Abstract
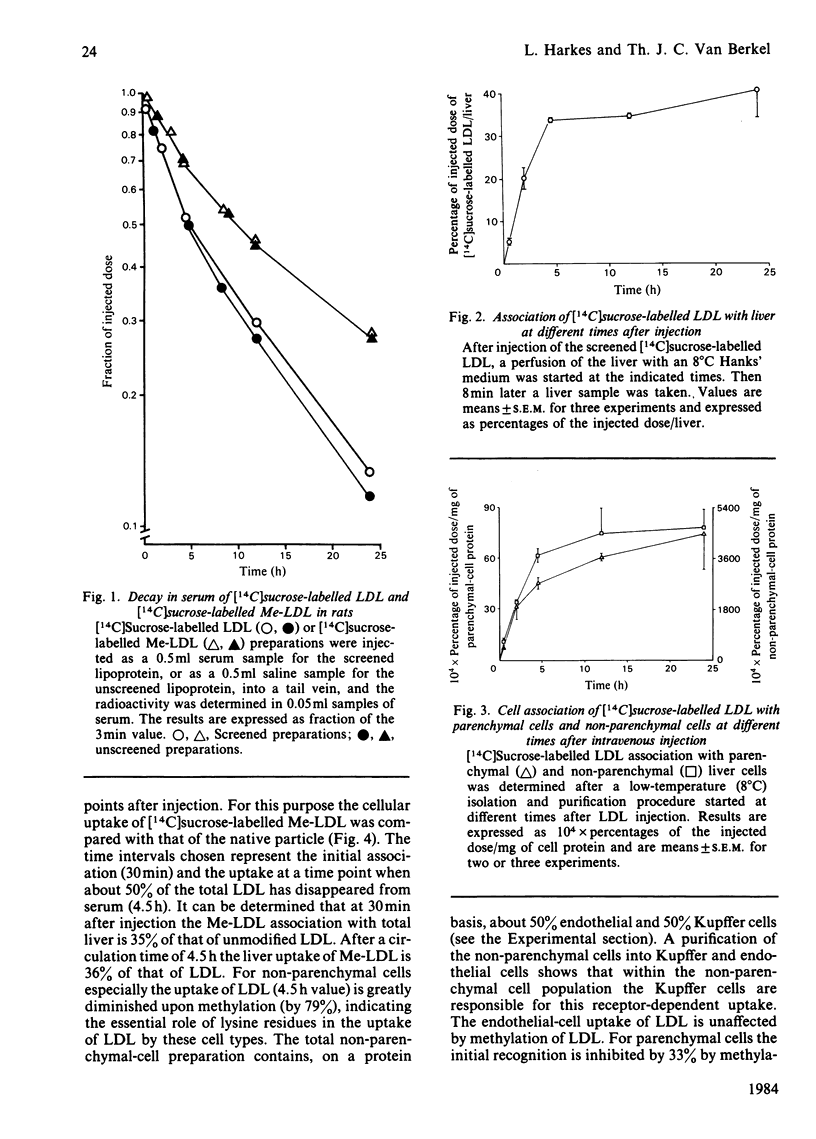
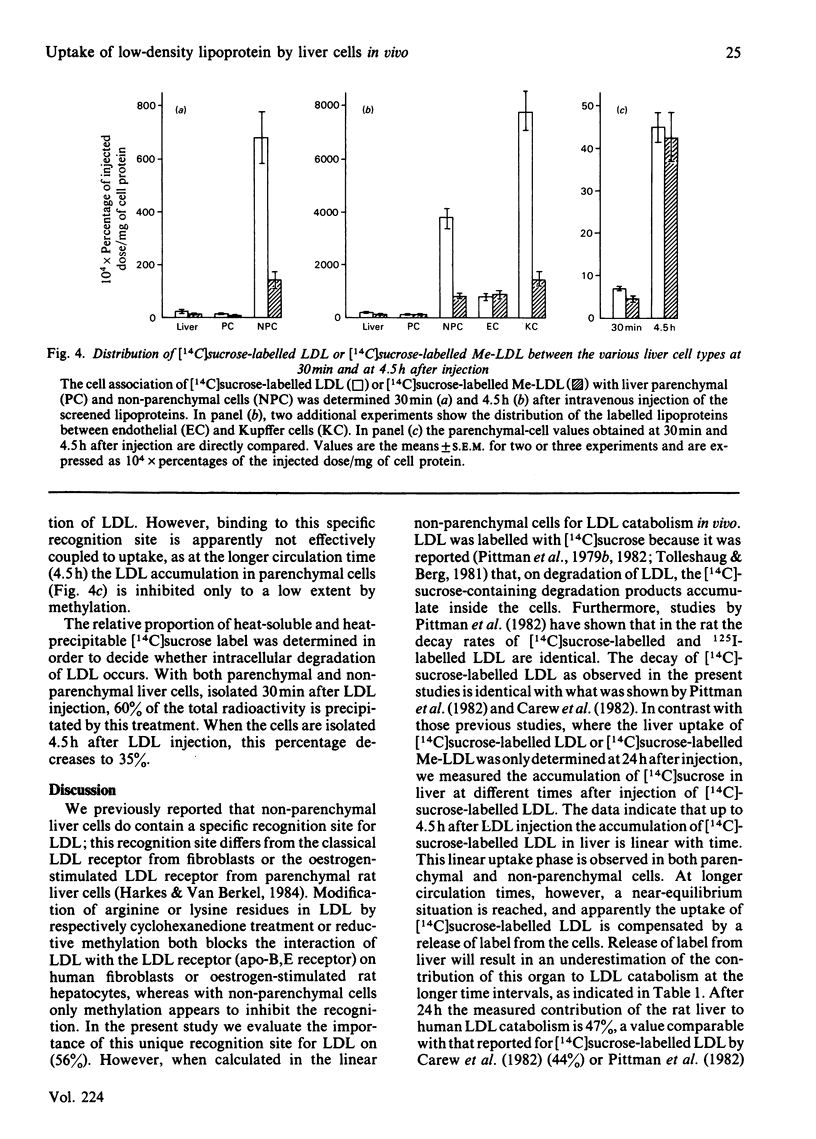
In order to assess the relative importance of the receptor for low-density lipoprotein (LDL) (apo-B,E receptor) in the various liver cell types for the catabolism of lipoproteins in vivo, human LDL was labelled with [14C]sucrose. Up to 4.5h after intravenous injection, [14C]sucrose becomes associated with liver almost linearly with time. During this time the liver is responsible for 70-80% of the removal of LDL from blood. A comparison of the uptake of [14C]sucrose-labelled LDL and reductive-methylated [14C]sucrose-labelled LDL ([14C]sucrose-labelled Me-LDL) by the liver shows that methylation leads to a 65% decrease of the LDL uptake. This indicated that 65% of the LDL uptake by liver is mediated by a specific apo-B,E receptor. Parenchymal and non-parenchymal liver cells were isolated at various times after intravenous injection of [14C]sucrose-labelled LDL and [14C]sucrose-labelled Me-LDL. Non-parenchymal liver cells accumulate at least 60 times as much [14C]sucrose-labelled LDL than do parenchymal cells accumulate at least 60 times as much [14C]sucrose-labelled LDL than do parenchymal cells when expressed per mg of cell protein. This factor is independent of the time after injection of LDL. Taking into account the relative protein contribution of the various liver cell types to the total liver, it can be calculated that non-parenchymal cells are responsible for 71% of the total liver uptake of [14C]sucrose-labelled LDL. A comparison of the cellular uptake of [14C]sucrose-labelled LDL and [14C]sucrose-labelled Me-LDL after 4.5h circulation indicates that 79% of the uptake of LDL by non-parenchymal cells is receptor-dependent. With parenchymal cells no significant difference in uptake between [14C]sucrose-labelled LDL and [14C]sucrose-labelled Me-LDL was found. A further separation of the nonparenchymal cells into Kupffer and endothelial cells by centrifugal elutriation shows that within the non-parenchymal-cell preparation solely the Kupffer cells are responsible for the receptor-dependent uptake of LDL. It is concluded that in rats the Kupffer cell is the main cell type responsible for the receptor-dependent catabolism of lipoproteins containing only apolipoprotein B.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blouin A., Bolender R. P., Weibel E. R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977 Feb;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys C. H., Dejong A. S., Bouma J. M., Gruber M. Rapid uptake by liver sinusoidal cells of serum albumin modified with retention of its compact conformation. Biochim Biophys Acta. 1975 May 5;392(1):95–100. doi: 10.1016/0304-4165(75)90169-5. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Pittman R. C., Steinberg D. Tissue sites of degradation of native and reductively methylated [14C]sucrose-labeled low density lipoprotein in rats. Contribution of receptor-dependent and receptor-independent pathways. J Biol Chem. 1982 Jul 25;257(14):8001–8008. [PubMed] [Google Scholar]
- Fahimi H. D. The fine structural localization of endogenous and exogenous peroxidase activity in Kupffer cells of rat liver. J Cell Biol. 1970 Oct;47(1):247–262. doi: 10.1083/jcb.47.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkes L., Van Berkel T. J. In vivo characteristics of a specific recognition site for LDL on non-parenchymal rat liver cells which differs from the 17 alpha-ethinyl estradiol-induced LDL receptor on parenchymal liver cells. Biochim Biophys Acta. 1984 Jul 6;794(2):340–347. doi: 10.1016/0005-2760(84)90165-6. [DOI] [PubMed] [Google Scholar]
- Harkes L., van Berkel T. J. A saturable, high-affinity binding site for human low density lipoprotein on freshly isolated rat hepatocytes. Biochim Biophys Acta. 1982 Sep 14;712(3):677–683. doi: 10.1016/0005-2760(82)90297-1. [DOI] [PubMed] [Google Scholar]
- Harkes L., van Berkel T. J. Cellular localization of the receptor-dependent and receptor-independent uptake of human LDL in the liver of normal and 17 alpha-ethinyl estradiol-treated rats. FEBS Lett. 1983 Apr 5;154(1):75–80. doi: 10.1016/0014-5793(83)80878-3. [DOI] [PubMed] [Google Scholar]
- Knook D. L., Sleyster E. C. Isolated parenchymal, Kupffer and endothelial rat liver cells characterized by their lysosomal enzyme content. Biochem Biophys Res Commun. 1980 Sep 16;96(1):250–257. doi: 10.1016/0006-291x(80)91207-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Pitas R. E., Weisgraber K. H., Brown J. H., Gross E. Inhibition of lipoprotein binding to cell surface receptors of fibroblasts following selective modification of arginyl residues in arginine-rich and B apoproteins. J Biol Chem. 1977 Oct 25;252(20):7279–7287. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Packard C. J., Slater H. R., Shepherd J. The reticuloendothelial system and low density lipoprotein metabolism in the rabbit. Biochim Biophys Acta. 1982 Aug 18;712(2):412–419. doi: 10.1016/0005-2760(82)90361-7. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Attie A. D., Carew T. E., Steinberg D. Tissue sites of catabolism of rat and human low density lipoproteins in rats. Biochim Biophys Acta. 1982 Jan 15;710(1):7–14. doi: 10.1016/0005-2760(82)90183-7. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Attie A. D., Carew T. E., Steinberg D. Tissue sites of degradation of low density lipoprotein: application of a method for determining the fate of plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5345–5349. doi: 10.1073/pnas.76.10.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Green S. R., Attie A. D., Steinberg D. Radiolabeled sucrose covalently linked to protein. A device for quantifying degradation of plasma proteins catabolized by lysosomal mechanisms. J Biol Chem. 1979 Aug 10;254(15):6876–6879. [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Slater H. R., Packard C. J., Shepherd J. Measurement of receptor-independent lipoprotein catabolism using 1,2 cyclohexanedione-modified low density lipoprotein. J Lipid Res. 1982 Jan;23(1):92–96. [PubMed] [Google Scholar]
- Slater H. R., Packard C. J., Shepherd J. Receptor-independent catabolism of low density lipoprotein. Involvement of the reticuloendothelial system. J Biol Chem. 1982 Jan 10;257(1):307–310. [PubMed] [Google Scholar]
- Tolleshaug H., Berg T. The effect of leupeptin on intracellular digestion of asialofetuin in rat hepatocytes. Exp Cell Res. 1981 Jul;134(1):207–217. doi: 10.1016/0014-4827(81)90478-x. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- van Berkel T. J., van Tol A. In vivo uptake of human and rat low density and high density lipoprotein by parenchymal and nonparenchymal cells from rat liver. Biochim Biophys Acta. 1978 Aug 25;530(2):299–304. doi: 10.1016/0005-2760(78)90015-2. [DOI] [PubMed] [Google Scholar]


