Abstract
Objective
In the present study, we aimed to explore the association between left atrial appendage emptying velocity (LAAEV) measured by transesophageal echocardiography and atrial fibrillation (AF) recurrence after radiofrequency catheter ablation (RFCA) in patients with early persistent atrial fibrillation (PeAF).
Methods
We retrospectively analyzed patients with early PeAF who underwent their initial ablation procedure. The echocardiographic and clinical data of the enrolled patients were collected and recorded prior to the operation. Following adjustment for confounding factors, we investigated the relationship between the LAAEV and AF recurrence in patients with early PeAF after radiofrequency ablation.
Results
The proportions of AF recurrence in the low, medium, and high LAAEV groups were 48.8%, 16.0%, and 13.1%, respectively. After adjusting for potential confounding factors, we observed a gradual decrease in the risk of AF recurrence with increasing LAAEV (odds ratio: 0.882, 95% confidence interval: 0.842—0.924, p<0.001). This trend was statistically significant (p<0.001), particularly when comparing the high and low LAAEV groups (odds ratio: 0.033, 95% confidence interval: 0.009―0.116, p<0.001). Curve fitting analysis demonstrated an approximate negative linear association between LAAEV and the probability of AF recurrence.
Conclusions
Among patients with early PeAF who successfully underwent their first RFCA, we found that the LAAEV within 24 h before the procedure was independently correlated with the risk of AF recurrence. Notably, this association was negative, indicating that higher LAAEV was associated with a lower risk of AF recurrence.
Keywords: Atrial fibrillation, Radiofrequency ablation, Recurrence, Left atrial appendage emptying velocity, Echocardiography
Introduction
Atrial fibrillation (AF) is recognized as the most prevalent cardiac arrhythmia, significantly impacting public health [1]. In 2017, the global number of individuals affected by AF was approximately 37.6 million, and it is projected that by 2050, the worldwide prevalence will surge by more than 60% [2]. Precisely in China, it is estimated that by 2050, among the population aged 60 and above, around 5.2 million men and 3.1 million women will be affected by AF [3]. AF can manifest with a range of symptoms, including palpitations, dizziness, shortness of breath, and fatigue. Furthermore, AF has been linked to increased rates of severe complications, such as stroke, heart failure, cognitive impairment, and sudden cardiac arrest, which not only have a significant impact on patient’s quality of life [4] but also contribute to rising healthcare costs [5]. The European Society of Cardiology guidelines strongly recommend radiofrequency catheter ablation (RFCA) as the initial treatment option for persistent atrial fibrillation (PeAF) [1]. However, despite this intervention, the recurrence rate within 1 year after the initial pulmonary vein isolation (PVI) procedure can be as high as 70%. Therefore, the identification of reliable predictive factors for AF recurrence prior to ablation treatment is of paramount importance. Such factors can greatly assist in enhancing treatment strategies and effectively managing patients with PeAF. It is noteworthy that there is a dearth of research specifically focusing on patients with early-stage PeAF, who represent the primary population undergoing RFCA and frequently exhibit more favorable outcomes [6] in comparison to those with long-standing PeAF [7]. This observation holds considerable clinical significance.
Increased left atrial volume (LAV) and fibrosis can predict the recurrence of AF after RFCA, and it is associated with the extent of left atrial remodeling [8]. Most AF patients undergo left atrial appendage (LAA) reconstruction, and the degree of LAA reconstruction is an independent risk factor for post-RFCA recurrence, with a higher degree of reconstruction correlating with a higher risk of recurrence [9]. Transesophageal echocardiography (TEE) is widely used to assess the morphology and function of the LAA, and the left atrial appendage emptying velocity (LAAEV) reflects the hemodynamic characteristics of the left atrium and LAA [10–12]. Patients with low LAAEV are at a higher risk of developing AF [13].
In the present study, we hypothesized that the LAAEV in patients with early-stage PeAF was correlated with the recurrence of AF after RFCA. We conducted a retrospective analysis of LAAEV measurements taken before RFCA in patients with early-stage PeAF to investigate the relationship between LAAEV and early postoperative recurrence, with the goal of providing new evidence for the precision treatment of AF.
Materials and methods
Research subject and clinical data
This study was a single-center retrospective cohort study that collected data from early PeAF patients who underwent RFCA treatment in our cardiology department from September 2018 to November 2022. The inclusion criterion for our study was the confirmation of AF rhythm through electrocardiogram (ECG) or Holter monitoring, with AF lasting from more than 7 days to less than 3 months. The exclusion criteria were as follows: (1) the presence of a left atrial thrombus or severe spontaneous echo contrast detected by TEE; (2) poor image quality on two-dimensional echocardiography, which would impede further analysis; (3) age below 18 or above 80 years; (4) history of congenital heart disease, coronary artery disease, moderate to severe rheumatic valve disease, hypertrophic obstructive cardiomyopathy, or other cardiac diseases; (5) prior surgical treatment for AF, atrioventricular node ablation, or coexistence of other arrhythmias requiring ablation; (6) AF caused by reversible factors, such as thyroid dysfunction, acute alcohol intoxication, or postoperative state; (7) failure to restore sinus rhythm during the RFCA procedure; and (8) patients with incomplete clinical data.
The patient’s medical history and clinical data were obtained by accessing and querying the medical records system. Accordingly, CHA2DS2.VASC score and body surface area were calculated for each patient. AF duration was defined as the duration from the first diagnosis of AF to the time of RFCA performed. This study adhered to the guidelines outlined in the revised 2013 Helsinki Declaration, and informed consent was obtained from all patients individually. The study protocol received approval from the Scientific Ethics Committee.
Measurement and analysis of echocardiographic parameters
Transthoracic echocardiography (TTE) and TEE were performed within 24 h before the RFCA procedure using a PHILIPS EPIQ 7 C color Doppler ultrasound diagnostic system (Philips Healthcare Royal Philips Electronics, Amsterdam, The Netherlands).
TTE was performed by an experienced senior physician with over 5 years of expertise in echocardiography, utilizing an X5-1 transducer. Simultaneously, the patient’s limb lead ECG was recorded. The measurement process commenced with routine M-mode and two-dimensional echocardiography. To ensure accuracy, five consecutive cardiac cycles were acquired from the parasternal long-axis, apical four-chamber, apical two-chamber, and apical long-axis views, with a frame rate of 50 Hz. The left ventricular ejection fraction (LVEF) was determined by applying the biplane Simpson’s method to the apical view. Three measurements were taken, and the average value was recorded. To obtain a clear two-dimensional image, the standard apical four-chamber view was acquired, and the “Full Volume” function was activated to capture the entire endocardium of the left atrium within the sample box. Patients were instructed to hold their breath, and the transducer position was stabilized. The dynamic images were saved in digital imaging and communications in medicine (DICOM) format for subsequent offline analysis.
TEE examinations were carried out by qualified ultrasound cardiologists using an X7-2 transducer. Various angles and planes were employed to visualize the LAA and evaluate the presence of thrombus or spontaneous echo contrast. Specifically, in the mid-esophageal 90-degree view, the LAAEV was measured. For pulsed-wave Doppler measurement, the sample volume was positioned 1 cm inside the entrance of the LAA. The sampling line was aligned as parallel as possible to the direction of blood flow. At least five cardiac cycles were recorded, and the average value was calculated from the obtained images.
In the same imaging plane, tissue Doppler imaging (TDI) mode was engaged. The pulsed-wave Doppler sample volume was positioned at the proximal 1/3 of the interatrial septum (TDI-S) and lateral wall (TDI-L) of the LAA. The sampling line was aligned to be as parallel as possible to the longitudinal contraction direction of the LAA. At least five cardiac cycles were recorded, and the irregular positive wave D2 and negative wave D3 in the spectrum were measured individually. The average values of D2 and D3 were determined from the recorded data.
The analysis of left atrial three-dimensional images was conducted using QLAB software (version 10.5, Philips Healthcare Royal Philips Electronics, Amsterdam, The Netherlands software pack). In the apical four-chamber view, during both end-diastole and end-systole, the 3DQ-A mode was selected. Sampling points were placed on the left atrial wall, and adjustments were made to ensure accurate left atrial morphology. The left atrial minimum volume (LAVmin) and maximum volume (LAVmax) were calculated. The left atrial reservoir function was determined using the following formulas [14]: Expansion Index (EI) = [(LAVmax - LAVmin) / LAVmin] × 100% and Diastolic Emptying Index (DEI) = [(LAVmax - LAVmin) / LAVmax] × 100%. Additionally, the left atrial volume index (LAVI) was calculated by normalizing the LAV based on BSA: LAVI = LAV / BSA.
All echocardiographic parameters were independently analyzed and calculated by two or more attending physicians. The average values of the measurements were obtained. Importantly, the two analyzing physicians were unaware of each other’s results, ensuring a blinded analysis process.
RFCA procedure
Before the procedure, a multi-electrode electrophysiological mapping system and the CARTO 3.0 three-dimensional mapping system were connected. Coronary sinus electrodes were inserted through the subclavian vein or femoral vein. Transseptal puncture was performed to introduce the ablation catheter and mapping catheter into the left atrium via the femoral vein. Subsequently, a three-dimensional reconstruction of the left atrium and pulmonary veins was conducted. All patients underwent PVI as part of the procedure. During the procedure, triggering foci located outside the pulmonary veins were identified and targeted for additional ablation. In some cases, patients also underwent linear ablation, ablation of complex fractioned electrograms, and substrate modification strategy guided by voltage mapping was employed. In all patients, high-density voltage mapping of the left atrium was conducted while in sinus rhythm. Regions exhibiting bipolar voltages ranging from 0.1 to 0.4 mV were identified as low-voltage areas and consistently targeted for ablation. The procedure was considered successful when a bidirectional conduction block between the pulmonary veins and the left atrium was achieved and confirmed through electrophysiological stimulation. This endpoint ensured the desired outcome of the ablation procedure.
Two experienced electrophysiologists consulted to determine every patient’s strategy of RFCA. Each electrophysiologist had relevant qualifications and performed RFCA procedures independently for more than 5 years.
Postoperative management and follow-up
All patients converted to sinus rhythm during RFCA procedure and completed their 3–6 months follow-up. Twelve-lead ECG was performed weekly and whenever symptoms of arrhythmia occurred. Monthly outpatient visits were scheduled for medical history review, physical examination, and recording of 12-lead ECG and 24-hour holter monitoring. All follow-up evaluations were conducted by individuals who were unaware of the treatment plan. Any episode of rapid atrial arrythmia (AF, atrial flutter, or atrial tachycardia) occurring after a blanking period of 3 months post-RFCA, with a duration > 30 s, was defined as AF recurrence.
Statistical analysis
All data were analyzed using R software (version 3.4.3, http://www.R-project.org). Normally distributed continuous variables were presented as mean ± standard deviation, skewed distributed continuous variables were presented as median (interquartile range), and categorical variables were presented as rates or proportions (%). Group differences were assessed using chi-square tests (for categorical variables), one-way analysis of variance (for normally distributed continuous variables), and Kruskal-Wallis tests (for skewed distributed continuous variables). Univariate logistic regression analysis was performed to examine the association between different variables and AF recurrence. Multivariate logistic regression analysis was conducted to identify independent risk factors influencing postoperative AF recurrence. Both unadjusted and adjusted estimates were obtained using exact and stepwise methods. If a covariate changed the risk estimate for LAAEV on AF recurrence by more than 10% or was significantly associated with AF recurrence risk (p < 0.1), it was included in the final model as a potential confounding factor. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Stratified analysis was performed using LAAEV first quartile group as the reference to further evaluate the association between LAAEV and AF recurrence risk within each subgroup. A generalized additive model (GAM) was used to test for nonlinear relationships between AF recurrence risk and LAAEV, aiming to detect nonlinearity and determine whether a threshold effect existed beyond the general linear regression. Two-sided tests were used, and a significance level of α = 0.05 was applied.
Results
General characteristics of included patients
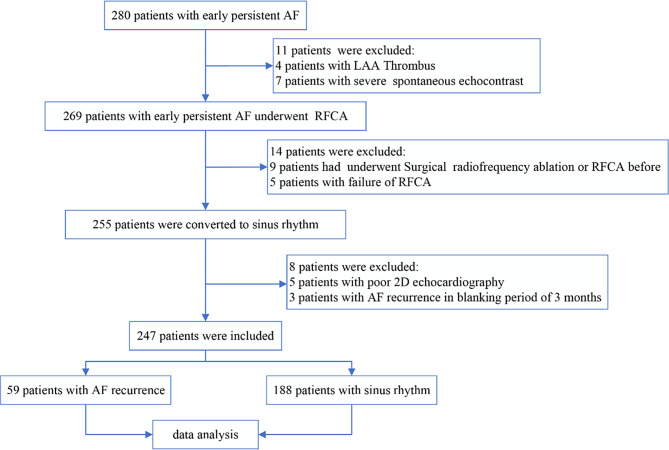
According to the inclusion criteria, a total of 280 patients with early PeAF were selected for this study. The following cases were sequentially excluded: 11 patients with LAA thrombus or significant spontaneous echo contrast, nine patients who underwent surgical or RFCA, five patients who did not successfully convert to sinus rhythm during RFCA, five patients with poor echocardiographic imaging conditions, and three patients who experienced recurrence within the 3-month blanking period after the procedure. Finally, a total of 247 patients with early PeAF were included in the study, including 203 males and 44 females, with a mean age of 62.95 ± 9.31 years. The process of patient selection is depicted in Fig. 1.
Fig. 1.
Patient selection flowchart
Comparison of baseline data among different LAAEV groups
All patients were averaged into three groups according to the left atrial emptying velocity from low to high (82 cases, 81 cases, 84 cases, respectively). The clinical and echocardiographic parameters among the three groups were compared and are shown in Table 1. After a follow-up of 3 to 6 months, a total of 59 patients experienced recurrence, with 40 in the low LAAEV group, 13 in the moderate LAAEV group, and 11 in the high LAAEV group. There were statistically significant differences among the three groups in terms of LAAEV, gender, serum creatinine (SCR), LAVImin, EI, AEI, brain natriuretic peptide (BNP), smoking history, and recurrence rate (all p < 0.05). However, there were no statistically significant differences in age, body mass index (BMI), AF duration, LAVImax, heart rate, CHA2DS2.VASC score, and various comorbidities among the groups (all p > 0.05).
Table 1.
Comparison of baseline data among three LAAEV groups
| LAAEV tertiles | Low | Medium | High | p-value |
|---|---|---|---|---|
| N(cases) | 82 | 81 | 84 | |
| Age | 63.89 ± 8.55 | 63.26 ± 8.59 | 61.74 ± 10.57 | 0.310 |
| Male, n (%) | 63 (76.8) | 75 (92.6) | 65 (77.4) | 0.012 |
| BMI | 23.71 ± 3.05 | 24.00 ± 2.83 | 23.11 ± 3.22 | 0.165 |
| AF duration (months) | 9.32 ± 3.97 | 10.35 ± 4.26 | 9.00 ± 3.72 | 0.080 |
| Heart rate | 79.93 ± 15.05 | 78.36 ± 17.66 | 75.20 ± 15.27 | 0.155 |
| SCR | 59.86 ± 18.03 | 68.79 ± 15.14 | 54.77 ± 16.17 | < 0.001 |
| BNP | 376.25 (254.15-584.80) | 410.00 (232.60-618.50) | 277.30 (189.57-417.15) | 0.001 |
| LVEF (%) | 54.46 ± 5.49 | 53.60 ± 4.80 | 53.93 ± 4.77 | 0.549 |
| LAAEV | 27.47 ± 3.35 | 38.43 ± 3.69 | 53.20 ± 4.93 | < 0.001 |
| LAVImax | 40.78 ± 4.95 | 41.56 ± 4.95 | 39.74 ± 5.07 | 0.065 |
| LAVImin | 26.64 ± 3.95 | 26.51 ± 3.51 | 24.38 ± 3.53 | < 0.001 |
| TDI-SD2 | 10.08 ± 2.59 | 10.39 ± 2.93 | 10.69 ± 2.71 | 0.359 |
| TDI-SD3 | 9.370 ± 2.338 | 9.107 ± 2.505 | 9.232 ± 2.353 | 0.784 |
| TDI-LD2 | 12.33 ± 2.99 | 12.62 ± 3.18 | 13.04 ± 3.18 | 0.332 |
| TDI-LD3 | 9.56 ± 2.37 | 9.32 ± 2.43 | 9.81 ± 2.43 | 0.433 |
| EI | 0.55 ± 0.20 | 0.58 ± 0.16 | 0.64 ± 0.19 | 0.003 |
| DEI | 0.34 ± 0.08 | 0.36 ± 0.06 | 0.38 ± 0.07 | 0.002 |
| Smoke | 21 (25.6) | 33 (40.7) | 14 (16.7) | 0.002 |
| Drinking | 22 (26.8) | 21 (25.9) | 11 (13.1) | 0.057 |
| Hypertension | 21 (25.6) | 17 (21.0) | 24 (28.6) | 0.528 |
| Diabetes mellitus | 20 (24.4) | 15 (18.5) | 14 (16.7) | 0.430 |
| CHF | 17 (20.7) | 10 (12.3) | 12 (14.3) | 0.305 |
| Hyperlipidemia | 13 (15.9) | 10 (12.3) | 18 (21.4) | 0.286 |
| History oh stroke or TIA | 15 (18.3) | 14 (17.3) | 10 (11.9) | 0.478 |
| Peripheral artery disease | 10 (12.2) | 5 (6.2) | 9 (10.7) | 0.401 |
| Recurrence, n (%) | 40 (48.8) | 13 (16.0) | 11 (13.1) | < 0.001 |
| CHA2DS2.VASC | 0.104 | |||
| 0 | 11.0 | 25.9 | 21.4 | |
| 1 | 43.9 | 30.9 | 22.6 | |
| 2 | 19.5 | 24.7 | 27.4 | |
| ≥ 3 | 24.7 | 18.5 | 28.6 |
The results are expressed as mean ± (SD) /midian (Q1, Q3) / n (%). LAAEV, LA appendage emptying velocity; BMI, body mass index; AF, atrial fibrillation; SCR, serum creatinine concentration; BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; LAVImax, maximum LA volume index; LAVImin, minimum LA volume index; TDI, tissue Doppler imaging; EI, expansion Index; DEI, diastolic emptying index; CHF, congestive heart failure; TIA, transient ischemia attack
Univariate analysis of the relationship between clinical and echocardiographic parameters and AF recurrence
A univariate logistic regression analysis was performed with AF recurrence as the dependent variable (Y = 1) and various clinical and echocardiographic parameters as independent variables. The results showed that SCR, AF duration, LAAEV, and the presence of stroke or transient ischemic attacks (TIA) were potentially associated with AF recurrence (p < 0.05) (Table 2).
Table 2.
Univariate analysis of the relationship between clinical and echocardiographic parameters and AF recurrence
| Exposure | Statistics | OR (95%CI) | p-value |
|---|---|---|---|
| Age | 62.95 ± 9.31 | 0.98 (0.95―1.01) | 0.243 |
| Male, n (%) | 203 (82.19%) | 1.63 (0.81―3.28) | 0.175 |
| BMI | 23.60 ± 3.05 | 1.02 (0.93―1.12) | 0.712 |
| AF duration(months) | 9.55 ± 4.01 | 0.86 (0.79―0.93) | < 0.001 |
| Heart rate | 77.81 ± 16.08 | 1.01 (0.99―1.03) | 0.345 |
| SCR | 61.06 ± 17.42 | 0.98 (0.96―0.99) | 0.008 |
| BNP | 357.60 (227.05-535.25) | 1.00 (1.00―1.00) | 0.469 |
| LVEF (%) | 54.00 ± 5.02 | 1.03 (0.97―1.09) | 0.321 |
| LAAEV | 39.81 ± 11.36 | 0.92 (0.89― 0.95) | < 0.001 |
| LAVImax | 40.68 ± 5.03 | 1.00 (0.95―1.06) | 0.986 |
| LAVImin | 25.83 ± 3.80 | 1.02 (0.96―1.10) | 0.583 |
| TDI-SD2 | 10.39 ± 2.75 | 0.95 (0.86―1.05) | 0.333 |
| TDI-SD3 | 9.24 ± 2.39 | 1.08 (0.96―1.22) | 0.213 |
| TDI-LD2 | 12.67 ± 3.12 | 1.05 (0.95―1.15) | 0.334 |
| TDI-LD3 | 9.56 ± 2.41 | 1.01 (0.89―1.13) | 0.930 |
| EI | 0.59 ± 0.19 | 0.61 (0.13―2.84) | 0.532 |
| DEI | 0.36 ± 0.07 | 0.26 (0.01―11.88) | 0.488 |
| Smoke | 68 (27.53) | 0.67 (0.34―1.31) | 0.241 |
| Drinking | 54 (21.86) | 0.59 (0.28―1.25) | 0.164 |
| Hypertension | 62 (25.10) | 0.976(0.46―1.99) | 0.922 |
| Diabetes mellitus | 49 (19.84) | 0.695 (0.42―1.72) | 0.592 |
| CHF | 39 (15.79) | 0.835 (0.37―1.87) | 0.660 |
| Hyperlipidemia | 41 (16.60) | 1.058 (0.50―2.26) | 0.883 |
| History oh stroke or TIA | 39 (15.79) | 0.282 (0.10―0.83) | 0.021 |
| Peripheral artery disease | 24 (9.72) | 1.491 (0.61―3.67) | 0.385 |
| CHA2DS2.VASC | |||
| 0 | 48 (19.43) | 1.0 | |
| 1 | 80 (32.39) | 0.667 (0.30―1.46) | 0.311 |
| 2 | 59 (23.89) | 0.682 (0.30―1.58) | 0.371 |
| ≥ 3 | 60 (14.98) | 0.552 (0.206―1.48) | 0.237 |
The results are expressed as mean ± (SD) /midian (Q1, Q3) / n (%).CI, confidence interval; BMI, body mass index; AF, atrial fibrillation; SCR, serum creatinine concentration; BNP, B-type natriuretic peptide; LVEF, left ventricular ejection fraction; LAAEV, LA appendage emptying velocity; LAVImax, maximum LA volume index; LAVImin, minimum LA volume index; TDI, tissue Doppler imaging; EI, expansion Index; DEI, diastolic emptying index; CHF, congestive heart failure; TIA, transient ischemia attack
Multivariable analysis of the relationship between LAAEV and AF recurrence
Table 3 presents the results of multivariable logistic regression analysis for the continuous variable LAAEV measurements and LAAEV categorized into three groups. The unadjusted covariates were equivalent to the univariate logistic regression analysis. The partially adjusted (Adjustment I) covariates included gender, age, BNP, SCR, LAVImin, EI, DEI, and smoking history. The fully adjusted (Adjustment II) covariates included gender, age, BNP, SCR, duration of AF, LAVImin, EI, DEI, smoking history, and the presence of stroke or TIA.
Table 3.
Multivariate analysis of the relationship between LAAEV and AF recurrence
| Non-adjusted | Adjustment I | Adjustment II | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | P-Value | OR (95%CI) | P-Value | OR (95%CI) | P-Value | |
| LAAEV | 0.916(0.887―0.947) | < 0.001 | 0.885(0.851―0.921) | < 0.001 | 0.882 (0.842―0.924) | < 0.001 |
| Low | 1.0 | 1.0 | 1.0 | |||
| Middle | 0.201 (0.096―0.418) | < 0.001 | 0.156 (0.058―0.419) | < 0.001 | 0.161 (0.048―0.541) | 0.0031 |
| High | 0.158 (0.073―0.341) | < 0.001 | 0.049 (0.017―0.138) | < 0.001 | 0.033 (0.009―0.116) | < 0.001 |
| P for trend | < 0.001 | < 0.001 | < 0.001 | |||
OR, odds ratio; CI, confidence interval; LAAEV, left atrial appendage emptying velocity
The results of the multivariable logistic regression analysis, considering unadjusted, Adjustment I, and Adjustment II models, showed that an increase in LAAEV was associated with a decreased risk of AF recurrence after RFCA. The ORs for LAAEV were 0.916, 0.885, and 0.882 in the unadjusted, Adjustment I, and Adjustment II models (p < 0.001 for all), respectively. Additionally, there was a statistically significant correlation between the increasing trend of LAAEV across the three groups and a decreased risk of post-ablation recurrence (p < 0.001 for all).
Curve fitting
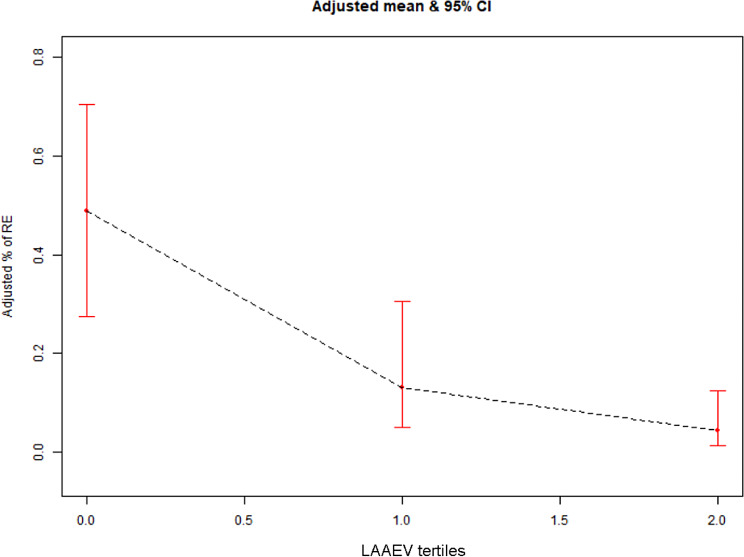
To examine the relationship between LAAEV and the risk of early PeAF recurrence following RFCA, we employed a GAM. After adjusting for gender, age, BNP, SCR, AF duration, LAVImin, EI, DEI, smoking history, and the presence of stroke or TIA, we observed a statistically significant correlation between increasing LAAEV and a decreasing probability of post-ablation recurrence. The probability was decreased from 48.78% (95% CI: 27.53–70.48%) in the lowest LAAEV group to 13.18% (95% CI: 4.96–30.64%) and 4.38% (95% CI: 1.44–12.56%) in the middle and high LAAEV groups, respectively (Fig. 2).
Fig. 2.
Relationship between three LAAEV groups and AF recurrence risk (The black dashed line represents the fitted line of the adjusted AF recurrence probability with three LAAEV groups, and the red line represents the 95% CI). LAAEV, left atrial appendage emptying velocity; AF, atrial fibrillation
Discussion
In this study, we found that there were differences in the recurrence rates of patients with early PeAF after RFCA among the three LAAEV groups. Even after adjusting for traditional risk factors, the multivariable logistic regression analysis showed a significant negative correlation between LAAEV and the risk of AF recurrence. The GAM demonstrated that the probability of post-ablation AF recurrence was gradually decreased with increasing LAAEV levels. The preoperative LAAEV measurement in patients with early PeAF can be used to differentiate their risk of post-ablation recurrence and predict which patients are more likely to benefit from RFCA. For early PeAF patients with lower LAAEV, additional linear ablation and/or substrate modification strategies during the procedure may be needed to reduce post-ablation recurrence. Patients with low LAAEV should be closely monitored postoperatively to detect AF recurrence early.
For patients with PeAF, RFCA is recommended as a first-line treatment according to guidelines. In studies encompassing both paroxysmal atrial fibrillation (PAF) and PeAF patients who undergo RFCA as their initial treatment, success rates at 6 or 12 months post-ablation have been reported to range from 59 to 80% [15, 16]. Our study aligned with these findings, as we observed a success rate of 76.11% at 6 months post-ablation. However, high recurrence rates after the procedure and the need for repeat ablation remain important challenges for both clinicians and AF patients.
Left atrial fibrosis, which induces structural remodeling of the left atrium, is a necessary condition for the development and continuation of AF. Fibrosis results in an increase in the abnormal extracellular matrix, leading to pathological alterations in atrial conduction and potentially playing a role in the initiation of AF [17, 18]. Left atrial enlargement, manifested by changes in its dimensions, is an important self-regulatory indicator for AF recurrence [19, 20]. Patients with left atrial enlargement are more prone to recurrent AF events. Our findings were consistent with previous studies, indicating that atrial remodeling was a crucial factor in the persistence of AF and its recurrence after ablation.
Previous studies have found that in patients with PAF, LAAEV is an independent predictor of recurrence after catheter ablation [21]. In PeAF patients undergoing initial RFCA, a decrease in LAAEV at 12or 24 months post-ablation increases the likelihood of recurrence, and low LAAEV is associated with AF recurrence and serves as an independent predictor of post-ablation recurrence [22–24], which is consistent with the results of our study. Nonetheless, the majority of studies examining risk factors for PeAF recurrence have typically encompassed both early and long-standing PeAF cases. In contrast, our study specifically concentrated on early PeAF patients with a duration of less than 3 months. The selection of patients in our study was based on the criteria of early PeAF as outlined in the 2017 h/ERA/ECAS/APHRS/SOLAECE expert consensus [6]. Stratified studies conducted in PeAF patients have the potential to provide more accurate predictions regarding the risk of recurrence after RFCA. Such studies enable personalized management of AF patients, facilitating precise adjustments to clinical strategies and ultimately enhancing the success rate of ablation procedures.
On the other hand, Ying Wei et al. have suggested that LAAEV in PeAF patients is not a reliable predictor of AF recurrence after catheter ablation [25], which differs from our results. These discrepancies in findings could be attributed to differences in the inclusion criteria, exclusion criteria, and baseline variables of the patients included in the two studies. In our present study, we only excluded AF patients with moderate to severe heart valve disease, while Ying Wei et al. have excluded AF patients with any degree of heart valve disease. However, our sample represented the majority of patients presenting to the hospital with this condition. Additionally, our sample had a shorter duration of AF compared to the sample in Ying Wei et al.‘s study. The prolonged duration of AF is associated with greater left atrial fibrosis [26], and AF duration is a risk factor for AF recurrence [27, 28]. Our multivariate regression analysis showed that even after adjusting for AF duration and other risk factors, LAAEV remained an independent risk factor for AF recurrence.
LAA is a finger-like projection originating from the main body of the left atrium, which can regulate left atrial pressure and volume [29]. LAAEV serves as an indicator of the emptying capability of the LAA and effectively reflects the contractile function of both the appendage and the left atrial wall [22, 30]. In PeAF patients, LAAEV exhibits a negative correlation with the extent of low voltage areas (LVA) within the left atrium [31]. LVA is recognized as a surrogate marker of atrial fibrosis [32] and is considered an important predictor of AF recurrence following catheter ablation [33, 34]. During cardiac contraction, when there is a significant increase in volume load, LAA serves as a reservoir to prevent excessive elevation of left atrial pressure [35]. A diastolic LAAEV > 46.4 cm/s in patients with AF is associated with good left atrial booster pump function [36]. Structural changes in LAA occur prior to left atrial remodeling, and both the morphology and function of the remodeled left atrium can predict AF recurrence after ablation [28, 37]. Based on the hemodynamic activity of LAA and our study results, we believed that LAAEV was a more sensitive predictor of AF recurrence than indicators of left atrial morphology or functional remodeling.
It is important to note that this study has some limitations. Firstly, it is a single-center retrospective study with a relatively small sample size, which may introduce selection bias. Secondly, the ablation approaches varied among patients, and different ablation methods may impact the recurrence rates in PeAF patients. Lastly, the use of electrocardiography and intermittent Holter monitoring may underestimate the actual recurrence rate. Future studies should focus on large-scale, multicenter, prospective clinical research to further validate the predictive value of LAAEV in RFCA-related recurrence among early PeAF patients.
Conclusion
In conclusion, this retrospective study analyzed early PeAF patients who underwent RFCA and successfully converted to sinus rhythm. After adjusting for confounding factors, we found that LAAEV was an independent predictor of AF recurrence, with a higher risk of recurrence associated with lower LAAEV. This finding might contribute to the development of personalized ablation strategies and improvement in treatment and care for early PeAF patients.
Acknowledgements
We thank all those who have been helpful to this manuscript.
Author contributions
Yuxia Miao performed the study and wrote the manuscript; Min Xu contributed to the conception of the study and performed the data analysis; Zhenni Yang contributed significantly to analysis and manuscript preparation; Mingxia Gong helped perform the analysis with constructive discussions.Ling Yang provided fund support.All authors reviewed the manuscript.All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Changzhou Municipal Health Commission (Grant No. QN202208), Changzhou City High-level and Top Talents Training Project of 14th Five-Year Plan (Grant No. KY20221362) and National Natural Science Foundation of China (Grant No. 82070405).
Data availability
The original data of our study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study adhered to the guidelines outlined in the revised 2013 Helsinki Declaration, and informed consent was obtained from all patients individually. The study protocol received approval from the Scientific Ethics Committee of The Third Affiliated Hospital of Soochow University. We followed all relevant guidelines and regulations during the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) [J]. European heart journal; 2020.
- 2.Lippi G, Sanchis-Gomar F Cervelling. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge [J]. Int J Stroke: Official J Int Stroke Soc. 2021;16(2):217–21. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Johnsen S P, Guo Y, et al. Epidemiology of Atrial Fibrillation: Geographic/Ecological risk factors, Age, Sex, Genetics [J]. Cardiac Electrophysiol Clin. 2021;13(1):1–23. [DOI] [PubMed] [Google Scholar]
- 4.Zoni-Berisso M, Lercari F, Carazza T et al. Epidemiology of atrial fibrillation: European perspective [J]. Clin Epidemiol, 2014, 6(213 – 20. [DOI] [PMC free article] [PubMed]
- 5.Burdett P, Lip G Y H. Atrial fibrillation in the UK: predicting costs of an emerging epidemic recognizing and forecasting the cost drivers of atrial fibrillation-related costs [J]. Eur Heart J Qual care Clin Outcomes. 2022;8(2):187–94. [DOI] [PubMed] [Google Scholar]
- 6.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation [J]. Heart Rhythm. 2017;14(10):e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poole Je, Bahnson T D, Monahan K H, et al. Recurrence of Atrial Fibrillation after catheter ablation or Antiarrhythmic Drug Therapy in the CABANA trial [J]. J Am Coll Cardiol. 2020;75(25):3105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marrouche N F Wilberd, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study [J]. JAMA. 2014;311(5):498–506. [DOI] [PubMed] [Google Scholar]
- 9.Suksaranjit P, Marrouche N F, Han F T, et al. Relation of left atrial appendage remodeling by Magnetic Resonance Imaging and outcome of ablation for Atrial Fibrillation [J]. Am J Cardiol. 2018;122(1):83–8. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y C, Lin L C, Lin MS, et al. Identification of good responders to rhythm control of paroxysmal and persistent atrial fibrillation by transthoracic and transesophageal echocardiography [J]. Cardiology. 2005;104(4):202–9. [DOI] [PubMed] [Google Scholar]
- 11.Hondo T, Okamoto M, Yamane T, et al. The role of the left atrial appendage. A volume loading study in open-chest dogs [J]. Jpn Heart J. 1995;36(2):225–34. [DOI] [PubMed] [Google Scholar]
- 12.Davis Ca 3rd, Rembert J C, Greenfield J C JR. Compliance of left atrium with and without left atrium appendage [J]. Am J Physiol. 1990;259(4 Pt 2):H1006–8. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima K, Fukushima N, Ejima K et al. Left atrial appendage flow velocity and time from P-wave onset to tissue Doppler-derived A’ predict atrial fibrillation recurrence after radiofrequency catheter ablation [J]. Echocardiography (Mount Kisco, NY), 2015, 32(7): 1101-8. [DOI] [PubMed]
- 14.Otani K, Takeuchi M, Kaku K, et al. Impact of diastolic dysfunction grade on left atrial mechanics assessed by two-dimensional speckle tracking echocardiography [J]. J Am Soc Echocardiogr. 2010;23(9):961–7. [DOI] [PubMed] [Google Scholar]
- 15.Berruezo A, Tamborero D. Pre-Procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation [J]. Eur Heart J. 2007;28(7):836–41. [DOI] [PubMed] [Google Scholar]
- 16.Seow S C, Lim T W, Koay C H, et al. Efficacy and late recurrences with wide electrical pulmonary vein isolation for persistent and permanent atrial fibrillation [J]. Europace. 2007;9(12):1129–33. [DOI] [PubMed] [Google Scholar]
- 17.Mikhailov A V, Kalyanasundaram A, LI N, et al. Comprehensive evaluation of electrophysiological and 3D structural features of human atrial myocardium with insights on atrial fibrillation maintenance mechanisms [J]. J Mol Cell Cardiol. 2021;151:56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begg Ga, Holden A V, Lip G Y et al. Assessment of atrial fibrosis for the rhythm control of atrial fibrillation [J]. Int J Cardiol, 2016, 220(155 – 61. [DOI] [PubMed]
- 19.Mohanty S, Della Rocca D G, Gianni C, et al. Predictors of recurrent atrial fibrillation following catheter ablation [J]. Expert Rev Cardiovasc Ther. 2021;19(3):237–46. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y G, Choi J I, Boo K Y, et al. Clinical and echocardiographic risk factors predict late recurrence after Radiofrequency catheter ablation of Atrial Fibrillation [J]. Sci Rep. 2019;9(1):6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang P, Liu Qm, Xu T, et al. The predictive value of P wave index and left atrial appendage emptying velocity in paroxysmal atrial fibrillation recurrence after catheter ablation procedure. Chin J Interventional Cardiol. 2019;27(02):90–9. [Google Scholar]
- 22.Ma X X, Zhang Y L Hub, et al. Association between left atrial appendage emptying velocity, N-terminal plasma brain natriuretic peptide levels, and recurrence of atrial fibrillation after catheter ablation [J]. J Interv Card Electrophysiol. 2017;48(3):343–50. [DOI] [PubMed] [Google Scholar]
- 23.Kanda T, Masuda M, Sunaga A, et al. Low left atrial appendage flow velocity predicts recurrence of atrial fibrillation after catheter ablation of persistent atrial fibrillation [J]. J Cardiol. 2015;66(5):377–81. [DOI] [PubMed] [Google Scholar]
- 24.Yang W, Zhao Q. The prognostic significance of left atrial appendage peak flow velocity in the recurrence of persistent atrial fibrillation following first radiofrequency catheter ablation [J]. J Thorac Disease. 2021;13(10):5954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y, Liu S, Yu H, et al. The predictive value of growth differentiation Factor-15 in recurrence of Atrial Fibrillation after Catheter ablation [J]. Mediators of inflammation; 2020. 2020(8360936. [DOI] [PMC free article] [PubMed]
- 26.Callegari S, Macchi E, Monaco R, et al. Clinicopathological Bird’s-Eye View of Left Atrial Myocardial Fibrosis in 121 patients with Persistent Atrial Fibrillation: developing Architecture and Main Cellular players [J]. Circ Arrhythm Electrophysiol. 2020;13(7):e007588. [DOI] [PubMed] [Google Scholar]
- 27.Yu, H T, Kim I S, Kim T H, et al. Persistent atrial fibrillation over 3 years is associated with higher recurrence after catheter ablation [J]. J Cardiovasc Electrophysiol. 2020;31(2):457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajraktari G, Bytyçi I, HENEIN M Y. Left atrial structure and function predictors of recurrent fibrillation after catheter ablation: a systematic review and meta-analysis [J]. Clin Physiol Funct Imaging. 2020;40(1):1–13. [DOI] [PubMed] [Google Scholar]
- 29.Thomas L, Abhayaratna Wp. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance [J]. JACC Cardiovasc Imaging. 2017;10(1):65–77. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida N, Okamoto M. Validation of transthoracic tissue Doppler assessment of left atrial appendage function [J]. J Am Soc Echocardiogr. 2007;20(5):521–6. [DOI] [PubMed] [Google Scholar]
- 31.Kiedrowicz R M, Wielusinski M, Wojtarowicz A, et al. Left and right atrial appendage functional features as predictors for voltage-defined left atrial remodelling in patients with long-standing persistent atrial fibrillation [J]. Heart Vessels. 2021;36(6):853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hohendanner F, Romero I, Blaschke F et al. Extent and magnitude of low-voltage areas assessed by ultra-high-density electroanatomical mapping correlate with left atrial function [J]. Int J Cardiol, 2018, 272(108 – 12. [DOI] [PubMed]
- 33.Huang D, Li Jb, Zghaib T, et al. The extent of Left Atrial Low-Voltage Areas included in pulmonary vein isolation is Associated with Freedom from Recurrent Atrial Arrhythmia [J]. Can J Cardiol. 2018;34(1):73–9. [DOI] [PubMed] [Google Scholar]
- 34.Vlachos K, Efremidis M, Letsas K P, et al. Low-voltage areas detected by high-density electroanatomical mapping predict recurrence after ablation for paroxysmal atrial fibrillation [J]. J Cardiovasc Electrophysiol. 2017;28(12):1393–402. [DOI] [PubMed] [Google Scholar]
- 35.Hoit B D. Left atrial size and function: role in prognosis [J]. J Am Coll Cardiol. 2014;63(6):493–505. [DOI] [PubMed] [Google Scholar]
- 36.Grimm R A Donale, Yamada H, et al. Usefulness of Doppler assessment of pulmonary vein and left atrial appendage flow following pulmonary vein isolation of chronic atrial fibrillation in predicting recovery of left atrial function [J]. Am J Cardiol. 2005;95(8):941–7. [DOI] [PubMed] [Google Scholar]
- 37.Ma X X, Boldt L H, Zhang Yl et al. Clinical relevance of left atrial strain to Predict recurrence of Atrial Fibrillation after catheter ablation: a Meta-analysis [J]. Echocardiography (Mount Kisco, NY), 2016, 33(5): 724–33. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data of our study are available from the corresponding author upon reasonable request.