Abstract
In most prion diseases, infectivity accumulates in lymphoreticular organs early after infection. Defects in hematopoietic compartments, such as impaired B-cell maturation, or in stromal compartments, such as abrogation of follicular dendritic cells, can delay or prevent lymphoreticular prion colonization. However, the nature of the compartment in which prion replication takes place is controversial, and it is unclear whether this compartment coincides with that expressing the normal prion protein (PrPc). Here we studied the distribution of infectivity in splenic fractions of wild-type and fetal liver chimeric mice carrying the gene that encodes PrPc (Prnp) solely on hematopoietic or on stromal cells. We fractionated spleens at various times after intraperitoneal challenge with prions and assayed infectivity by bioassay. Upon high-dose challenge, chimeras carrying PrPc on hematopoietic cells accumulated prions in stroma and in purified splenocytes. In contrast, after low-dose challenge ablation of Prnp in either compartment prevented splenic accumulation of infectivity, indicating that optimal prion replication requires PrPc expression by both stromal and hematopoietic compartments.
Prion diseases are invariably lethal, transmissible neurodegenerative conditions that affect humans and many animal species. The causative infectious agent, termed prion (37), was proposed to be identical with PrPSc, a pathological conformer of the cellular protein PrPc encoded by the cellular gene Prnp.
While the central nervous system is the only site of histologically discernible damage, PrPc is expressed in many other sites, notably including lymphocytes. Intracerebral (i.c.) or peripheral administration of prions to mice causes a rise of infectivity in spleen and in other lymphatic organs long before the development of neurological symptoms and neuropathological changes (21). Moreover, peripheral inoculation routes are likely to initiate most forms of spongiform encephalopathies such as sheep scrapie, bovine spongiform encephalopathy, iatrogenic Creutzfeldt-Jakob disease (CJD) and new-variant CJD. Intraperitoneal (i.p.) inoculation has been extensively used to study the pathogenesis of transmissible spongiform encephalopathies because it causes rapid accumulation of infectivity in secondary lymphatic organs (12, 16, 17). The immune system is important for pathogenesis: development of scrapie disease after i.p. inoculation, in contrast to i.c. challenge, is impaired in SCID mice (30) and B-cell-deficient mice (24) and, to a lesser extent, after splenectomy (21). These and many other studies argue for an active role of the lymphoreticular system in the transport of scrapie infectivity from the periphery to the central nervous system.
The question of which compartments within lymphoreticular tissues support prion replication is of immediate relevance to public health: contamination with new-variant CJD prions of germinal centers in lymph node and tonsillar follicles, for example, might call for precautionary measures in handling, reusage, and sterilization of surgical instruments. Conversely, infection of germinal center lymphocytes with prions may raise the question of whether these cells carry infectivity into the bloodstream, a question of great importance to transfusion medicine (1).
Here we have investigated the role of various spleen cell subsets in the preclinical phase of mouse scrapie. We demonstrate that—within the potential limitations of experiments with fetal liver chimeras—prion infectivity can be associated with splenic lymphocytes devoid of Prnp and that congruently with previous results (5), chimeras of PrP-deficient hosts with PrP-expressing hematopoetic cells are able to accumulate chronically prions in the spleen for at least 200 days after inoculation. Furthermore, we show that efficient lymphoreticular prion propagation requires PrPc in stromal and hematopoietic cells.
MATERIALS AND METHODS
Preparation of the RML standard inoculum.
Rocky Mountain Laboratory (RML; passage 4.1) mouse-adapted scrapie prion inoculum was prepared from brains of terminally sick CD-1 mice (incubation time, 154 ± 12 days) as described previously (10). Brains (11.5 g, total) of terminally sick mice were pooled and homogenized with 1.2- and 0.7-mm syringes in 103.5 ml of sterile 0.32 M sucrose (without heat inactivation or Sarkosyl treatment). This 10% homogenate was defined as the standard RML inoculum. To determine the infectivity titer, serial 10-fold dilutions of our standard inoculum were prepared in sterile phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA) and injected i.c. into 4 tga20 indicator mice (15) (30 μl per mouse). The titer of the standard inoculum (7.9 log10 of 50% lethal dose [LD50]/ml, corresponding to 8.9 log LD50/g of brain tissue) was determined by the 50% endpoint calculation method (42). The relationship y = 11.45 − 0.088x (y, log LD50 per milliliter of homogenate; x, incubation time in days to terminal disease) was calculated by linear regression (38). All animal experiments were performed according to the law of the Kanton of Zürich and were approved by the Committee on Animal Experimentation.
Construction of fetal liver chimeric mice.
Eight-week-old mice were reconstituted with lymphohemopoietic stem cells (LSCs) derived from fetal livers. Timed pregnancies of wild-type and Prnpo/o mice served to produce mouse embryos. Fetal livers were collected at embryonic day 14.5 to 15.5 in Dulbecco's modified Eagle's medium (DME) and dissociated using 1.2- and 0.7-mm syringes. After a short spin (10 s, 170 × g), the supernatant containing fetal liver cells (FLCs) was collected and diluted in DME. FLCs (2 × 107 to 3 × 107 cells) were injected into the recipient's tail vein (injection volume, 100 μl). Recipients had been lethally irradiated with 900 rad 24 h earlier. Prnp+/+ ([129/Sv × C57BL/6]F1) and Prnpo/o (10) ([129/Sv × C57BL/6]Fn) mice served as recipients and as donors.
Flow cytometric analysis of PBLs of fetal liver cell-reconstituted mice.
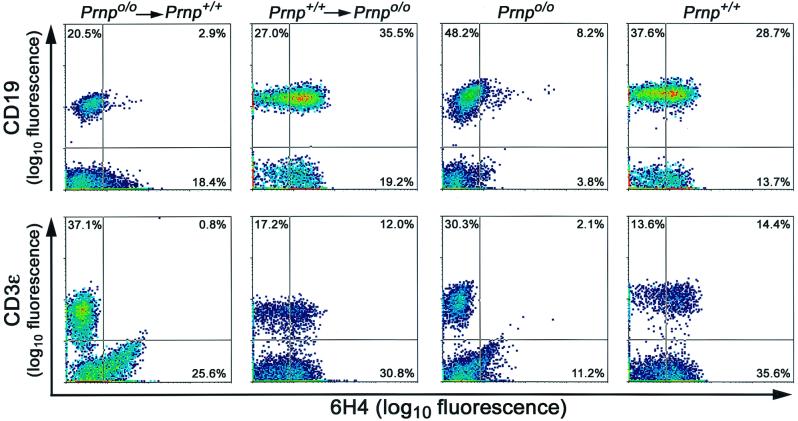
Six to eight weeks after grafting, successful reconstitution was assessed by the PrP status of B and T cells (Fig. 2). Peripheral blood lymphocytes (PBLs) were incubated with monoclonal 6H4 mouse anti-PrP antibody (28) (Prionics) diluted 1:200 in fluorescence activated cell sorter (FACS) buffer (PBS, 2% fetal calf serum, 10 mM EDTA [pH 8], 15.3 mM NaN3) and detected with fluorescein isothiocyanate (FITC)-goat anti-mouse immunoglobulin G1 (IgG1). Cells were separated in two fractions and additionally stained for B or T cells. We used phycoerythrin (PE)-anti-mouse CD19 (clone 1D3) for B cells and PE-anti-mouse CD3ɛ (clone 500A2) antibodies for T cells. Lysis of the red blood cells was performed with FACS lysing solution (Becton Dickinson) according to the manufacturer's protocol. All data were acquired on a FACSCalibur (Becton Dickinson). Analyses were done with CELLQuest (Becton Dickinson), WinList (Verity Software House, Inc.), and WinMDI 2.8 (Scripps Research Institute; http://facs.scripps.edu) analysis software. PBLs of Prnpo/o and of wild-type mice as well as omission of primary antibody and unstained cells served as controls. A total of 66 successfully reconstituted and control mice (Table 1) were inoculated i.p. with 100 μl of RML inoculum containing either 6 or 3 log LD50 of prions.
FIG. 2.
Flow cytometric analysis of PrPc expression on PBLs. Reconstitution resulted consistently in CD19-positive B and CD3ɛ-positive T lymphocytes of graft origin, as confirmed by the expression of PrPc and detected by 6H4 antibody staining. Numbers indicate the percentage of cells in the quadrants. Analyses were done on WBCs 6 to 8 weeks after the grafting procedure described. Ordinate, logarithm of fluorescence intensity for B cells (CD19) or T cells (CD3ɛ); abscissa, logarithm of fluorescence intensity for PrP (6H4).
TABLE 1.
Prion load of spleens in individual FLC-reconstituted mice
| Mice | RML inoculum (log LD50) | Days after inoculation | No. of animals inoculated | Splenic infectivity (log LD50) | No. of indicator animals succumbing to scrapie/no. inoculated (mean incubation time ± SD [days]) |
|---|---|---|---|---|---|
| Prnp+/+→Prnpo/o | 6 | 34 | 2 | 5.5, <1.5a | 4/4 (80.8 ± 9.0), 2/8 (133, 135) |
| 60 | 3 | 5.6, 5.0, 4.3 | 4/4 (80.3 ± 4.5), 4/4 (87.5 ± 9.1), 3/3 (95.7 ± 12.2) | ||
| 90 | 3 | NDb | ND | ||
| 200 | 4 | 5.0, 4.9, 4.0, ND | 4/4 (88.8 ± 8.1), 4/4 (89.3 ± 5.9), 4/4 (101.5 ± 9.0), ND | ||
| Prnp+/+→Prnpo/o | 3 | 34 | 3 | <1.5, <1.5, ND | 0/4, 0/3, ND |
| 60 | 3 | <1.5, <1.5, <1.5 | 0/4, 0/3, 0/3 | ||
| 90 | 3 | ND | ND | ||
| 360 | 5 | ND | ND | ||
| Prnpo/o→Prnp+/+ | 6 | 34 | 3 | 7.1, 6.1, ND | 4/4 (65.8 ± 3.5), 4/4 (77.0 ± 3.8), ND |
| 60 | 2 | 6.6, 6.5 | 3/3 (71.0 ± 11.1), 4/4 (72.3 ± 3.0) | ||
| 90 | 2 | 6.6, 6.1 | 4/4 (69.5 ± 7.9), 3/3 (75.3 ± 5.5) | ||
| 200 | 2 | 7.0, 7.2 | 4/4 (64.8 ± 1.5), 4/4 (62.0 ± 2.3) | ||
| Prnpo/o→Prnp+/+ | 3 | 34 | 3 | <1.5, <1.5, <1.5 | 0/4, 0/4, 0/4 |
| 60 | 2 | ND | ND | ||
| 90 | 2 | ND | ND | ||
| 360 | 2 | <1.5, ND | 0/4, ND | ||
| Prnpo/o→Prnpo/o | 6 | 34 | 3 | <1.5, <1.5,a ND | 0/4, 3/4 (155, 163, 178),c ND |
| 60 | 2 | <1.5, <1.5 | 0/4, 0/4 | ||
| 200 | 2 | ND | ND | ||
| Prnpo/o→Prnpo/o | 3 | 360 | 5 | ND | ND |
| Prnp+/+ | 6 | 34 | 2 | 6.5, 5.3 | 4/4 (69.0 ± 2.0), 3/3 (83.0 ± 6.2) |
| 200 | 3 | ND | ND | ||
| Prnp+/+ | 3 | 34 | 2 | 5.8, 5.8 | 3/3 (77.3 ± 5.9), 4/4 (77.3 ± 4.7) |
| 230 | 3 | ND | ND |
If one or more animals survived >180 days, the titer was assumed to be close to the detection limit of the bioassay (<1.5).
ND, not determined.
The development of scrapie disease in these indicator animals is most likely due to residual inoculum of the primary inoculation.
Immunofluorescence analyses of spleen sections.
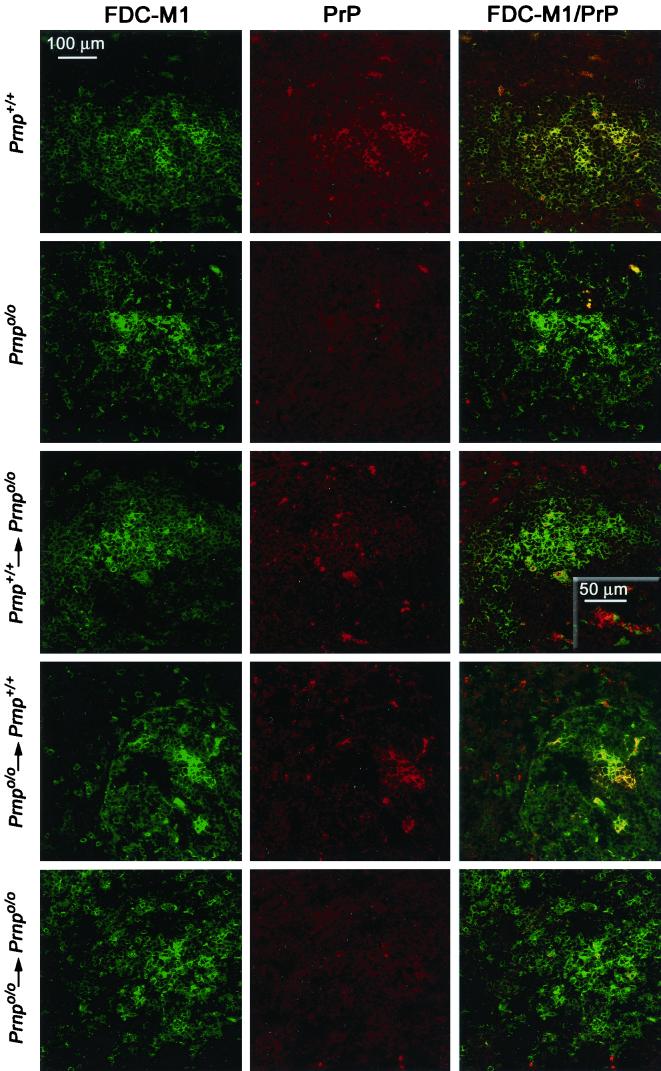
At various time points (34, 60, 90, 200, and 360 days), groups of two to three animals per experimental setup were analyzed. Spleens were sampled, and tissue for preparation of whole spleen homogenates, immunofluorescence analyses, and cellular fractions was collected. Two-color immunofluorescence of frozen sections was performed by using rat anti-mouse FDC-M1 antibody (29) (clone 4C11; 1:300 in PBS–0.15% BSA; a gift from M. Kosco-Vilbois, Glaxo-Wellcome Research and Development S.A., Plan-les-Ouates, Switzerland) for follicular dendritic cells (FDCs) and a polyclonal rabbit anti-mouse PrP serum (XN; 1:1,000 in PBS–0.15% BSA). Primary antibodies were detect with FITC-F(ab′)2 fragments of goat anti-rat IgG (heavy plus light chain [H+L]) (1:100 in PBS) or Alexa 546-goat anti rabbit IgG (H+L) (1:500 in PBS). Omission of primary antibodies or rabbit preimmune serum served as a control. Sections were then analyzed on an immunofluorescence microscope, and representative samples were selected for confocal laser scanning microscopy (Fig. 3) with Leica model DM IRB E inverted microscope and confocal laser scanning system TCS4D.
FIG. 3.
Confocal double-color immunofluorescence analysis of splenic germinal centers of reconstituted and inoculated mice and noninoculated controls. Thirty-four days after i.p. scrapie challenge, spleen sections of fetal liver chimeras were stained with FDC-M1 antibody to FDCs (green color, left column) and XN polyclonal antiserum to PrP (red color, middle column). Noninoculated Prnp+/+ ([129Sv × C57BL/6]F1) and Prnpo/o ([129Sv × C57BL/6]Fx) mice served as controls. In spleens of noninoculated wild-type mice or prion-infected wild-type mice grafted with Prnpo/o FLCs (Prnpo/o→Prnp+/+), the signal for PrP is confined to FDC-M1-positive areas (yellow color, first and fourth rows). Instead, Prnpo/o mice reconstituted with Prnp+/+ FLCs (Prnp+/+→Prnpo/o) show PrP-positive cells both in FDC-M1-positive areas (yellow color, third row) and in FDC-M1-negative compartments surrounding the follicular dendritic network (red color, third row).
Preparation of total spleen homogenates and splenic cellular and stromal fractions.
To prepare total spleen homogenates, spleen tissue was hand-homogenized as a 10% (wt/vol) suspension in 0.32 M sucrose with a microhomogenizer (Pellet Mixer 1.5 ML; Treff AG) for 10 to 15 min, then passed several times through 0.7-mm needles, and spun for 1 min at 230 × g in 1.5-ml Eppendorf tubes. The supernatant was diluted 10 times with PBS–5% BSA to produce a 1% homogenate.
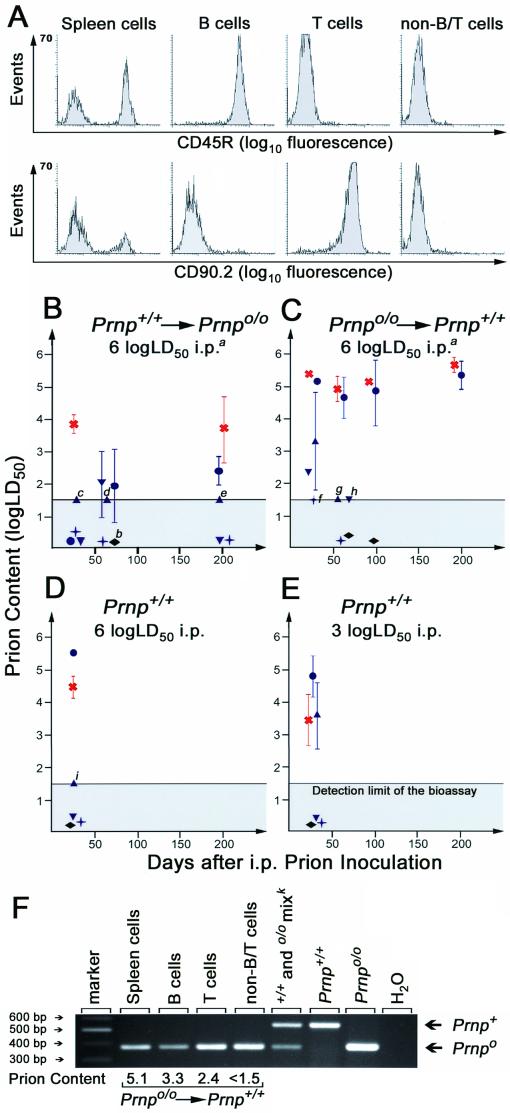
Spleen samples of mice belonging to the same reconstitution group and killed after the same incubation time were pooled to produce cellular fractions. Spleen cells were obtained by forcing spleens through a 40-μm-mesh nylon net into a petri dish prefilled with DME. The stromal fraction was recovered from the net by scraping, and a 10% (wt/vol) homogenate in PBS–5% BSA containing 0.1% Sarkosyl was prepared by forcing the suspension through an 1.2-mm needle. The homogenate was digested with collagenase D (1 mg/ml) for 30 min at 37°C, sonicated (Branson Sonifier; maximal output) 7 to 14 times for 2 min in 1.5-ml Micro Tubes, and then diluted 10 times with PBS–5% BSA to give a 1% (wt/vol) homogenate. Spleen cells were centrifuged (300 × g, 10 min, 4°C), counted, and dissolved in magnetic cell sorting (MACS) buffer (PBS, 0.5% BSA, 2 mM EDTA). To purify B cells, spleen cells were incubated with Mouse CD45R (B220) MicroBeads (catalog no. 495-01; Miltenyi Biotec Inc.) according to the manufacturer's protocol. After washing, cells were resuspended in MACS buffer and applied to a prefilled and washed MACS VS+ separation column (Miltenyi Biotec) fixed onto a VarioMACS (Miltenyi Biotec). Unlabeled cells passed through and were collected as the negative fraction. The column was removed from the VarioMACS, and B cells were flushed out. For purification of T cells, the negative fraction recovered from the B-cell sorting was incubated with mouse CD90.2 (Thy-1.2) MicroBeads (catolog no. 491-01; Miltenyi Biotec), and T cells were purified as described above. Unlabeled cells were again rinsed out with MACS buffer and collected as non-B/T cells. All purified cells were counted and diluted in the appropriate volume of PBS–5% BSA to adjust the concentration to 2 × 105 or 106 cells per 30 μl, depending on the number of total cells. The purity of the collected cell fractions was confirmed by FACS analysis (Fig. 4A). Each splenic fraction was labeled for B cells (PE-anti-mouse CD45R) and for T cells (PE-anti-mouse CD90.2); unstained samples served as controls. Data were acquired with a MoFlo cell sorter (Cytomation). Analyses were done with WinList.
FIG. 4.
Distribution of prions in spleens of scrapie-infected mice. (A) FACS analysis of MACS-purified spleen cell fractions originating from scrapie-infected mice. MACS sorting resulted in >97% of the sorted cell type; therefore cross-contamination could be excluded to a large extent. Anti-CD45R (B220) antibodies were used to label B cells, and anti-CD90.2 (Thy 1.2) was used for T cells. Gating for lymphocytes by forward and side scattering did not interfere with the readout. (B to E) Infectivity levels in splenic fractions and WBCs of FLC-reconstituted and control mice. Fractions were transmitted i.c. in groups of three to four tga20 indicator mice. Symbols: blue circles, spleen cells; blue triangles, B cells; blue inverted triangles, T cells; blue crosses, non-B/T cells; red crosses, stromal fraction; black rhombi, WBCs. Within-group standard deviations are indicated only if they exceed ±0.15 log LD50. Infectivity contents relate to each cellular fraction of individual spleens. (Footnotes) a, similar groups of cell subsets were transmitted after primary inoculation with 3 log LD50; all these fractions were devoid of prions. b, in each of the two separate transmissions of the stromal fraction of this group, all four indicator animals died of intercurrent death within 72 h after i.c. administration, most likely due to brain emboli or toxic contaminations of this homogenate. If one or more animals survived >180 days, the titer was assumed to be close to the detection threshold of the bioassay. For these samples (labeled with small italic letters), the number of animals succumbing to scrapie out of the number of indicator animals inoculated and, in parentheses, incubation time (days) to terminal scrapie in individual mice were as follows: c, one of three (157); d, two of four (110, 116); e, one of four (128); f one of four (115); g, two of four (90, 145); h, two of four (87, 90); i, two of three (90, 93). (F) PCR of spleen cells and splenic B, T, and non-B/T cells. Infectious splenic lymphocytes, derived 34 days after primary inoculation from Prnp+/+ mice reconstituted with Prnp null FLCs, were tested for the presence of the Prnp gene by PCR. Only the Prnpo/o genotype of reconstituting cells was identified in these infectious splenic cell fractions. Prion contents are indicated in log LD50 per fraction of each spleen. k, sample derived from spleen cells known to contain Prnp+/+ and Prnpo/o DNA.
Isolation of WBCs from whole blood.
White blood cells (WBCs) were isolated from whole blood by density gradient centrifugation using Lympholyte-M medium (Cedarlane Laboratories). At the time points indicated, blood was taken and samples of the same experimental group were pooled. Cells were adjusted to maximally 2 × 107 nucleated cells/ml in MACS buffer, and gradient centrifugation was performed as specified by the manufacturer (Cedarlane Laboratories). The lymphocyte layer at the interface was recovered, counted, and diluted to the appropriate concentration (2 × 105, 5 × 105, or 106 cells per 30 μl).
Infectivity bioassays with tga20 indicator animals.
All assays were done with 1% spleen homogenates, spleen cell purifications, stromal homogenates, and WBCs. Whole spleen homogenates (1%, wt/vol), spleen cells (106 cells), stromal fractions (1%, wt/vol), spleen cell purifications (B cells, T cells, and non-B/T cells; 2 × 105 or 106 cells per fraction), and WBCs (2 × 105 or 106 cells per fraction) were inoculated i.c. (30 μl of each) into groups of three or four tga20 indicator mice (15). Animals were monitored for neurological symptoms and killed after development of terminal scrapie. Scrapie disease in indicator mice was confirmed by typical histopathology. The relationship y = 11.45 − 0.088x (y, log LD50 per milliliter of homogenate; x, incubation time in days to terminal disease) was used to calculate the level of infectivity (15, 38) within these homogenates. Prion contents are indicated per spleen; spleen weights were calculated according to the determined weight of the stromal fraction, which was considered to be 10% of the weight of a total spleen (13). Spleen cells consist approximately of 80% lymphocytes (30% T cells and 70% B cells) and 20% nonlymphocytic cells (4, 6).
ELISA for detection of anti-PrP antibodies.
Sera were analyzed by indirect enzyme-linked immunosorbent assay (ELISA). Wells of 384-well plates were coated with 0.105 μg of recombinant PrP23–231 diluted in 50 μl of PBS overnight at 4°C. After three washes with ELISA buffer (PBS containing 0.1% Tween), the residual binding capacity of the plate was blocked with 100 μl of ELISA buffer containing 5% milk powder for 2 h at room temperature (RT). Extensive washing with ELISA buffer removed the unbound protein. Then the plates were incubated for 2 h at RT with three dilutions of each mouse serum (1:25, 1:75, and 1:225, in 50 μl of ELISA buffer containing 1% BSA), using three wells for every dilution. Plates were washed four times with ELISA buffer before incubating with the second antibody (horseradish peroxidase-conjugated rabbit anti-mouse IgG-IgA-IgM [H+L]; 0.075 μg per well in 50 μl of ELISA buffer–1% BSA for 2 h at RT). The plates were developed with 50 μl of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS; 5 mg of ABTS in 50 ml of 0.1 M NaH2PO4) [pH 4] containing 0.055% H2O2 for 50 min at RT, and optical density was then measured with a LAMBDA E ELISA reader (MWG Biotech AG) at 405 nm. The antibody titer in the serum of a terminally sick 129Sv × C57BL/6 mouse was considered to be the background titer. Omission of the sera, omission of the secondary antibody, uncoated wells, dilutions of 6H4 antibody (Prionics) (28), and a serum of a Prnp-null mouse with a PrPc-expressing brain graft (8) known to contain high levels of anti PrP antibodies served as controls.
PCR of splenic fractions.
Analyses were performed postmortem on spleen cells, B cells, T cells, and non-B/T cells. Cells (3 × 106) were digested with proteinase K (18 μg/ml) overnight at 55°C in 100 μl of digestion buffer (50 mM KCl, 10 mM Tris HCl [pH 9], 0.4% NP-40, 0.4% Tween 20). Proteinase K was inactivated for 12 min at 75°C, and the samples were centrifuged at 5,000 × g for 12 min. PCR was carried out with primers P3 (ATT CGC AGC GCA TCG CCT TCT ATC GCC), complementary to the 3′ end of the Neo cassette of the knockout allele; P10 (GTA CCC ATA ATC AGT GGA ACA AGC CCA GC), complementary to the 5′ end of the Prnp reading frame; and 3′nc (ATA ACC CCC TCC CCC AGC CTA GAC CAC GAG), complementary to genomic sequences adjacent to the 3′ end of the Prnp reading frame (15). PCR conditions were 94°C for 5 min 30 s, then 35 cycles of 94°C for 45 s, 55°C for 45 s, and 72°C for 1 min, followed by extension at 72°C for 7 min. Amplification with primer pair P10-3′nc resulted in a 550-bp DNA fragment representing the Prnp+ allele; amplification with primer pair P3-3′nc resulted in a 350-bp DNA fragment showing the presence of the Prnp0 allele.
RESULTS
Establishment of a standard prion inoculum.
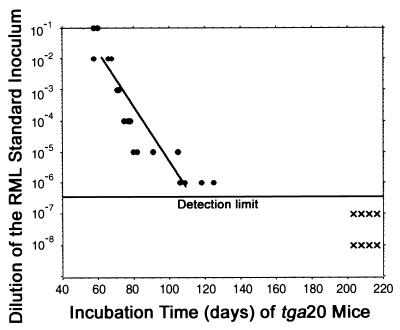
All inoculation experiments described in this study were performed with the RML 4.1 prion inoculum, which was derived by fourfold serial passage in CD-1 mice of a brain homogenate originally donated by S. Prusiner (San Francisco, Calif.). This homogenate originates from the Chandler mouse-adapted scrapie strain. The prion titer of the RML 4.1 inoculum was determined by the endpoint dilution assay. We transmitted serial dilutions (10−1 to 10−8 in log10 steps) of the isolate into tga20 indicator mice (Fig. 1). We calculated the level of infectivity according to the 50% endpoint (42) and found it to be 8.9 log LD50/g of brain tissue. Because the standard inoculum was derived by diluting brain homogenate 1:10 in 0.32 M sucrose, the titer of the inoculum is 7.9 log LD50/ml. The transmission results evidenced a linear relationship between the logarithmic size of inoculum and incubation time over a dilution range of 10−2 to 10−6 (Fig. 1) according to the following empirically derived formula: y = 11.45 − 0.088x (y, log LD50 per milliliter of homogenate; x incubation time in days to terminal disease) (38). This relationship was then used to calculate the level of prions in materials containing an unknown amount of infectivity.
FIG. 1.
Endpoint titration of the standard inoculum used for this study. Serially diluted brain homogenate of terminally sick CD-1 mice (30 μl) was injected i.c. into tga20 mice. Each circle represents one indicator mouse that succumbed to scrapie disease; each cross corresponds to a healthy animal. The 50% endpoint served to ascertain the titer of the 10% standard inoculum (42). The relationship y = 11.45 − 0.088x (y, log LD50 per milliliter of homogenate; x, incubation time in days to terminal disease) was determined by linear regression (38).
Expression of PrPc in lymphocyte subsets of fetal liver chimeras.
To produce chimeric mice expressing PrPc on lymphocytes but not on stromal cells such as FDCs, or vice versa, FLCs of the Prnpo/o or Prnp+/+ genotype, were administered intravenously to Prnpo/o or Prnp+/+ mice that had been lethally irradiated.
Radioresistant splenic cells, including FDCs, were expected to display the PrP phenotype of the host (49), whereas cells derived from the incoming LSCs showed the PrP phenotype of the FLC donors. This was studied by flow cytometric analysis of PBLs 6 to 8 weeks after grafting using monoclonal anti-PrP antibody 6H4 (Fig. 2) (28). Reconstitution of Prnpo/o mice with Prnp+/+ FLCs (creating Prnp+/+→Prnpo/o mice) yielded 35.5% 6H4-positive B lymphocytes and 12.0% 6H4-positive T lymphocytes in peripheral blood, and 6H4 two-color FACS profiles were superimposable to those of wild-type mice. In contrast, wild-type mice reconstituted with PrP deficient FLCs (Prnpo/o→Prnp+/+ mice) exhibited 2.9% 6H4-positive B cells and 0.8% 6H4-positive T cells when the same instrument settings and gates were used (Fig. 2). The latter findings were identical to the staining pattern of Prnpo/o PBLs and were most probably due to a small amount of nonspecific binding of the 6H4 antibody to lymphocytes.
PrP topography in spleens of scrapie-infected chimeras.
Groups of 10 to 15 chimeric mice and corresponding controls (Table 1) were inoculated i.p. with either a saturating dose (1 mg of RML 4.1 brain homogenate diluted in 100 μl 0.32 M sucrose, corresponding to 6 log LD50 as calculated using the data shown in Fig. 1) or a limiting dose (3 log LD50) of RML scrapie prions. At various time points after inoculation (34, 60, 90, 200, and 360 days), two to three mice per group were analyzed. Cryoconserved spleen sections (10 μm in thickness) were stained with antibody FDC-M1 to FDCs (29) and polyclonal serum against PrP and then analyzed by immunofluorescence microscopy. Representative sections were selected for confocal laser scanning microscopy (Fig. 3). In spleens of noninoculated or scrapie-infected wild-type mice (naive or grafted with Prnpo/o FLCs), signals for FDCs and PrP colocalized precisely (Fig. 3, first and fourth rows) FDCs are situated in germinal centers: accordingly, the staining for PrP was confined to these structures. In contrast, Prnpo/o mice grafted with Prnp+/+ FLCs (Prnp+/+→Prnpo/o), which express PrPc on LSCs but not on stromal cells, displayed PrP-positive cells both in FDC-M1-positive areas and in FDC-M1-negative compartments that surrounded the germinal centers (Fig. 3, third row).
Distribution of prions in spleens of fetal liver chimeric mice.
The findings described above indicate that 6H4 immunoreactivity, at least in fetal liver chimeras, is not confined to germinal centers. We therefore set out to determine the nature of 6H4+ non-germinal-center cells and assess whether these cells are associated with prion infectivity in addition to PrP immunoreactivity.
Spleens were fractionated into stroma and spleen cells (here defined as cells that can be recovered as a suspension after passing spleens through a mesh). Spleen cells were then separated into B cells, T cells, and a non-B/T-cell fraction. Each fraction was tested for purity by FACS analysis with anti-CD45R (B220) and anti-CD90.2 (Thy1.2) antibodies. In each fraction, purity was >97% of the sorted cell type (Fig. 4A). Cross-contamination of the purified cell fractions (e.g., T cells contaminating the B-cell fraction, or vice versa) could be excluded to a large extent. Whole spleen homogenates and cell subsets were then transmitted i.c. to groups of 3 to 4 tga20 indicator mice. The relationship y = 11.45 − 0.088x (y, log LD50/per milliliter of homogenate; x, incubation time in days to terminal disease) was used to calculate the level of infectivity (8, 38) within these homogenates and, consequently, the distribution of prions within spleens.
In wild-type mice, prions replicate in secondary lymphatic organs and a plateau level of splenic infectivity is reached early after i.p. inoculation (20, 40). In this study, we recovered 5.3 to 6.5 log LD50 prions per spleen 34 days after inoculation with a saturating (6 log LD50) and a limiting (3 log LD50) dose (Table 1), indicating that both inoculum sizes were sufficient to establish chronic spleen infection of wild-type mice. In agreement with previous experiments (5) the infectious agent was captured and stored in spleens of Prnpo/o mice carrying LSCs from Prnp+/+ mice (Prnp+/+→Prnpo/o; Table 1). Prion amounts ranging between 4 and 5.5 log LD50 per spleen could be measured throughout a time frame of 34 to 200 days after peripheral inoculation with 6 log LD50. We observed only one exception: one spleen of a Prnp+/+→Prnpo/o chimera sacrificed 34 days after scrapie challenge contained very little prion infectivity (Table 1). When prion loads were measured in individual spleen cell fractions, the highest loads were detected in stromal fractions, whereas Prnp+/+ spleen cells contained only low amounts of prion infectivity (Fig. 4B). The prion load in MACS-sorted splenic B and T cells amounted to only 10- to 100 infectious units and was therefore close to the detection threshold of the assay. In the same experimental group (Prnp+/+→Prnpo/o), i.p. inoculation with 3 log LD50 of scrapie prions resulted in noninfectious spleens (Table 1).
In wild-type mice grafted with FLCs derived from Prnp-null (Prnpo/o→Prnp+/+) mice and inoculated with 6 log LD50 of primary inoculum, higher splenic infectivity loads than in the reciprocal (Prnp+/+→Prnpo/o) chimeras were detected at all time points. Infectivity rose to ca. 7 log LD50 per total spleen 34 days after i.p. challenge and stayed constant throughout the experimental observation time (Table 1). As in Prnp+/+→Prnpo/o chimeras, in Prnpo/o→Prnp+/+ fetal liver chimeras 3 log LD50 i.p. failed to produce disease in indicator animals (Table 1). In Prnpo/o→Prnp+/+ chimeras, stroma and PrP-deficient spleen cells contained similar amounts of scrapie prions, as assessed by bioassay with indicator animals (Fig. 4C): infectivity ranged from 4.8 to 5.8 log LD50 in both fractions. To exclude contamination of spleen cells with components of the stroma which might nonspecifically pollute fractions with prions, we performed PCR analyses of these subsets and transmitted purified B, T, and non-B/T cells to tga20 mice. Whereas B and T cells contained 3.3 and 2.4 log LD50 34 days after i.p. inoculation, very low amounts of infectivity could be recovered in the non-B/T cells, as well as in B- and T-cell fractions at later time points (Fig. 4C). PCR analysis of these infectious spleen cells, B cells, T cells, and non-B/T cells confirmed the Prnpo/o genotype of these fractions (Fig. 4F). Prions could not be detected in WBCs of any of the reconstituted mice or in WBCs of control mice (Fig. 4B to E).
Prion replication requires PrPc, and mice deficient in PrPc are unable to accumulate prion infectivity in the spleen and brain (10, 43). However, scrapie developed in three of four tga20 indicator animals challenged with a whole spleen homogenate derived from a Prnpo/o mouse that had been reconstituted with Prnpo/o FLCs 34 days after inoculation (Table 1). No infectivity was detected at later time points in any of the mice belonging to the same experimental group. This is in agreement with earlier findings (43) and is most probably explained by the occasional persistence of traces of residual inoculum.
Detection of anti-PrP antibodies in sera of inoculated mice.
We searched for anti-PrP antibodies in mice devoid of PrPc 200 days after inoculation to investigate whether the clearance of the infectious agent might be due to a specific immune response to the RML inoculum. The sera of seven PrP-deficient mice reconstituted with Prnpo/o FLCs were assayed. Only one chimera had a titer higher than two times above the background titer. Two additional mice had a titer close to twice the background titer. The remaining four animals did not display detectable anti-PrP antibody titers (data not shown).
DISCUSSION
The nature of cells replicating prions in the periphery, and the precise role of Prnp expression in these cells, is still unclear. A likely candidate for a lymphoreticular prion replication site is the FDC (9, 22, 36), but experimental evidence has so far not been conclusive. FDCs are sessile cells located in germinal centers of lymphoid organs which act as antigen traps and support affinity maturation of B lymphocytes. Although their origin remains controversial, there is general agreement that they are not significantly replaced by transfer of FLCs into adult mice (19, 49) but, due to the expression of fibroblast antigens (7), may originate from the fibroblastic reticulum. It has not been possible to recover prions from FDC-deficient spleens derived, e.g., from mice deficient in tumor necrosis factor alpha (TNF-α−/− mice) (33), nor could PrPSc be visualized in spleens of lymphotoxin β-deficient (LTβ−/−) mice (35). Notwithstanding the strong experimental evidence that FDCs are involved in prion pathogenesis, there are several findings that point to an additional role of lymphocytes in prion neuroinvasion after peripheral infection. While the presence of B cells is crucial for neuroinvasion after peripheral challenge (24), the main role of B cells in pathogenesis may consist of supporting FDCs by providing lymphotoxin signaling. In tumor necrosis factor receptor 1-deficient (TNFR1−/−) mice, which lack mature FDCs in their spleens (31), development of cerebral disease after peripheral challenge with RML scrapie is unaffected (24), and spleens of TNFR1−/− mice contain only traces of infectivity (M. A. Klein, F. Montrasio, M. Prinz, and A. Aguzzi, unpublished results). In addition, it was reported that LTβ−/− mice, which suffer from a similar defect in FDC maturation (26), may be as susceptible to infection with CJD prions as wild-type mice, although pathologic PrP could not be visualized in spleens and lymph nodes (35). However, the latter study may not be fully representative, since no transmission studies of spleens or lymph nodes were reported. Further, in vivo ablation of FDCs by administration of a soluble lymphotoxin β receptor, while efficiently preventing the buildup of a splenic prion burden (36), does not fully shelter the brain from neuroinvasion (32, 36).
In view of the uncertainties delineated above, we first assessed whether prions actively replicate, or just accumulate, at peripheral sites after i.p. infection. Regardless of the dose of the primary inoculum administered (6 or 3 log LD50 i.p.), wild-type spleens contained 5 to 7 log LD50 of scrapie infectivity at early time points after peripheral challenge (Table 1), yielding strong evidence for peripheral prion replication. This is in line with the data recorded in transgenic mouse lines showing peripheral replication of a scrapie agent in mice (41).
To investigate the significance of the PrP status of immune cells during peripheral pathogenesis of scrapie disease, we constructed reciprocal fetal liver chimeras with wild-type (Prnp+/+) mice and with a genetically altered mouse line of the Prnpo/o genotype. A limiting dose of prions (3 log LD50) never produced detectable levels of prion infectivity in spleens of animals with compartment-restricted Prnp expression, indicating that these chimeras were not able to effectively replicate or store the infectious agent. We therefore conclude that expression of PrPc both in LSCs and in a sessile, radioresistant cellular compartment (most likely consisting of FDCs) is needed for efficient splenic capture and replication of prion infectivity.
Spleens with PrPc expression confined to LSCs are able to chronically accumulate prions over a time course of at least 200 days after i.p. inoculation with a saturating dose of prions (6 log LD50). These findings confirm and extend our previously published experiments in which long-term prion persistence was detected in Prnpo/o mice bearing a Prnp+/+ brain graft and Prnp+/+ hematopoietic cells (5). Therefore, PrPc expression by the host is not absolutely required for splenic prion accumulation. This result is in contrast with the findings reported by Brown et al. (9), who performed similar experiments with ME7 prions, and may point to differences in the cellular tropism of different prion strains.
Astonishingly, the bulk of prion infectivity in Prnp+/+→Prnpo/o chimeras was located within the stromal compartment, even if the Prnp+/+ genotype was confined to hematopoietic cells. At least two hypotheses could explain this finding: (i) Prnp-deficient FDCs could acquire PrPc or prions from LSCs, or (ii) the Prnp+/+→Prnpo/o FLC reconstitution could have induced development of Prnp+ FDCs from hypothetical precursors within FLCs (19, 49), which may acquire and replicate prion infectivity. We consider the second hypothesis to be improbable since previous analyses do not indicate that FDCs can be efficiently reconstituted by FLCs (19, 49). Conversely, after i.p. challenge with a saturating dose of prions (6 log LD50), spleens of wild-type mice grafted with Prnpo/o FLCs contained 6.1 to 7.2 log LD50 of infectivity. The prion loads of spleen cells and stromal fractions were similar, i.e., 5.0 to 6.0 log LD50. Therefore, Prnp-deficient spleen cells (whose pure Prnpo/o genotype was confirmed by PCR analysis) were able to transmit relatively high infectivity titers to indicator animals. When separated B, T, and non-B/T cells of the same chimeras were transmitted, the resulting findings were consistent with this PrP-independent association of infectivity in spleen cells: highly purified B and T lymphocytes of Prnpo/o origin were able to infect indicator animals. Consequently, splenic lymphocytes of mice infected i.p. with large amounts of prions do not require a Prnp+ allele in order to transmit prions to indicator animals.
The mechanism of chronic spleen infection in mice with compartment-restricted expression of Prnp after challenging with a saturating dose of prions remains unclear. It is possible that under these circumstances prions undergo very slow splenic replication and reach an equilibrium with elimination of infectivity. PrPc is a glycophosphatidylinositol-anchored protein (46), and since these proteins can easily transfer from one cell to another (3, 27), it is conceivable that “painting” of the Prnpo/o compartment by PrPc-positive cells results in acquisition of a PrPc-positive phenotype and, possibly, in restored support of low-level prion replication.
Alternatively, the original inoculum may be chronically stored: other antigens, such as human immunodeficiency virus type 1 virions (44, 45) and human serum albumin (47), are known to be retained on the plasma membrane of FDCs for months or even years (23, 34, 48). The immunological or cellular mechanism of such retention may be independent of PrPc: molecules acting as partners of antigen presentation like complement factors (25) or Fcγ receptors (11) are likely to be involved. In fact, it has been recently reported that disease-specific PrPSc is detectable ultrastructurally on the surface of FDCs, where it colocalizes with complement and Fcγ receptors (18).
The mismatch between the level of infectivity in spleen cells and the sum of the corresponding subpopulations in all experimental groups is most probably due to the extensive washing procedure associated with MACS and could represent a consequence of the interaction between lymphocytes and prions. Therefore, the association of prions with lymphocytes may be rather loose.
After inoculation with 4.5 log LD50 of prions (30 times less than in the present report), infectivity was associated with spleen cells expressing PrPc but not with Prnpo/o spleen cells (40). The present results indicate that upon a primary i.p. inoculation with 6 log LD50, prions were associated with spleen cells and with highly purified lymphocyte fractions regardless of PrPc expression. This discrepancy points to a possible dose dependence of the Prnp-independent capture of prions by lymphocytes.
Furthermore, the present study shows that inoculation with 3 log LD50 of scrapie prions, which corresponds to a 30-fold dilution of the dose used by Raeber and colleagues (40), results in a loss of splenic infectivity both in fetal liver chimeras, with ablation of Prnp in the donor, and in reciprocal chimeras with a Prnpo/o recipient. To investigate whether the loss of prions in mice devoid of the Prnp gene might be due to a humoral immune response (2) to the prion protein, we screened sera for anti-PrP antibodies. In only one of seven animals, we found a titer that was more than twice the background titer. We conclude that the infectivity clearance in these animals is unlikely to be due to a persisting humoral immune response to PrP. While the precise mechanism of clearance remains unclear, removal of Prnp in either compartment appears to decrease the overall efficiency of the lymphoreticular phase of prion replication.
After replicating in the germinal center, prions find access to the central nervous system. As indicated in previous studies (39), peripheral nerves are likely to be the final common pathway for neuroinvasion. How interactions between the peripheral nervous system and FDCs occur (14) and whether lymphocytes may contribute to the transport of scrapie prions within the spleen remain to be elucidated.
ACKNOWLEDGMENTS
P. S. Kaeser and M. A. Klein contributed equally to this study.
This work is supported by the Kanton of Zürich, the Bundesamt für Bildung und Wissenschaft, and the Migros and Coop Foundations and by grants from the Swiss National Foundation.
We thank R. Zinkernagel, C. Weissmann, and F. Montrasio for critical reading of the manuscript; M. Höchli for help with confocal microscopy; and M. König for technical help.
REFERENCES
- 1.Aguzzi A. Prion diseases, blood and the immune system: concerns and reality. Haematologica. 2000;85:3–10. [PubMed] [Google Scholar]
- 2.Aguzzi A, Weissmann C. Spongiform encephalopathies. The prion's perplexing persistence. Nature. 1998;392:763–764. doi: 10.1038/33812. [DOI] [PubMed] [Google Scholar]
- 3.Anderson S M, Yu G, Giattina M, Miller J L. Intercellular transfer of a glycosylphosphatidylinositol (GPI)-linked protein: release and uptake of CD4-GPI from recombinant adeno-associated virus-transduced HeLa cells. Proc Natl Acad Sci USA. 1996;93:5894–5898. doi: 10.1073/pnas.93.12.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder D, Fehr J, Hengartner H, Zinkernagel R M. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185:517–530. doi: 10.1084/jem.185.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blättler T, Brandner S, Raeber A J, Klein M A, Voigtländer T, Weissmann C, Aguzzi A. PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature. 1997;389:69–73. doi: 10.1038/37981. [DOI] [PubMed] [Google Scholar]
- 6.Boes M, Esau C, Fischer M B, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–4787. [PubMed] [Google Scholar]
- 7.Bofill M, Akbar A N, Amlot P L. Follicular dendritic cells share a membrane-bound protein with fibroblasts. J Pathol. 2000;191:217–226. doi: 10.1002/(SICI)1096-9896(200006)191:2<217::AID-PATH586>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, Kobayashi Y, Marino S, Weissmann C, Aguzzi A. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 9.Brown K L, Stewart K, Ritchie D L, Mabbott N A, Williams A, Fraser H, Morrison W I, Bruce M E. Scrapie replication in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nat Med. 1999;5:1308–1312. doi: 10.1038/15264. [DOI] [PubMed] [Google Scholar]
- 10.Büeler H R, Aguzzi A, Sailer A, Greiner R A, Autenried P, Aguet M, Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 11.Carroll M C. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 12.Clarke M C, Haig D A. Multiplication of scrapie agent in cell culture. Res Vet Sci. 1970;11:500–501. [PubMed] [Google Scholar]
- 13.Clarke M C, Kimberlin R H. Pathogenesis of mouse scrapie: distribution of agent in the pulp and stroma of infected spleens. Vet Microbiol. 1984;9:215–225. doi: 10.1016/0378-1135(84)90039-7. [DOI] [PubMed] [Google Scholar]
- 14.Felten D L, Ackerman K D, Wiegand S J, Felten S Y. Noradrenergic sympathetic innervation of the spleen. I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res. 1987;18:28–36. doi: 10.1002/jnr.490180107. [DOI] [PubMed] [Google Scholar]
- 15.Fischer M, Rülicke T, Raeber A, Sailer A, Moser M, Oesch B, Brandner S, Aguzzi A, Weissmann C. Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J. 1996;15:1255–1264. [PMC free article] [PubMed] [Google Scholar]
- 16.Hill A F, Zeidler M, Ironside J, Collinge J. Diagnosis of new variant Creutzfeldt-Jakob disease by tonsil biopsy. Lancet. 1997;349:99. doi: 10.1016/S0140-6736(97)24002-X. [DOI] [PubMed] [Google Scholar]
- 17.Hilton D A, Fathers E, Edwards P, Ironside J W, Zajicek J. Prion immunoreactivity in appendix before clinical onset of variant Creutzfeldt-Jakob disease. Lancet. 1998;352:703–704. doi: 10.1016/S0140-6736(98)24035-9. [DOI] [PubMed] [Google Scholar]
- 18.Jeffrey M, McGovern G, Goodsir C M, Brown K L, Bruce M E. Sites of prion protein accumulation in scrapie-infected mouse spleen revealed by immuno-electron microscopy. J Pathol. 2000;191:323–332. doi: 10.1002/1096-9896(200007)191:3<323::AID-PATH629>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Kapasi Z F, Qin D, Kerr W G, Kosco-Vilbois M H, Shultz L D, Tew J G, Szakal A K. Follicular dendritic cell (FDC) precursors in primary lymphoid tissues. J Immunol. 1998;160:1078–1084. [PubMed] [Google Scholar]
- 20.Kimberlin R H, Walker C A. Pathogenesis of mouse scrapie: dynamics of agent replication in spleen, spinal cord and brain after infection by different routes. J Comp Pathol. 1979;89:551–562. doi: 10.1016/0021-9975(79)90046-x. [DOI] [PubMed] [Google Scholar]
- 21.Kimberlin R H, Walker C A. The role of the spleen in the neuroinvasion of scrapie in mice. Virus Res. 1989;12:201–211. doi: 10.1016/0168-1702(89)90039-7. [DOI] [PubMed] [Google Scholar]
- 22.Kitamoto T, Muramoto T, Mohri S, Doh-Ura K, Tateishi J. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J Virol. 1991;65:6292–6295. doi: 10.1128/jvi.65.11.6292-6295.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaus G G, Humphrey J H, Kunkl A, Dongworth D W. The follicular dendritic cell: its role in antigen presentation in the generation of immunological memory. Immunol Rev. 1980;53:3–28. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 24.Klein M A, Frigg R, Flechsig E, Raeber A J, Kalinke U, Bluethmann H, Bootz F, Suter M, Zinkernagel R M, Aguzzi A. A crucial role for B cells in neuroinvasive scrapie. Nature. 1997;390:687–690. doi: 10.1038/37789. [DOI] [PubMed] [Google Scholar]
- 25.Klein M A, Kaeser P S, Schwarz P, Weyd H, Xenarios I, Zinkernagel R M, Carroll M C, Verbeek J S, Botto M, Walport M J, Molina H, Kalinke U, Acha-Orbea H, Aguzzi A. Complement facilitates early prion pathogenesis. Nat Med. 2001;7:488–492. doi: 10.1038/86567. [DOI] [PubMed] [Google Scholar]
- 26.Koni P A, Sacca R, Lawton P, Browning J L, Ruddle N H, Flavell R A. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 27.Kooyman D L, Byrne G W, McClellan S, Nielsen D, Tone M, Waldmann H, Coffman T M, McCurry K R, Platt J L, Logan J S. In vivo transfer of GPI-linked complement restriction factors from erythrocytes to the endothelium. Science. 1995;269:89–92. doi: 10.1126/science.7541557. [DOI] [PubMed] [Google Scholar]
- 28.Korth C, Stierli B, Streit P, Moser M, Schaller O, Fischer R, Schulz-Schaeffer W, Kretzschmar H, Raeber A, Braun U, Ehrensperger F, Hornemann S, Glockshuber R, Riek R, Billeter M, Wuthrich K, Oesch B. Prion (PrPSc)-specific epitope defined by a monoclonal antibody. Nature. 1997;390:74–77. doi: 10.1038/36337. [DOI] [PubMed] [Google Scholar]
- 29.Kosco M H, Pflugfelder E, Gray D. Follicular dendritic cell-dependent adhesion and proliferation of B cells in vitro. J Immunol. 1992;148:2331–2339. [PubMed] [Google Scholar]
- 30.Lasmezas C I, Cesbron J Y, Deslys J P, Demaimay R, Adjou K T, Rioux R, Lemaire C, Locht C, Dormont D. Immune system-dependent and -independent replication of the scrapie agent. J Virol. 1996;70:1292–1295. doi: 10.1128/jvi.70.2.1292-1295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Hir M, Bluethmann H, Kosco-Vilbois M H, Muller M, di Padova F, Moore M, Ryffel B, Eugster H P. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J Exp Med. 1996;183:2367–2372. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mabbott N A, Mackay F, Minns F, Bruce M E. Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat Med. 2000;6:719–720. doi: 10.1038/77401. [DOI] [PubMed] [Google Scholar]
- 33.Mabbott N A, Williams A, Farquhar C F, Pasparakis M, Kollias G, Bruce M E. Tumor necrosis factor alpha-deficient, but not interleukin-6-deficient, mice resist peripheral infection with scrapie. J Virol. 2000;74:3338–3344. doi: 10.1128/jvi.74.7.3338-3344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandel T E, Phipps R P, Abbot A, Tew J G. The follicular dendritic cell: long term antigen retention during immunity. Immunol Rev. 1980;53:29–59. doi: 10.1111/j.1600-065x.1980.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 35.Manuelidis L, Zaitsev I, Koni P, Yun Lu Z, Flavell R A, Fritch W. Follicular dendritic cells and dissemination of Creutzfeldt-Jakob disease. J Virol. 2000;74:8614–8622. doi: 10.1128/jvi.74.18.8614-8622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montrasio F, Frigg R, Glatzel M, Klein M A, Mackay F, Aguzzi A, Weissmann C. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science. 2000;288:1257–1259. doi: 10.1126/science.288.5469.1257. [DOI] [PubMed] [Google Scholar]
- 37.Prusiner S B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 38.Prusiner S B, Cochran S P, Groth D F, Downey D E, Bowman K A, Martinez H M. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982;11:353–358. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- 39.Race R, Oldstone M, Chesebro B. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J Virol. 2000;74:828–833. doi: 10.1128/jvi.74.2.828-833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raeber A J, Klein M A, Frigg R, Flechsig E, Aguzzi A, Weissmann C. PrP-dependent association of prions with splenic but not circulating lymphocytes of scrapie-infected mice. EMBO J. 1999;18:2702–2706. doi: 10.1093/emboj/18.10.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raeber A J, Sailer A, Hegyi I, Klein M A, Rulicke T, Fischer M, Brandner S, Aguzzi A, Weissmann C. Ectopic expression of prion protein (PrP) in T lymphocytes or hepatocytes of PrP knockout mice is insufficient to sustain prion replication. Proc Natl Acad Sci USA. 1999;96:3987–3992. doi: 10.1073/pnas.96.7.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hygi. 1938;27:493–497. [Google Scholar]
- 43.Sailer A, Büeler H, Fischer M, Aguzzi A, Weissmann C. No propagation of prions in mice devoid of PrP. Cell. 1994;77:967–968. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 44.Schmitz J, van Lunzen J, Tenner-Racz K, Grossschupff G, Racz P, Schmitz H, Dietrich M, Hufert F T. Follicular dendritic cells retain HIV-1 particles on their plasma membrane, but are not productively infected in asymptomatic patients with follicular hyperplasia. J Immunol. 1994;153:1352–1359. [PubMed] [Google Scholar]
- 45.Smith B A, Gartner S, Liu Y, Perelson A S, Stilianakis N I, Keele B F, Kerkering T M, Ferreira-Gonzalez A, Szakal A K, Tew J G, Burton G F. Persistence of Infectious HIV on follicular dendritic cells. J Immunol. 2001;166:690–696. doi: 10.4049/jimmunol.166.1.690. [DOI] [PubMed] [Google Scholar]
- 46.Stahl N, Borchelt D R, Hsiao K, Prusiner S B. Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell. 1987;51:229–240. doi: 10.1016/0092-8674(87)90150-4. [DOI] [PubMed] [Google Scholar]
- 47.Tew J G, Mandel T E, Burgess A W. Retention of intact HSA for prolonged periods in the popliteal lymph nodes of specifically immunized mice. Cell Immunol. 1979;45:207–212. doi: 10.1016/0008-8749(79)90378-2. [DOI] [PubMed] [Google Scholar]
- 48.Tew J G, Phipps R P, Mandel T E. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 49.Tkachuk M, Bolliger S, Ryffel B, Pluschke G, Banks T A, Herren S, Gisler R H, Kosco-Vilbois M H. Crucial role of tumor necrosis factor receptor 1 expression on nonhematopoietic cells for B cell localization within the splenic white pulp. J Exp Med. 1998;187:469–477. doi: 10.1084/jem.187.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]