Abstract
Background
Local anaesthesia in dental procedures is generally safe, although the occurrence of transient bradycardia (TB) has occasionally been reported. TB is often associated with two reflexes, the trigeminal cardiac reflex (TCR) and the vasovagal reflex (VVR) and is characterised by a rapid decrease in heart rate (HR) and blood pressure (BP). The prevalence of TCR is considered low, and its predictors have not been thoroughly investigated, although an association with the gag reflex has been suggested in recent years.
Methods
This prospective study assessed TB occurrence during local anaesthesia and its potential associated factors. A comprehensive questionnaire was used to categorise discomforts during dental treatment, and various anxiety scales were used to measure patients’ anxiety levels. We investigated HR variability during local anaesthesia administration under sedation and the association between the incidence of TB and gag reflex. Subsequently, logistic regression analysis was performed to assess factors associated with TB occurrence.
Results
The prospective analysis included 188 patients of 234 initial patients. The analysis revealed a high TB incidence rate of 41% during local anaesthesia administration under sedation. No severe hypotensive events occurred, indicating a relatively benign nature of TB during local anaesthesia. TB occurrence was significantly higher in the group of patients with the gag reflex. Further analysis revealed that both gag reflex and trait anxiety were significantly associated with TB occurrence, whereas dental phobia did not directly correlate with TB.
Conclusion
This study highlights the prominent occurrence of TB during local anaesthesia in dental treatment, which is primarily attributed to TCR activation. The identification of gag reflex and trait anxiety as independent factors associated with TB development may pave the way for TB prevention measures. Further research is required to clarify the mechanisms of TCR and perform safer dental procedures under sedation. Future studies should also aim to elucidate the precise mechanisms underlying TB during local anaesthesia through direct measurements of neural activity. A better understanding of TB in dentistry is crucial for improving patient safety and optimising dental practice protocols.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-04940-0.
Keywords: Local anaesthesia, Bradycardia, Trigeminal cardiac reflex, Vasovagal reflex, Dental procedures, Sedation, Anxiety, Gag reflex, State-trait anxiety inventory, Phobia
Background
The incidence of hypotension during local dental anaesthesia is less than 0.1%. Reports have stated that discomfort or transient ischaemic-like symptoms observed during dental procedures or the administration of local anaesthesia are often attributed to a decline in heart rate (HR) and blood pressure (BP) resulting from an emotional syncope, known as the vasovagal reflex (VVR) [1, 2]. Another mechanism implicated in hypotension induced by stimulation in the maxillofacial-oral region is the trigeminal cardiac reflex (TCR) [3]. This distinctive brainstem reflex is characterised by abrupt reductions in HR, BP, cardiac arrhythmias, asystole, and other autonomic responses, such as apnoea and gastric hypermotility [4]. In dental procedures or oral surgery, TCR is triggered by stimulation of the maxillary and mandibular branches and their corresponding innervated area [5–7]. Consequently, TCR can be elicited through invasive procedures during dental treatments. TCR frequently occurs during ophthalmic surgeries (25–90%) and skull base surgeries (8–18%) under general anaesthesia, although the incidence of TCR during oral surgery ranges from 1–2%. The reasons for this low incidence remain unclear [8, 9]. Previous studies on TCR generally employed a threshold of a 20% decrease in HR from baseline. This threshold could reduce the risk of misinterpreting minor HR changes influenced by anaesthetics or patient movements, which are frequently observed in surgical settings. Nevertheless, this criterion may potentially underestimate the incidence of TCR [4, 10, 11].
We conducted a retrospective analysis to confirm the incidence and potential precipitating factors contributing to a ≥ 10% decrease in HR during local anaesthesia [12]. Our findings indicated that a decrease in HR during local anaesthesia is comparable to that reported for TCR occurrences in skull base surgery and that a history of gag reflex during dental treatment is associated with transient bradycardia (TB). However, nausea may also be associated with anxiety, stress, and depression, and anxiety about dental treatment might influence nausea and TB occurrence [13–15]. Additionally, unresolved questions remain because of the retrospective nature of the study. Firstly, the study could not verify that only cases in which actual HR reduction occurred during local anaesthesia were included. Furthermore, it did not ensure the accuracy of the classification of discomfort symptoms during past dental treatment, which was the reason for using intravenous sedation. Therefore, the previous study did not completely exclude the possibility that anxiety about dental treatment may trigger TB [14, 15]. To accurately assess the factors associated with TB occurrence, we required an objective quantitative assessment of anxiety during dental treatment, as well as an accurate classification of past discomfort, including the gag reflex.
In this study, we conducted a prospective analysis to assess HR fluctuations during local anaesthesia administration in patients receiving intravenous sedation for dental treatment. A questionnaire was used to obtain comprehensive data on the discomfort experienced during dental treatment (Additional File). We utilised the Dental Fear Survey (DFS) and Modified Dental Anxiety Scale (MDAS) to assess specific anxiety in detail during dental treatment. Additionally, the State-Trait Anxiety Inventory (STAI) was used to assess anxiety, which can evaluate both the intensity of situational temporary anxiety and relatively stable anxiety, independent of the environment and based on individual characteristics [16]. The primary outcome is the incidence of TB during local anaesthesia under sedation, with or without the gag reflex. The secondary outcome focuses on identifying the associations between TB occurrence and accurately categorising dental treatment-associated discomfort and various anxiety scales.
Materials and methods
This study included patients scheduled for oral surgery under intravenous sedation between March 2022 and March 2023 at the dental anaesthesia outpatient clinic of the hospital. Eligible participants were adults classified under the American Society of Anesthesiologists Physical Status (ASA PS) 1–2. Exclusion criteria included individuals aged < 17 years, those with artificial pacemakers, recipients of catecholamine agonists/blockers or circulatory medications, users of antipsychotic/antidepressant medications, and those with a history of cardiovascular disease. Informed consent was obtained from all participants after thoroughly explaining the study’s purpose.
Upon providing consent to participate in the study, a comprehensive questionnaire was administered to document the occurrence and characteristics of the discomfort experienced during dental treatment and their detailed symptoms. Demographic data of participants were also collected. Subsequently, a certified dental anaesthesiologist categorised each discomfort based on the questionnaire responses. The classification included dental phobia, hyperventilation, gag reflex, VVR, intraoperative hypertension, drug allergy, and allergic reactions to local anaesthesia. Symptoms that could not be classified into these categories were classified as ‘other’ (invasive). Anxiety related to dental phobia was assessed using DFS (60 or more points positive) and MDAS (19 or more points positive). We used the Japanese translations of the DFS and MDAS, and each threshold was defined based on previous publications [17–20]. Additionally, the anxiety scale was assessed using STAI and was considered positive if it met or exceeded certain thresholds: for State Anxiety (Y-1), men who scored 41 or higher and women who scored 42 or higher, and for Trait Anxiety (Y-2), men who scored 44 or higher and women who scored 45 or higher were considered positive. We used the Japanese version of the STAI, and the thresholds were based on its manual [21].
For intravenous sedation, a combination of midazolam and propofol constituted the anaesthetic regimen, and the attending anaesthesiologist determined the dosage. The target sedation depth for the participants was maintained within the range of 4–5 on the Ramsay Sedation Scale (RSS), indicating brisk to sluggish responses to stimuli. Vital signs, including electrocardiogram, HR, non-invasive blood pressure, and peripheral capillary oxygen saturation, were monitored and recorded by the patient monitors (BSM3562, Nihon-kohden, Tokyo, Japan) and documented through the anaesthesia record system (paperChart; https://paperchart.net/ech/) at 2-s intervals. Following the stabilisation of BP and HR, the baseline HR before local anaesthesia (HRLA) was recorded alongside the sedation level assessment. HRLA was defined as the mean value over 2 min before local anaesthesia administration. Subsequently, a local anaesthetic injection was administered by the treating dentist, and the largest decrease in HR (HRmin) within 2 min immediately after the start of the local anaesthetic injection was recorded. Sedation management and the recording of HR variability (HRR) were performed by a dental anaesthesiologist who did not participate in this study. HRR after local anaesthesia was determined according to a previous publication per the following equation [12]:
A ≥ 10% decrease in HRR was defined as TB. The patients were divided into two groups: those with observed TB (TB group) and those without observed TB (normal group).
Sample size estimation and statistical analysis
To prove the hypothesis that a history of nausea is not associated with TB, we established a significance level of 0.05, with a power of 0.8 and an effect size of 0.27. The effect size was determined based on the results of a previous retrospective study. Assuming an expected dropout rate of 25%, a total of 235 cases were required.
Comparative analyses of the experience of nausea and TB occurrence or other variables were conducted using the chi-square test or Fisher’s two-tailed exact probability test for categorical variables and an independent-sample t-test for continuous variables. Furthermore, a binary logistic regression model was used to identify risk factors associated with TB occurrence. The dependent variable was TB, and the independent variables were selected using a likelihood-based variable reduction method. Initially, all candidate factors were included in the model. A two-tailed P-value of < 0.05 indicated statistical significance.
Results
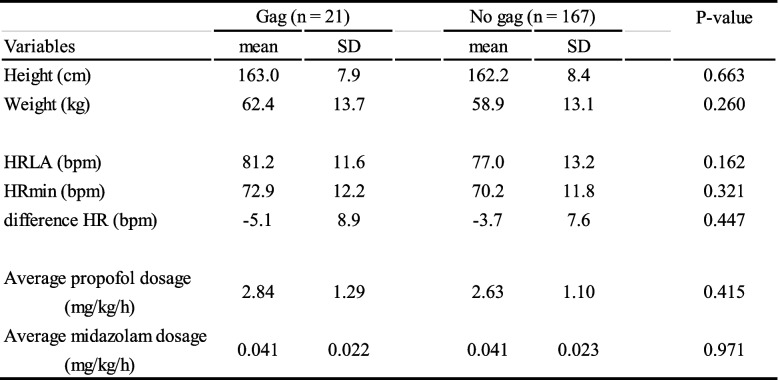
This study was conducted between March 2022 and March 2023. Figure 1 illustrates the patient flow. Of the initial 234 patients attending our outpatient dental anaesthesia clinic during this period, 46 were excluded due to incomplete questionnaire responses, insufficient anaesthesia records, or the absence of local anaesthetic administration, leaving a final cohort of 188 patients for data analysis. Table 1 presents the patients’ demographic, cardiac, and pharmacological characteristics in both cohorts according to the presence or absence of gag reflex. Among the 188 patients, 21 (11.2%) experienced vomiting reflexes during dental treatment. No significant differences were observed in height, weight, HRR, or sedative doses between the two groups.
Fig. 1.
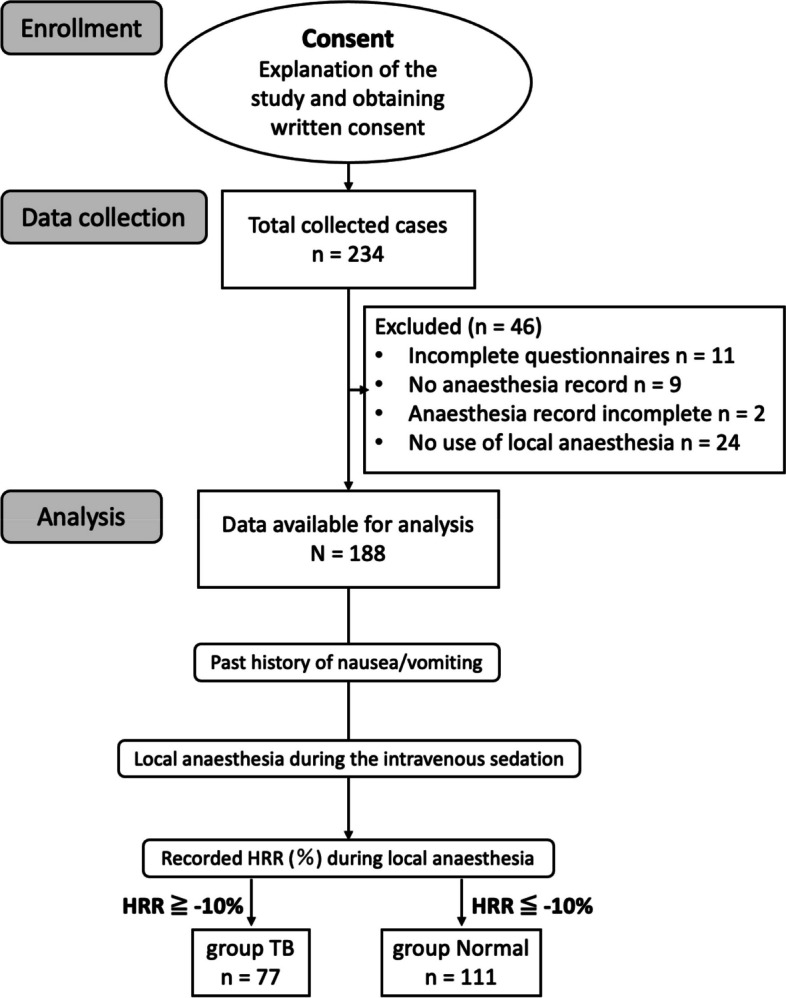
Flowchart of data collection, with patient allocation to groups for analysis. After providing consent, participants completed a comprehensive questionnaire documenting discomfort and symptoms experienced during dental treatment. Discomfort was categorised by a certified dental anaesthesiologist into specific types such as dental phobia, gag reflex/nausea, and allergies. Participants were allocated to two groups based on the presence or absence of gag reflex/nausea. Sedation was maintained at levels 4–5 on the Ramsay Sedation Scale. HR was monitored before and after administration of local anaesthesia, recording the maximum HR reduction within two min. HRR was calculated to assess the TB occurrence, subsequently classifying patients into either the TB group or the normal group. HR, heart rate; HRR, heart rate variability; TB, transient bradycardia
Table 1.
Demographic and pharmacologic characteristics of patients with gag reflex
HR heart rate, HRLA heart rate before local anesthesia, HRmin minimum heart rate after local anesthesia, SD standard deviation
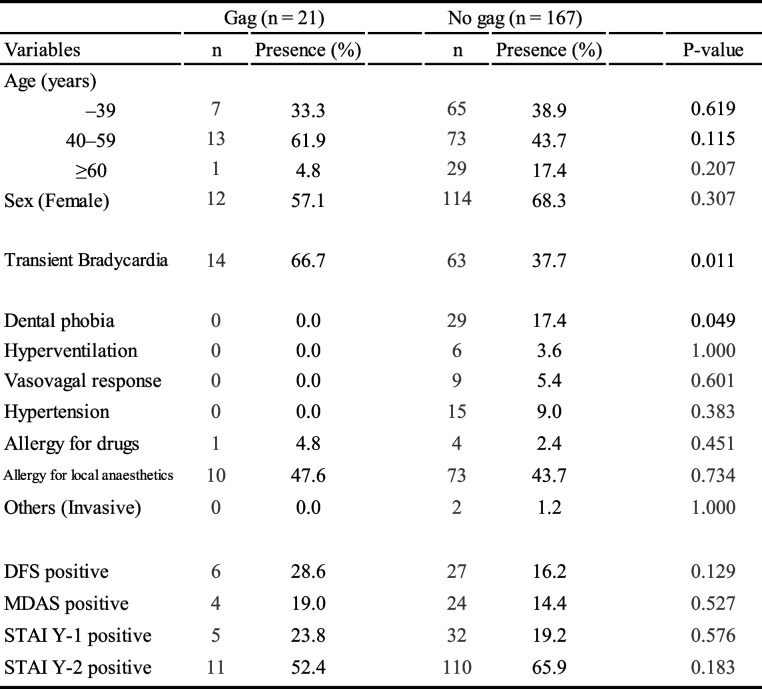
Table 2 presents the relationship between the presence or absence of the gag reflex and age distribution, categories of discomfort during dental treatment, and positive findings of DFS, MDAS, and STAI. TB occurrence was significantly higher in the group of patients with the gag reflex. Furthermore, the prevalence of dental phobia was lower in this group. No significant differences were found between the presence or absence of gag reflex and age group. No apparent differences were observed in other discomfort symptoms experienced during dental treatment between the groups with and without the gag reflex. Positive findings on DFS and MDAS, in addition to positive findings on STAI, were not significantly different between the two groups.
Table 2.
Age distribution and clinical characteristics of patients with gag reflex
Values are presented as number and %
DFS Dental Fear Survey, MDAS Modified Dental Anxiety Inventory, STAI Y-1 State-TraitAnxiety Inventory (State Anxiety), STAI Y-2 State-Trait Anxiety Inventory (Trait Anxiety)
Among the 188 participants, 77 (41.0%) exhibited TB symptoms, as illustrated in Fig. 1. The largest decrease in HR recorded was 30 beats per minute (bpm), whereas the lowest HRmin observed was 41 bpm. Additionally, 32 (17.0%) patients had an HR below 60 bpm. Notably, no patients experienced severe hypotension accompanied by loss of or decreased consciousness, nor did they require pharmacological intervention or therapeutic measures. Furthermore, none of the patients had postoperative memories of the administration of local anaesthesia.
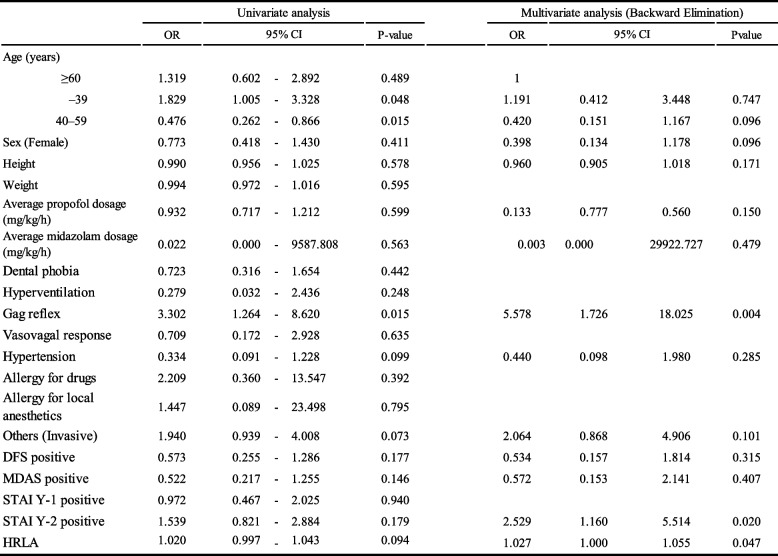
Table 3 presents the results of the univariate and binary logistic regression analyses performed to identify the independent factors associated with the occurrence of TB. A significant association was found between the prevalence of the gag reflex (odds ratio [OR], 5.578; 95% confidence interval [CI], 1.726–18.025; P = 0.004) and positive findings for trait anxiety on the STAI (Y-2) (OR, 2.529; 95% CI, 1.160–5.514; P = 0.020) with TB occurrence. Additionally, HRLA expression was slightly higher in the TB group (OR, 1.027; 95% CI, 1.000–1.055; P = 0.047). Age and sex exhibited no association with TB occurrence.
Table 3.
Binary logistic regression analysis of variables on patients with transient bradycardia (TB)
CI confidence interval, DFS Dental Fear Survey, HRLA heart rate before local anaesthesia, MDAS Modified Dental Anxiety Inventory, OR odds ratio, STAI Y-1 State-Trait Anxiety Inventory (State Anxiety), STAI Y-2 State-Trait Anxiety Inventory (Trait Anxiety)
Discussion
Our results revealed a higher occurrence of TB in patients with a history of nausea. Furthermore, a history of gag reflex was identified as an independent factor associated with the occurrence of TB. These results are consistent with those of a previous retrospective analysis; however, the present study was conducted using a prospective approach, ensuring the results were more robust. One possible explanation for our results is that afferent stimuli through the glossopharyngeal nerve overstimulate the emetic centre in individuals with an enhanced gag reflex, efferently activating the parasympathetic nervous system [15, 22]. As neurally modulated syncope also induces bradycardia via afferent vagal branches in response to various stimuli, symptoms such as the swallowing reflex and nausea could precede the bradycardia reflex [23]. Excitation of the upper gastrointestinal tract by vomiting stimuli can also increase vagal activity, leading to a vagal reflex (known as vomiting syncope) [24, 25]. As stimulation of the gastrointestinal vagal afferent nerve can cause nausea, bradycardia may occur more frequently in patients with frequent episodes of the gag reflex. Moreover, animal studies suggest that the activation of gastrointestinal vagal afferent fibres may be a pathway that could initiate the prodromal symptoms of vomiting and activate the efferent vagus nerve [26]. However, vagus nerve stimulators reportedly cause a sustained vomiting reflex [27]. Afferent stimulation via the trigeminal nerve may induce vomiting or nausea by stimulating the efferent gastrointestinal vagus nerve, in addition to generating bradycardia [28].
In this study, trait anxiety tendency emerges as an independent predictor of TB occurrence. Unlike state anxiety, which fluctuates rapidly in response to situational factors, such as dental treatment or local anaesthesia, trait anxiety remains relatively stable regardless of the situation [16, 29]. Patients with trait anxiety experience persistent anxiety and continuously significant psychological stress. In such situations, the sympathetic nervous system is activated, and the parasympathetic nervous system is hyperactive, leading to vagal reflexes in case of disruption of this delicate balance by minor intraoral stimuli during dental treatment [15, 22, 30]. In our investigation, we observed a slight elevation in HR among the TB group before local anaesthesia administration. This finding may indicate trait anxiety, characterised by chronic hyperactivity of the sympathetic nervous system. Moreover, the gag reflex increases when the parasympathetic activity is preponderant [26, 31]. This may provide a physiological basis for the development of vomiting and nausea. In a study of haematopoietic stem cell transplant recipients, participants with frequent nausea had higher anxiety levels, and the severity and frequency of nausea were predictors of trait anxiety [32]. In addition, persistent stress caused by anxiety affects the autonomic nervous system and increases the secretion of stress hormones. This may cause nausea by decreasing blood flow to the gastrointestinal tract to prepare for the ‘fight or flight’ response [14]. Conversely, we investigated the association between dental phobia and TB, and no correlation with TB was established using quantitative measures, such as DFS or MDAS, or qualitative assessments of dental phobia classification by dental anaesthetists using structured questionnaires [33, 34]. This finding is consistent with our previous study [12]. Dental phobia is considered a risk factor for bradycardia and associated syncope during local anaesthesia administration; however, it may not directly affect bradycardia caused by TCR.
Our study revealed a 41% incidence of TCR during local anaesthesia under sedation, which exceeds that of previous reports and our previous retrospective study. In this study, we evaluated the depth of intravenous sedation and confirmed that the patients had no memory of local anaesthesia after arousal. Thus, the present study indicates that TCR, rather than VVR, is the primary inducing factor [30, 35]. Variations in TCR incidence among surgical fields might be due to factors, including the intensity of the stimulation and whether the stimulation originates from direct stimulation of the nerve branches [9, 10, 15, 16]. Dental treatment can provide only relatively low-intensity stimulation, resulting in a minor decrease in HR compared with otolaryngological or ophthalmological procedures. Another reason for the low incidence of TCR is that local anaesthesia operations do not directly stimulate nerve branches but rather nerve endings distributed over the innervated area, which may result in minor fluctuations in HR [3, 10, 36].
This study had some limitations. First, the study did not distinguish between bradycardia occurrence induced by the gag reflex and bradycardia-induced vomiting or nausea. Therefore, the actual or potential likelihood of bradycardia in patients with a gag reflex remains unexplored. Second, because autonomic nerve activity has not been directly assessed during local anaesthesia, an increase in the parasympathetic tone of activity has not been proven. Finally, the study cohort comprised patients who required intravenous sedation during dental treatment for various reasons. Therefore, they might have encountered more discomfort during previous dental treatments than the cohort who visited a general dental clinic. This may have influenced the analysis of this study.
Conclusion
The results of this study indicate a high frequency of occurrence of local anaesthesia-induced TB, likely caused by TCR. Furthermore, TB was associated with gag reflex and the presence of trait anxiety, as identified by the STAI. Whether the occurrence of TB during local anaesthesia is solely the result of TCR remains controversial. Therefore, future studies are required, including the direct measurement of neural activity.
Supplementary Information
Acknowledgements
We gratefully appreciate all hospital staff who contributed to the data recording process. We would also like to thank Editage (www.editage.jp) for English language editing.
Abbreviations
- HR
Heart Rate
- BP
Blood Pressure
- VVR
Vasovagal Reflex
- TCR
Trigeminal Cardiac Reflex
- ASA PS
American Society of Anesthesiologists Physical Status
- DFS
Dental Fear Survey
- MDAS
Modified Dental Anxiety Scale
- STAI
State-Trait Anxiety Inventory
- RSS
Ramsay Sedation Scale
- HRR
Heart Rate variability
- TB
Transient Bradycardia
- HRLA
HR Before Local Anaesthesia
- OR
Odds Ratio
- CI
Confidence Interval
Authors’ contributions
RW contributed to conception, design, collection, analysis, and interpretation of the patient data and was a major contributor in writing the manuscript. JA contributed to data analysis and critically revised the manuscript. YB contributed to data acquisition and interpretation and critically revised the manuscript. NU contributed to collection and interpretation of the patient data. AN contributed to collection and interpretation of the patient data. SM contributed to data interpretation and critically revised the manuscript. All authors read and approved the final manuscript.
Funding
No funding.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The ethics committee of Tokyo Medical and Dental University (No. D2021-001) approved the study procedures for sample collection and analyses. Informed consent was obtained from all participants after thoroughly explaining the study’s purpose.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Greenwood M, Meechan JG. General medicine and surgery for dental practitioners: part 3. Management of specific medical emergencies in dental practice. Br Dent J. 2014;217:21–6. [DOI] [PubMed] [Google Scholar]
- 2.Girdler NM, Smith DG. Prevalence of emergency events in British dental practice and emergency management skills of British dentists. Resuscitation. 1999;41:159–67. [DOI] [PubMed] [Google Scholar]
- 3.Arakeri G, Arali V. A new hypothesis of cause of syncope: trigeminocardiac reflex during extraction of teeth. Med Hypotheses. 2010;74:248–51. [DOI] [PubMed] [Google Scholar]
- 4.Meuwly C, Golanov E, Chowdhury T, Erne P, Schaller B. Trigeminal cardiac reflex: new thinking model about the definition based on a literature review. Med (Baltim). 2015;94:e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhury T, Mendelowith D, Golanov E, Spiriev T, Arasho B, Sandu N, et al. Trigeminocardiac reflex: the current clinical and physiological knowledge. J Neurosurg Anesthesiol. 2015;27:136–47. [DOI] [PubMed] [Google Scholar]
- 6.Bohluli B, Bayat M, Sarkarat F, Moradi B, Tabrizi MHS, Sadr-Eshkevari P. Trigeminocardiac reflex during le Fort I osteotomy: a case-crossover study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:178–81. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama S, Iwai T, Honda K, Mitsudo K. Trigeminocardiac reflex during bilateral sagittal split osteotomy. J Dent Sci. 2021;16:782–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Precious DS, Skulsky FG. Cardiac dysrhythmias complicating maxillofacial surgery. Int J Oral Maxillofac Surg. 1990;19:279–82. [DOI] [PubMed] [Google Scholar]
- 9.Schaller B, Cornelius JF, Prabhakar H, Koerbel A, Gnanalingham K, Sandu N, et al. The trigemino-cardiac reflex: an update of the current knowledge. J Neurosurg Anesthesiol. 2009;21:187–95. [DOI] [PubMed] [Google Scholar]
- 10.Yorgancilar E, Gun R, Yildirim M, Bakir S, Akkus Z, Topcu I. Determination of trigeminocardiac reflex during rhinoplasty. Int J Oral Maxillofac Surg. 2012;41:389–93. [DOI] [PubMed] [Google Scholar]
- 11.Sadr-Eshkevari P, Schaller BJ, Bohluli B. Trigeminocardiac reflex: some thought to the definition. Surg Neurol Int. 2014;5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakita R, BaBa Y, Fukayama H, Maeda S. Factors associated with transient bradycardia during local anesthesia administration to the oral cavity under intravenous sedation: a retrospective cohort study. J Dent Sci. 2024;19:878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.So WK, Marsh G, Ling WM, Leung FY, Lo JC, Yeung M, et al. Anxiety, depression and quality of life among Chinese breast cancer patients during adjuvant therapy. Eur J Oncol Nurs. 2010;14:17–22. [DOI] [PubMed] [Google Scholar]
- 14.Choi JM, Yang JI, Kang SJ, Han YM, Lee J, Lee C, et al. Association between anxiety and depression and gastroesophageal reflux disease: results from a large cross-sectional study. J Neurogastroenterol Motil. 2018;24:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassi GS, Humphris GM, Longman LP. The etiology and management of gagging: a review of the literature. J Prosthet Dent. 2004;91:459–67. [DOI] [PubMed] [Google Scholar]
- 16.Kim WS, Byeon GJ, Song BJ, Lee HJ. Availability of preoperative anxiety scale as a predictive factor for hemodynamic changes during induction of anesthesia. Korean J Anesthesiol. 2010;58:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinknecht RA, Klepac RK, Alexander LD. Origins and characteristics of fear of dentistry. J Am Dent Assoc. 1973;86:842–8. [DOI] [PubMed] [Google Scholar]
- 18.Sano T, Tanabe Y, Noda T. Assessment on dental fear in Japan - Part 1 results of dental fear survey in young adults -. The Japanese. J Pediatr Dent. 2001;39:865–71. [Google Scholar]
- 19.Humphris GM, Freeman R, Campbell J, Tuutti H, D’Souza V. Further evidence for the reliability and validity of the modified dental anxiety scale. Int Dent J. 2000;50:367–70. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa H, Hosaka K. Development of the Japanese version of the Modified Dental Anxiety Scale (MDAS-J): investigation of the eliability and the validity. Jpn J Psychosom Dent. 2010;25:2–6. [Google Scholar]
- 21.Hidano T, Fukuhara M, Iwawaki S, Soga S, Spielberger CD. New STAI manual. 1st ed. Tokyo: Jitsumukyoikusyuppan; 2000. [Google Scholar]
- 22.Nesheiwat Z, Ghanim M, Eid J, Patel N, Burmeister C, Eltahawy E. Gag reflex-mediated restoration of sinus rhythm during TEE probe insertion for atrial fibrillation: a word of caution. Case Rep Cardiol. 2020;2020:6398196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alboni P, Brignole M, Menozzi C, Raviele A, Del Rosso A, Dinelli M, et al. Diagnostic value of history in patients with syncope with or without heart disease. J Am Coll Cardiol. 2001;37:1921–8. [DOI] [PubMed] [Google Scholar]
- 24.Mehta D, Saverymuttu SH, Camm AJ. Recurrent paroxysmal complete heart block induced by vomiting. Chest. 1988;94:433–5. [DOI] [PubMed] [Google Scholar]
- 25.Lewis NP, Fraser AG, Taylor A. Syncope while vomiting during migraine attack. Lancet. 1988;2:400–1. [DOI] [PubMed] [Google Scholar]
- 26.Andrews PL, Wood KL. Vagally mediated gastric motor and emetic reflexes evoked by stimulation of the antral mucosa in anaesthetized ferrets. J Physiol. 1988;395:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder HE, Pai N, Meaney B, Sloan Birbeck C, Whitney R, Johnson N, et al. Significant vomiting and weight loss in a pediatric epilepsy patient secondary to vagus nerve stimulation: a case report and review of the literature. Epilepsy Behav Rep. 2023;24:100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong W, Shahbaz O, Teskey G, Beever A, Kachour N, Venketaraman V, et al. Mechanisms of nausea and vomiting: current knowledge and recent advances in intracellular emetic signaling systems. Int J Mol Sci. 2021;22:5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spielberger CD, Auerbach SM, Wadsworth AP, Dunn TM, Taulbee ES. Emotional reactions to surgery. J Consult Clin Psychol. 1973;40:33–8. [DOI] [PubMed] [Google Scholar]
- 30.Win NN, Kohase H, Miyamoto T, Umino M. Decreased bispectral index as an indicator of syncope before hypotension and bradycardia in two patients with needle phobia. Br J Anaesth. 2003;91:749–52. [DOI] [PubMed] [Google Scholar]
- 31.Uchino M, Kuwahara M, Ebukuro S, Tsubone H. Modulation of emetic response by carotid baro- and chemoreceptor activations. Auton Neurosci. 2006;128:25–36. [DOI] [PubMed] [Google Scholar]
- 32.Pasyar N, Rambod M, Zahedi F, Ramzi M. Pain, fatigue, nausea, and vomiting as the predictors of anxiety in patients undergoing hematopoietic stem cell transplantation: a prospective cohort study. Support Care Cancer. 2022;30:5871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haukebø K, Skaret E, Ost LG, Raadal M, Berg E, Sundberg H, et al. One- vs. five-session treatment of dental phobia: a randomized controlled study. J Behav Ther Exp Psychiatry. 2008;39:381–90. [DOI] [PubMed] [Google Scholar]
- 34.Sreenivasagan S, Sneha P, Ravi P, Raja VBK. To assess the prevalence of dental anxiety and assess the efficacy of Vibraject and to assess prevalence of dental phobia. Dentistry. 2018;8:1122–2161. [Google Scholar]
- 35.Graham DT, Kabler JD, Lunsford L Jr. Vasovagal fainting: a diphasic response. Psychosom Med. 1961;23:493–507. [DOI] [PubMed] [Google Scholar]
- 36.Schaller B, Probst R, Strebel S, Gratzl O. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg. 1999;90:215–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.