned with the six components of the COM-B model. Themes that did not align with any TDF domains would be reported separately. In cases of differences in the placement of themes, both researchers reached agreement through discussions. If a disagreement persisted, a senior researcher (SN) would provide the decisive opinion.
Results
Participants’ characteristics
Overall, eight doctors and eight pharmacists participated in two focus group discussions, while twenty doctors and two doctor associates participated in in-depth interviews. On interview days, four participants opted not to be recorded, prompting the interviewer (NTHY) to document discussions through field notes. Data saturation was achieved after twenty interviews, as subsequent interviews did not yield new themes. Table 3 provides details of the participants’ characteristics, including gender, qualification, and length of service.
Table 3.
Participant characteristics
| Characteristics | Focus group discussions | In-depth interviews | Total (%) |
|---|---|---|---|
| Gender | |||
| Male | 9 | 9 | 18 (47) |
| Female | 7 | 13 | 20 (53) |
| Qualification | |||
| Medical doctor | 8 | 20 | 28 (74) |
| Doctor associate | 0 | 2 | 2 (5) |
| Pharmacist | 8 | 0 | 8 (21) |
| Length of service (years) | |||
| 1–10 | 2 | 3 | 5 (13) |
| 11–20 | 6 | 5 | 11 (29) |
| − 30 | 6 | 8 | 14 (37) |
| > 30 | 2 | 6 | 8 (21) |
Determinants of antibiotic prescribing according to the Theoretical Domains Framework
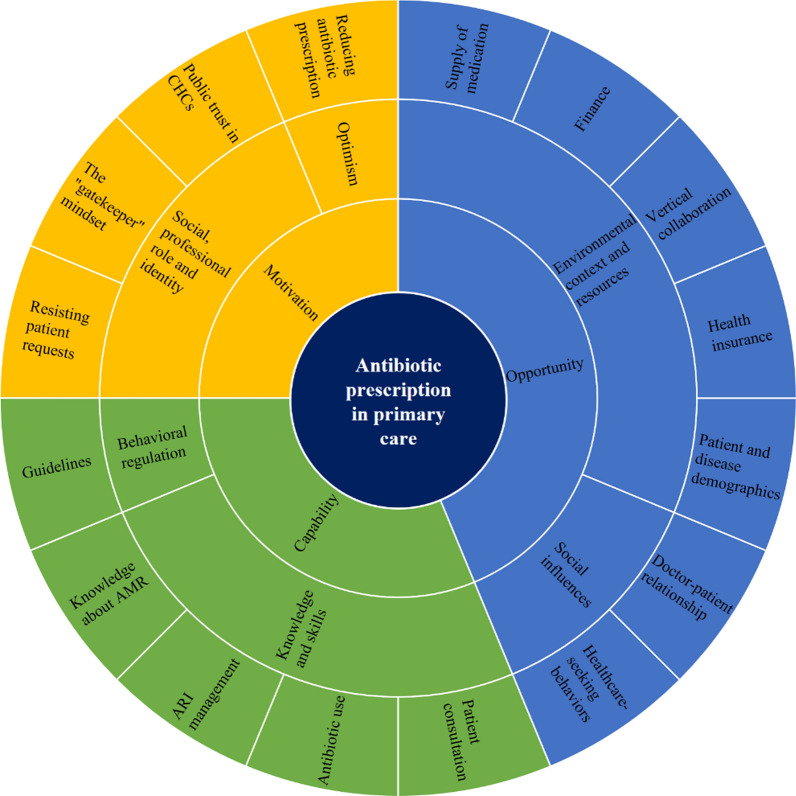
We identified sixteen themes, directly mapping onto seven TDF domains and three pillars of the COM-B model (Fig. 1). Four COM-B components associated with antibiotic prescribing in primary care included: I—Psychological capability, II—Physical opportunity, III—Social opportunity, and IV—Reflective motivation. Due to the blurred distinctions between knowledge and skills through participants’ narrative, we merged these two TDF domains into a single COM-B component (under Psychological capability).
Fig. 1.
Identification of themes and mapping onto TDF domains and COM-B model [25]. The behavior of interest—antibiotic prescription in primary care, is centered around by three COM-B pillars: capability, opportunity, and motivation. Surrounding each pillar are the TDF domains linked to the behavior. The outermost layer represents the specific themes identified through interviews and focus groups, aligned with each respective domain
Psychological capability
Knowledge and skills
Knowledge about AMR—Participants exhibited diverse knowledge and perceptions of AMR, with some showing insufficient understanding. In the doctors' focus group, one doctor estimated AMR prevalence at 10% in his practice, characterized by prolonged symptoms and referral needs. However, in another interview, a doctor stated that she had never encountered a patient with AMR and considered the issue of AMR of no concern, while another doctor associate mistakenly defined AMR as an immunological phenomenon.
“…there are cases where antibiotics don't work because the body develops antibodies against them. It's similar to situations where people live in an area with a disease outbreak, but they never get infected.” (INT_1, doctor associate, 12 years in service).
-
(2)
ARI management – All participants were aware that viral infections do not necessitate antibiotics and could articulate a symptom-based treatment strategy for ARIs caused by viruses. However, this awareness did not significantly affect their practice, as most admitted to prescribing antibiotics in nearly all ARI cases.
“You know, for example, common cold does not require antibiotics, but in order to prevent infections, treat symptoms, kill pain, reduce fever then we might use antibiotics.” (INT_14, doctor, 29 years in service).
Some interviewees expressed difficulty in differentiating bacterial infections from other infectious symptoms, and the absence of diagnostic tests added challenges to clinical practice, with three metaphorically describing the situation as "catching the enemy with bare hands."
“So, we often joke that doctors at the commune level it’s like “tay khong bat giac” (catching enemies with bare hands). Apart from the stethoscope, the otoscope for examining ears, noses, and throats, and a thermometer, there are not much else… right? [laughter].” (INT_2, doctor, 28 years in service).
-
(3)
Use of antibiotics—Regarding choice of antibiotics for ARI treatment, consistent responses were noted, typically comprising 500 mg of Amoxicillin or Cephalexin per dose, twice a day, over 5 to 7 days. Participants expressed a preference for newer generations of cephalosporins over older ones and favored the cephalosporin group for its perceived broader spectrum of activity and higher efficacy than the penicillin group. However, a doctor associate described inappropriate approaches to reduce antibiotic use.
“So, in that case (a patient actively asks for antibiotics when not necessary), we can potentially reduce the dosage. Typically, it is prescribed for five days, but we can shorten the duration. If the patient still doesn't agree, we decrease the dose. […] Let’s say if they were taking 500, we can lower it to 250, and it still works effectively.” (INT_1, doctor associate, 12 years in service).
-
(4)
Patient communication—Public health communication is a crucial aspect of CHC healthcare workers' responsibilities, with the content originating from either the district health center (DHC) or CHC doctors. They employed various channels, including loudspeakers, village health worker teams, and in-person engagements during women’s union and farmers’ union meetings. However, the majority of participants, except for one, did not report communicating content related to AMR to the public. The consensus among all participants was that in-person patient education is more impactful than mass communication, and there is a need for additional AMR training for effective patient consultations.
“Now we need instructions on how to use antibiotics. For instance, when to use high doses, or there are cases that antibiotics are not necessary. Then, instruct us how to explain (AMR and antibiotic use) to the public, so that they can understand. […] If we don’t explain well, they would not understand.” (INT_8, doctor associate, 10 years in service).
CHC staff need to use computers for a variety of tasks, such as prescribing, managing drugs, making reports, and creating health education materials. However, two older doctors acknowledged challenges with their IT skills and often sought assistance from younger colleagues for their daily tasks.
“To be honest, we don't know how to do it unless someone guides us. Nowadays, young people can do it, but for us, the older generation, we never had the opportunity to learn about computers.” (INT_9, doctor, 32 years in service).
Behavioral regulation
-
(5)
Guidelines—Unlike the hospital setting, there are currently no indicators or monitoring mechanisms for antibiotic prescribing practices in primary healthcare facilities [31–33]. Doctors primarily rely on knowledge acquired during their medical school education, often decades ago, and their own professional experience. Interviewees in Hai Hau district acknowledged the provision of a guidebook on the diagnosis and treatment of illnesses at CHCs by the DHC (released by MoH in 2014). However, they paid little attention to it due to it being almost ten years old, perceived similarity to their practice, and the availability of more convenient information sources like consulting with colleagues or referring to product information leaflets. None of interviewees in other districts could recall the name of a guidebook.
“Actually, upon reading it (the MoH guidelines), there wasn’t really any particular concern, so… It just provides instructions for antibiotic treatment in certain infections. Nothing special.” (INT_5, doctor, 16 years in service).
Physical opportunity
Environmental context and resources
-
(6)
Patient and disease demographics—According to interviewees, health insurance coverage exceeds 90% in all their communes, leading to almost all patient visits at CHCs being covered by insurance. Participants’ estimates of the proportion of patients presenting with ARIs ranged from 40 to 70%. The predominant patient demographic comprises the elderly, who encounter limitations in traveling long distances, and those experiencing financial constraints.
Participants in 2023 interviews observed a decreasing trend in their patient pool due to factors such as the rise of private clinics, pharmacies, informal healthcare providers, and changes in referral policies allowing insured care at district hospitals without a CHC referral. They noted that excessive antibiotic use is common in these settings, leading to patients visiting CHCs expressing a strong preference for antibiotics.
“They admitted that they not only used oral antibiotics but also took antibiotic injection from retired medical staff or those who had participated in a medical school but they do not work in any official health establishment.” (INT_13, doctor, 5 years in service).
“I see that higher-level healthcare facilities use combinations of many antibiotic classes.” (INT_7, doctor, 31 years in service).
-
(7)
Health insurance—Health insurance guidelines significantly affect CHC doctors' decision-making, as they are constrained by rigid diagnosis codes that may not fully cover all ARI conditions. Uncertainty in the reimbursement cap, estimated by interviewees to range from 40,000 to 100,000 VND per treatment, prompted doctors to limit prescriptions to 50,000 VND (~ 2 USD) to avoid the need for complicated explanation to the health insurance office.
“Prescription for each patient visit can cost at most 50,000 dong, so if patients have more than one disease, doctors have to prioritize the main one. Patients can come back the following week to receive medicines for the other disease they might have.” (INT_19, doctor, 28 years in service).
“In general, if you keep exceeding the limits too much, they will remind you. […] It goes beyond the control of the insurance reimbursement, and we are afraid of that, so they remind you [laughs].” (INT_7, doctor, 31 years in service).
For doctors to be able to claim reimbursement for a consultation, the policy requires that a prescription be made, which may encourage unnecessary antibiotic prescribing due to the lack of alternative medications available at primary care (e.g. anti-inflammation, cough suppressants, expectorants). Furthermore, health insurance does not allow a patient to have two consecutive visits within seven calendar days.
“Now we are only paid if we prescribe medicine. If providing patient consultation only can be paid, then doctors have no need to prescribe antibiotics.” (FGD_1/P8, doctor, 33 years in service).
-
(8)
Finance—Three doctors expressed concern that they had received partial or no payment for medical consultations over extended periods, intensifying the discouragement felt by CHC health workers amid already modest remuneration.
“Health insurance (payment) is a big issue; the examination fees for 3–4 years have been collected by the Department of Health and not reimbursed (to CHCs).” (FGD_1/P1, doctor, 30 years in service).
-
(9)
Vertical collaboration—DHCs are responsible for providing medications to their managed CHCs upon request. Yet, CHCs’ supply requests are bound by an inventory list, and discrepancies often arise between the requested and supplied quantities.
“Starting from the Covid [pandemic], the medication supplies have been increasingly limited. Because now… when we inquire, they [the DHC] say that they cannot procure it now.” (INT_1, doctor associate, 12 years in service)
.
DHCs also provide the day-to-day operational budget for CHCs, with ad-hoc expenditures, such as renovations, funded by the Commune People’s Committee upon application and approval. Five participants indicated that the amounts provided were insufficient to cover basic expenses. Additionally, one doctor mentioned that, despite financial constraints, complications in the process discouraged them to request for additional funding.
“The DHC allocates only a few hundred thousand VND per month for office supplies. The main expenditure is on facility maintenance, which is mainly funded by the local administrative committee. […] Requesting funds for facility improvements is a challenging process. Sometimes, it's so discouraging that we hesitate to request more. Financial constraints are extremely difficult. […] Sometimes, we run out of money and have to borrow from our staff to buy paper, pens, and refill ink.” (INT_10, doctor, 24 years in service).
-
(10)
Supply of medication—Two doctors stated that, though generally adequate in quantity, the antibiotic supply posed challenges for their practice due to a limited variety in dosage forms and strengths, particularly affecting pediatric treatments. Participants also observed regular disruptions in the supply of symptom relief medications, potentially leading to the excessive use of corticosteroids and antibiotics. In cases of insured medication unavailability, non-insurance drugs, supplied by CHC staff themselves, which serve as an additional source of income for them, could be an alternative at some CHCs, albeit at a non-competitive price.
“The health insurance’s drug list only has packaged (solid) medicine, not the suspension form for children like syrups. So, the parents bring their children out (to pharmacies).” (INT_6, doctor, 33 years in service).
“Even the most basic items like chlorpheniramine (anti-histamine) or vitamins are not available. Medications for symptomatic relief are lacking. Only Prednisolone (steroid) for anti-allergy but overusing it is not good either.” (FGD_2/P4, pharmacist, 12 years in service).
“If patients want to buy service (non-insurance) drugs at CHCs, we need to release an invoice, so the drug price here would be much more expensive than at pharmacies.” (FGD_2/P4, pharmacist, 12 years in service).
Social opportunity
Social influences
-
(11)
Healthcare seeking behaviors—Participants identified diverse patient behaviors affecting their prescribing decisions. Some patients viewed CHC medication as health insurance benefits not to be wasted, leading to antibiotic requests even without medical reasons. Patients might indicate a specific antibiotic worked for them previously. Many had a strong desire for quick, short, and cheap treatments. If not given antibiotics at CHCs, they might purchase antibiotics themselves or seek care from other providers.
“Sometimes, one person's visit prompts others to go to the CHC to request medication. Some people don’t even visit for medical check-up but for requesting medicine only.” (FGD_2/P3, pharmacist, two years in service).
“Many patients tend to seek high-dose medications. It's ingrained in their mindset. They often want the “best” medication or the highest dose of medicine, thinking that just a few doses will cure them.” (INT_1, doctor associate, 12 years in service).
-
(12)
Doctor – patient relationship—Two participants mentioned a potential undesirable consequence of not meeting patients’ demands – being reported through the hotline, a telephone number of the managing DHC. This pressured them to prescribe antibiotics as requested. Another doctor considered the close relationship with local people a barrier to resisting their antibiotic requests.
"Because of the relationship with them, we could not refuse. If we do not give them the medicines, they would scold us and make a scene here. You know, they are already old and we know each other well. We could not refuse them. So, sometimes, we had to make up something like respiratory infections so that they could receive antibiotics from the health insurance scheme.” (INT_20, doctor, 31 years in service).
Reflective motivation
Social, professional role and identity
-
(13)
The “gatekeeper” mindset—Participants were aware of their professional role as the gatekeepers of the health system. They attend to patients within their capacity and refer them to higher levels of care when necessary. Patients with more severe ARI conditions, such as pneumonia, are hardly kept at CHCs for treatment.
“I still tell my colleagues at the station not to claim to be experts because we don't have in-depth expertise. We don't specialize deeply, so there's no need to claim to be highly skilled. We just talk about what we know to categorize patients well, and that's when we can be considered skilled.” (INT_11, doctor, 30 years in service).
-
(14)
Public trust in CHCs—The pharmacists’ focus group revealed that public trust in CHCs relies on the providers’ education and length of services. It affects patients’ adherence to doctors’ advice and the patient pool of that CHC.
“Patients tend to have more trust in local health station because it's not just about getting medicine; there are doctors there too. […] In my CHC, the doctor has been treating patients for many years, and he has a lot of experience and knowledge.” (FGD_2/P2, pharmacist, 28 years in service).
-
(15)
Resisting patient requests—Some doctors showed high confidence in resisting patient requests for antibiotics.
“I am the doctor, they are my patients; so the patient should listen to the doctor, not the other way around.” (INT_2, doctor, 28 years in service).
Optimism
-
(16)
Reducing antibiotic prescription—However, even the most confident interviewees were quite pessimistic about the possibility of reducing antibiotic prescription in their practice, in particular citing diagnostic uncertainty.
“There is no chance to reduce it. It means that when we treat patients, the symptoms have been there for a couple of days, some may have had infections. Therefore, the use of antibiotics is quite common." (INT_4, doctor, 23 years in service).
"In general, it's (reducing antibiotic use) difficult. Although I know it, but sometimes I can't do it. Because we are catching the enemy with bare hands." (INT_2, doctor, 28 years in service).
Discussion
To our knowledge, this is the first study using the Theoretical Domains Framework to investigate factors influencing the antibiotic prescribing behavior of primary care practitioners in Vietnam. The study identifies seven relevant domains—knowledge, skills, behavioral regulation, environmental context and resources, social influences, social/professional role and identity, and optimism.
Our study found that primary care workers hold diverse perspectives on AMR. Contrasting views of AMR as a local problem were also observed in a multi-country qualitative study in Europe; and those perceiving AMR as less concerning attributed treatment failure to either poor patient adherence or the probability of viral infections [34]. In our study, the observed disparity can be attributed to variations in participants’ definitions of AMR and their exposure to relevant information in mass media. Our investigation of sources of information for treatment practices aligns with findings from a local survey-based study, indicating a significant reliance on original training (91.1%) and a low dependence on post-qualifying training courses (28.6%) [22]. This, combined with incorrect understanding of AMR mechanisms and bacterial infection symptoms, inappropriate dose-adjustment strategies, and the need for improving patient consultation skills identified in our study, underscores the critical importance of continuous education and training on these matters through AMS programs in primary care. Moreover, given the high reliance on original training, greater integration of rational antibiotic use into undergraduate pharmacy and medical education should be targeted for long-term impacts in addressing AMR [35].
Various structural factors contribute to unnecessary antibiotic prescription. Our study aligns with Hoang et al. (2019), finding that the health insurance cap and a fear of confronting health insurance officials hinder primary care doctors from giving optimal treatment [22]. The majority of patients visiting CHCs are elderly with multiple comorbidities. Consequently, given the perceived low insurance cap, doctors have to prioritize primary diseases, choosing the "strongest" and "cheapest" medications, with antibiotics emerging as the preferred choice for infectious conditions [36]. The "wait and see" approach is also impractical in this context as patients are unlikely to wait for seven days (two consecutive patient visits within seven days are not allowed) to revisit the CHC and receive medications if their symptoms worsen [37]. These limitations make accessing antibiotics from alternative sources like private pharmacies and clinics more appealing to patients, even if it requires paying out of pocket [38]. In cases of patient visits with a singular infectious condition, the prescription-based reimbursement policy fosters the over-prescription of antibiotics due to frequent unavailability of symptom relief medications.
Environment restructuring and enablement interventions are appropriate approaches to addressing structural challenges, as suggested by Michie et al. [30]. Some doctors in our study advocated for the prompt implementation of the Family Medicine framework in commune health facilities. The framework, currently in a pilot phase across eight provinces, does not mandate medication prescriptions for reimbursement and allows primary care workers to provide additional services, including home visits, to enhance both CHCs and personal income [39, 40]. Potential context-specific enablement measures include providing primary care practitioners with consistent clarifications on health insurance caps and fostering trust and collaboration between them and health insurance officers.
By the time our second phase of interviews was conducted in 2023, significant shortages of drugs and medical supplies had emerged post-Covid-19, impacting public healthcare facilities across all levels of Vietnam’s health system [41]. DHCs, tasked with supplying drugs to CHCs, often prioritize hospital usage, resulting in frequently inadequate supplies to CHCs, particularly for symptom relief medications for ARIs. The unavailability of alternatives for antibiotics represents a significant missed opportunity, as many doctors acknowledge their potential efficacy in mild conditions and expressed willingness to prescribe them instead of antibiotics if they were available. Recent government legislation may help healthcare facilities secure better supplies of medications by granting them more autonomy in the procurement process [42]. However, it may not fully resolve the power imbalance between the district (supplier) and commune (receiver) levels. Despite frequent complaints from participants about medication shortages and their impacts on antibiotic prescribing, there was little evidence that they had raised these concerns with their managing body—the DHCs. To enhance medication supply for CHCs, DHCs are encouraged to actively involve CHC staff in the planning process and make efforts to accommodate their subordinates’ requests.
Similar to existing literature, patients' perceived efficacy of prior use and social constructions of antibiotics as a trusted remedy were also reported as contributing to increased pressure on practitioners in our study [36, 43]. Patients convey diverse forms of pressure. Some explicitly request antibiotics, even in generic names of “Amox” (Amoxicillin) or “Cipro” (Ciprofloxacin). Others prime doctors for an antibiotic prescription in their problem presentations or resist non-antibiotic recommendations. Our finding of familiarity with patients as a significant influence on doctors’ prescribing preferences was also found elsewhere [44]. In addition, we found that commune citizens, especially the elderly, view health insurance as a “common good” entitling them to antibiotics when requested [45]. Although participants believe that the best way to lessen patient pressure is through patient education, either at CHCs or other public areas, they have had limited involvement in such initiatives. Future AMS interventions should address barriers to patient education found in our studies such as a lack of guidance and monitoring from DHCs, uncertainty about what messages to deliver, and low IT skills.
While looking at challenges, primary care AMS planners and policy makers should not overlook other important opportunities. Firstly, primary care doctors have ample time available for in-person consultations, an advantage that their higher-level counterparts do not possess. In addition, their local residency fosters strong connections with the community, and their experience in public communication through various health initiatives place them in good position to educate patients about AMR and proper antibiotic use. Despite some skepticism about reducing antibiotic prescription in their practice, most doctors express willingness to act if the managing DHC prioritizes the same in their directions to CHCs.
Our qualitative study displays strengths in triangulation, combining in-depth interviews and focus group discussions, involving doctors, doctor associates, and pharmacists, with data collectors from various disciplines, ensuring diverse perspectives. Data coding was robust with independent analysis by a pharmacist and an anthropologist. The use of the Theoretical Domains Framework added structure to the analysis. However, it is important to note that the tool was not incorporated into the formulation of the interview and discussion guides, which may have restricted our capacity to explore unintended yet consequential themes. Nevertheless, the study opted for open-ended enquiry, addressing salient facets of antibiotic prescribing within regular practice. Another limitation is that interview transcripts were not returned to participants for confirmation. To address this, we sought for clarifications when responses were unclear or contradictory during interviews and focus group discussions, and cross-checked information with different participants for confirmation. For four interviews where recording was not allowed, we used field notes to meticulously document participants' discussions. Finally, given the nature of a qualitative study, we cannot assume that the number of participants mentioning specific points signifies the importance or salience of the theme. Future quantitative research would address this.
Conclusion
This study demonstrates that primary care workers' antibiotic prescribing behavior is shaped by a combination of interconnected capability, opportunity, and motivation factors. Barriers to the appropriate use of antibiotics include insufficient comprehension of AMR and the need for prudent antibiotic usage, limited use of guidelines, inadequate medication supplies, complex and conflicting health insurance regulations, insufficient financing, doctor-patient relationship, and patient demand. In contrast, willingness to act and extended experience in patient communication position primary care workers well to address AMR in their communities. Utilizing the Theoretical Domains Framework to systematically analyze and present these behavioral determinants offers a structured foundation for designing impactful antimicrobial stewardship interventions in primary care. The findings underscore the importance of not only enhancing knowledge and skills but also implementing environmental restructuring, regulation, and enablement measures to effectively tackle unnecessary antibiotic prescribing in this context.
Supplementary Information
Acknowledgements
The authors wish to thank the National Institute of Hygiene and Epidemiology, Department of Health in Ha Nam and Nam Dinh province, coordinators in Hai Hau, Xuan Truong, Nghia Hung, Thanh Liem, and Binh Luc district for their support and assistance with this study. We thank participants for their insights and time.
Author contributions
DVM, YNTH, JVN, SN, NDTT, HA, HTH, and SL contributed to conceptualization and methodology; TNTC and YNH contributed to piloting the interview guide; DVM, YNTH, NNV, NDTT, HCNT, and HTT were responsible for data collection; DVM and YNTH analyzed data; NNY, HDTT, and VKT was responsible for study administration; Collective Action against AMR investigators contributed to funding acquisition and supervision; DVM drafted and finalized the manuscript; SL, SN, NDTT, HVTL, and YNTH reviewed the manuscript.
Funding
This research was funded Wellcome Africa Asia Programme Grant (2015–2022) [Grant Number: 106680/Z/14/Z] and Wellcome Trust [Grant Number: 213920/Z/18/Z). SN is funded by a Wellcome Trust Senior Fellowship awarded to Professor Mike English [Grant Number: 207522].
Data availability
Data cannot be shared openly but are available on request from authors.
Declarations
Ethics approval and consent to participate
Ethics approval was obtained from relevant ethical review committees prior to conduct of this study: Hanoi University of Public Health Ethics Committee (HUPH, Reference number: 392/2019/YTCC-HD3), Oxford University Tropical Research Ethics Committee (OxTREC, Reference number: 52819), and National Institute of Hygiene and Epidemiology (NIHE, Reference: HDDD-30/2019).
Competing interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The World Bank, Drug-resistant infections: A threat to our economic future. International Bank for Reconstruction and Development, Editor. 2017: Washington DC. [PubMed]
- 3.Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119(6, Supplement 1):S3–10. [DOI] [PubMed] [Google Scholar]
- 4.Cantón R, Morosini MI. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol Rev. 2011;35(5):977–91. [DOI] [PubMed] [Google Scholar]
- 5.Goossens H, et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet (London, England). 2005;365(9459):579–87. [DOI] [PubMed] [Google Scholar]
- 6.Ardillon A, et al. Inappropriate antibiotic prescribing and its determinants among outpatient children in 3 low- and middle-income countries: a multicentric community-based cohort study. PLoS Med. 2023;20(6): e1004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saleem Z, et al. Pattern of inappropriate antibiotic use among hospitalized patients in Pakistan: a longitudinal surveillance and implications. Antimicrob Resist Infect Control. 2019;8(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costelloe C, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340: c2096. [DOI] [PubMed] [Google Scholar]
- 9.WHO, Antimicrobial resistance and primary care. 2018. Available from: https://www.who.int/docs/default-source/primary-health-care-conference/amr.pdf
- 10.Lam TT, et al. What are the most effective community-based antimicrobial stewardship interventions in low- and middle-income countries? A narrative review. J Antimicrob Chemother. 2021;76(5):1117–29. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira Rodrigues A, et al. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–12. [DOI] [PubMed] [Google Scholar]
- 12.Dyar OJ, et al. How can we improve antibiotic prescribing in primary care? Expert Rev Anti Infect Ther. 2016;14(4):403–13. [DOI] [PubMed] [Google Scholar]
- 13.Borek AJ, et al. How can behavioural science contribute to qualitative research on antimicrobial stewardship in primary care? JAC-Antimicrob Resis. 2022;4(1):dlac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Monetary Fund, Vietnam. 2023 [cited 2023 December 27th]; Available from: https://www.imf.org/external/datamapper/profile/VNM.
- 15.Taylor-Robinson A, Quan NK. Vietnam’s Evolving healthcare system: notable successes and significant challenges. Cureus. 2023;15. [DOI] [PMC free article] [PubMed]
- 16.Vietnam Ministry of Health, Indicators of Population, Economics, Society, and Environment. 2020 [cited 2024 10th April]; Available from: https://moh.gov.vn/thong-ke-y-te?p_p_id=useryearbook_WAR_yearbookserviceportlet&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&p_p_col_id=row-0-column-2&p_p_col_count=1&_useryearbook_WAR_yearbookserviceportlet_jspPage=%2Fhtml%2Fyearbook%2Fuser%2Fview.jsp&_useryearbook_WAR_yearbookserviceportlet_yearbookId=1009&_useryearbook_WAR_yearbookserviceportlet_datatableGroupId=409.
- 17.Vietnamplus.vn. Over 92% of Vietnam’s population covered by health insurance: VSS. 2023 [cited 2023 December 27]; Available from: https://en.vietnamplus.vn/over-92-of-vietnams-population-covered-by-health-insurance-vss/246914.vnp.
- 18.Torumkuney D, et al. Country data on AMR in Vietnam in the context of community-acquired respiratory tract infections: links between antibiotic susceptibility, local and international antibiotic prescribing guidelines, access to medicines and clinical outcome. J Antimicrob Chemother. 2022;77(Supplement_1):i26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nga DTT, et al. Antibiotic sales in rural and urban pharmacies in northern Vietnam: an observational study. BMC Pharmacol Toxicol. 2014;15(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen KV, et al. Antibiotic use and resistance in emerging economies: a situation analysis for Viet Nam. BMC Public Health. 2013;13(1):1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsson M, et al. Overprescribing of antibiotics to children in rural Vietnam. Scand J Infect Dis. 2005;37(6–7):442–8. [DOI] [PubMed] [Google Scholar]
- 22.Hoang NH, et al. Current use of antibiotics among Vietnamese people in the first level of healthcare system in Nam Dinh Province. Am J Public Health Res. 2019;7(3):87–93. [Google Scholar]
- 23.Nam Vinh N, et al. Outpatient antibiotic prescribing for acute respiratory infections in Vietnamese primary care settings by the WHO AWaRe (Access, Watch and Reserve) classification: an analysis using routinely collected electronic prescription data. Lancet Reg Health Western Pacific. 2023;30: 100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pope C, Mays N. Qualitative Research in Health Care. Newark: Wiley; 2020. [Google Scholar]
- 25.Atkins L, et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. 2017;12(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flannery C, et al. Enablers and barriers to physical activity in overweight and obese pregnant women: an analysis informed by the theoretical domains framework and COM-B model. BMC Preg Childbirth. 2018;18(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangurian C, et al. Utilization of the Behavior Change Wheel framework to develop a model to improve cardiometabolic screening for people with severe mental illness. Implement Sci. 2017;12(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson M, et al. Using the theoretical domains framework and the behavioural change wheel in an overarching synthesis of systematic reviews. BMJ Open. 2019;9(6): e024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sargent L, et al. Using theory to explore facilitators and barriers to delayed prescribing in Australia: a qualitative study using the Theoretical Domains Framework and the Behaviour Change Wheel. BMC Fam Pract. 2017;18(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vietnam Government, Vietnam National Strategies on Drug Resistance 2023 - 2030, vision to 2045. 2023.
- 32.Vietnam Ministry of Health, Decision 5631/QD-BYT Guidance on implementing antimicrobial stewardship in hospital. 2020.
- 33.Vietnam Ministry of Health, Decision 2115/QD-BYT Handbook on Implementing antimicrobial stewardship programs in district hospital. 2023.
- 34.Wood F, et al. Primary care clinicians’ perceptions of antibiotic resistance: a multi-country qualitative interview study. J Antimicrob Chemother. 2012;68(1):237–43. [DOI] [PubMed] [Google Scholar]
- 35.Mubarak N, et al. How are we educating future physicians and pharmacists in Pakistan? A survey of the medical and pharmacy student’s perception on learning and preparedness to assume future roles in antibiotic use and resistance. Antibiotics. 2021;10(10):1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pechère JC. Patients’ interviews and misuse of antibiotics. Clin Infect Dis. 2001;33(Supplement_3):S170–3. [DOI] [PubMed] [Google Scholar]
- 37.Spurling GKP et al, Immediate versus delayed versus no antibiotics for respiratory infections. Cochrane Database System Rev. 2023;10. [DOI] [PMC free article] [PubMed]
- 38.Do N et al. Community-based antibiotic access and use in six low-income and middle-income countries: a mixed-method approach. Lancet Glob Health. 2021;9. [DOI] [PMC free article] [PubMed]
- 39.Vietnam Ministry of Health, Circular 21/2019/TT-BYT Guidance on piloting Family Medicine practice . 2019.
- 40.Quy, H., Trạm y tế hoạt động theo nguyên lý y học gia đình: Mô hình mới - hiệu quả mới, in Dân tộc và Phát triển. 2019. Available from: https://baodantoc.vn/tram-y-te-hoat-dong-theo-nguyen-ly-y-hoc-gia-dinh-mo-hinh-moi-hieu-qua-moi-42213.htm
- 41.vietnam.vn. The Minister of Health explained and clarified the shortage of drugs and medical supplies. 2023. Available from: https://www.vietnam.vn/en/bo-truong-y-te-giai-trinh-lam-ro-tinh-trang-thieu-thuoc-vat-tu-y-te/.
- 42.Vietnam Ministry of Health, Circular 07/2024/TT-BYT Regulations on drug bidding at public health facilities. 2024.
- 43.McKinn S, et al. Drivers of antibiotic use in Vietnam: implications for designing community interventions. BMJ Glob Health. 2021;6(7): e005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lum EPM, et al. Antibiotic prescribing in primary healthcare: Dominant factors and trade-offs in decision-making. Infect Dis Health. 2018;23(2):74–86. [DOI] [PubMed] [Google Scholar]
- 45.Peck J, et al. Caring for the commons: using psychological ownership to enhance stewardship behavior for public goods. J Mark. 2020;85(2):33–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared openly but are available on request from authors.