Abstract
Background
The impact of dynamic changes in the degree of atherosclerosis on the development of prediabetes remains unclear. This study aims to investigate the association between cumulative atherogenic index of plasma (CumAIP) exposure during follow-up and the development of prediabetes in middle-aged and elderly individuals.
Methods
A total of 2,939 prediabetic participants from the first wave of the China Health and Retirement Longitudinal Study (CHARLS) were included. The outcomes for these patients, including progression to diabetes and regression to normal fasting glucose (NFG), were determined using data from the third wave. CumAIP was calculated as the ratio of the average AIP values measured during the first and third waves to the total exposure duration. The association between CumAIP and the development of prediabetes was analyzed using multivariable Cox regression and restricted cubic spline (RCS) regression.
Results
During a median follow-up period of 3 years, 15.21% of prediabetic patients progressed to diabetes, and 22.12% regressed to NFG. Among the groups categorized by CumAIP quartiles, the proportion of prediabetes progressing to diabetes gradually increased (Q1: 10.61%, Q2: 13.62%, Q3: 15.65%, Q4: 20.95%), while the proportion regressing to NFG gradually decreased (Q1: 23.54%, Q2: 23.71%, Q3: 22.18%, Q4: 19.05%). Multivariable-adjusted Cox regression showed a significant positive linear correlation between high CumAIP exposure and prediabetes progression, and a significant negative linear correlation with prediabetes regression. Furthermore, in a stratified analysis, it was found that compared to married individuals, those who were unmarried (including separated, divorced, widowed, or never married) had a relatively higher risk of CumAIP-related diabetes.
Conclusion
CumAIP is closely associated with the development of prediabetes. High CumAIP exposure not only increases the risk of prediabetes progression but also hinders its regression within a certain range. These findings suggest that monitoring and maintaining appropriate AIP levels may help prevent the deterioration of blood glucose levels.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02449-y.
Keywords: Cumulative atherogenic index of plasma, Atherogenic index of plasma, Progression to diabetes, Regression to normal fasting glucose
Background
Prediabetes is a common metabolic disorder during aging, representing the earliest identifiable stage of glucose dysregulation, characterized by plasma glucose levels between normal and diabetic thresholds [1, 2]. According to the American Diabetes Association (ADA) criteria, recent nationwide epidemiological surveys in China indicate a prediabetes prevalence of approximately 35.2%, with the rate approaching 50% among Chinese adults over 50 years old [3]. The development of prediabetes may result in progression to diabetes, maintenance of the prediabetic state, or regression to NFG [4, 5]. A recent meta-analysis of 103 prospective cohort studies reported that the cumulative incidence of prediabetes progressing to diabetes within five years ranges from 18 to 39%, while the rate of regression to NFG ranges from 33 to 59% [6]. It is noteworthy that the progression of prediabetes can exacerbate various cardiovascular and metabolic diseases, leading to severe adverse outcomes, whereas regression can significantly improve these outcomes [2, 7–9]. Therefore, it is essential to systematically evaluate the key factors influencing the progression and regression of prediabetes.
Contrary to the common belief that atherosclerosis is merely a complication of diabetes, atherosclerosis might also be a contributing factor to diabetes, as atherosclerotic burden has been found to exist before the onset of diabetes, accompanied by pro-atherosclerotic inflammation and vascular constriction [10–13]. In terms of lipid patterns, prediabetic individuals typically exhibit atherogenic dyslipidemia, characterized by increased triglycerides (TG), very low-density lipoprotein cholesterol (vLDL-C), and decreased high-density lipoprotein cholesterol (HDL-C) [14, 15]. Moreover, clinical evidence has revealed that the AIP, representing atherosclerosis, is a risk factor for both prediabetes and diabetes in adults [16–22] and is negatively associated with prediabetes regression [22]. However, previous findings have overlooked the dynamic changes in atherosclerosis over time, which may significantly influence the transition of glycemic states. A previous cohort study of adolescents with type 1 diabetes suggested that changes in arterial stiffness might affect glycemic status. Lower insulin sensitivity at baseline appeared to be an important risk factor for increased arterial stiffness over time in these adolescents [23]. This finding further indicates the importance of assessing atherosclerosis dynamics over time for glucose metabolism. However, the association between cumulative exposure to atherosclerosis during follow-up and the progression or regression of prediabetes in adults remains unclear. To address this limitation, the current study aimed to analyze the CHARLS national survey follow-up data to assess the impact of CumAIP exposure during follow-up on the development of prediabetes in middle-aged and elderly Chinese.
Methods
Study population and design
CHARLS is a prospective national cohort study conducted in China, utilizing a complex probabilities proportional to size sampling method to collect nationally representative health-related data from middle-aged and elderly populations in China [24]. The detailed study design is summarized in the online supplementary methods, with Supplementary Fig. 1 showing the screening process for the CHARLS cohort. The current study uses data from the first wave (2011–2012) of CHARLS as the baseline and data from the third wave (2015–2016) as the study endpoint, as these waves included blood samples from the participants. The entire study process adhered to the Declaration of Helsinki, and the study results were reported following the STROBE guidelines. The protocol for the CHARLS cohort was authorized by the Ethics Review Committee of Peking University (IRB00001052–11015), and all participants provided written informed consent at the time of participation.
Figure 1 shows the screening process for the study population in the current study. Among the 14,226 participants from the first wave who were followed up in the third wave, we excluded those lacking baseline blood glucose and glycated hemoglobin (HbA1c) information (n = 4,338), those diagnosed with diabetes (n = 1,479), and those with hypoglycemia or normal blood glucose (n = 4,461). Additionally, participants lacking blood glucose and HbA1c information during follow-up (n = 999) and those missing AIP information (n = 10) were also excluded. Ultimately, 2,939 prediabetic participants were included in the current analysis.
Fig. 1.
Flow chart of study participants
Calculation of CumAIP
AIP = log10 [TG (mg/dL)/HDL-C(mg/dL)] [16].
CumAIP was calculated as the ratio of the average AIP values measured during the first and third waves to the total exposure duration, specifically as follows: (AIP2012 + AIP2015)/2 * time (2012 − 2015) [25].
Determination and definition of prediabetes and its development
The determination of prediabetes and its development referred to the ADA diagnostic criteria. The development of prediabetes included progression to diabetes and regression to NFG. In the current study, we primarily assessed blood glucose status based on the ADA standards for impaired fasting glucose. NFG is defined as fasting plasma glucose (FPG) < 5.6 mmol/L and HbA1c < 5.7%; prediabetes is defined as FPG 5.6–6.9 mmol/L or HbA1c 5.7–6.4%; diabetes is defined as FPG ≥ 7.0 mmol/L, HbA1c ≥ 6.5%, or a self-reported history [26]. Additionally, for participants with random plasma glucose (RPG) measurements, RPG < 7.8 mmol/L indicated normal glucose, while RPG > 11.1 mmol/L indicated diabetes.
Covariates
As previously described [24], CHARLS utilized face-to-face computer-assisted personal interviews to collect data. A standardized structured questionnaire recorded demographic information (gender, age), disease information [hypertension, cardiovascular disease (CVD), stroke; detailed diagnostic information available in the online supplementary methods], body measurements (height, weight, and blood pressure), health behaviors (smoking and drinking status), registered residence, and laboratory parameters. Laboratory parameters were analyzed using venous blood samples of subjects after they overnight fasting collected by professional healthcare workers. The blood samples were sent to the central laboratory in Beijing for biochemical analysis using standard methods, including measurements of total cholesterol (TC), TG, LDL-C, HDL-C, blood urea nitrogen (BUN), high-sensitivity C-reactive protein (hs-CRP), uric acid (UA), serum creatinine (Cr), plasma glucose, and HbA1c levels.
In addition, we also evaluated whether the study population engaged in physical exercise and drug treatment during follow-up based on the physical activity questionnaire and drug use information questionnaire, respectively, of the study population in the third wave of the survey. Drug treatment included antihypertensive and antidiabetic drug treatment, and physical exercise was divided into light activity, moderate activity, and intensive activity according to the intensity of exercise (See Supplementary Methods for details).
Missing data handling
Given the substantial amount of missing blood glucose measurement data in the CHARLS dataset, we first compared the baseline characteristics of participants who provided blood glucose/HbAIc measurement information with those who did not, in both the first and third waves of the survey, before conducting the research analysis. The comparison results in Supplementary Tables 1 and 2 showed that most of the baseline characteristics of the group with missing blood glucose/HbAIc measurement information and the group without missing information exhibited similar distribution patterns. (P > 0.05 or Standardized difference < 0.1). This result suggested that the missing blood glucose/HbAIc in these two waves of surveys was completely random.
Supplementary Table 3 shows the missing baseline data in the current analysis, including 4 subjects with missing educational information, 3 subjects with missing marital status, 4 subjects with missing registered residence, 31 subjects with missing CVD information, 23 subjects with missing stroke information, 11 subjects with missing hypertension information, 13 subjects with missing drinking status, 369 subjects with missing blood pressure measurements, 354 subjects with missing height, 346 subjects with missing weight, and 1 subjects with missing LDL-C. To minimize potential bias due to missing data, we used multiple imputations by fully conditional specification to address the missing data issue [27].
Statistical analysis
For descriptive data, we provided flexible data presentation formats; qualitative variables were presented as frequencies and percentages, while quantitative variables were presented as medians and interquartile ranges or means and standard deviations. One-way ANOVA, Kruskal-Wallis H test, and Chi-square test were used to examine differences between groups.
Multivariable Cox regression models were used to examine the association between CumAIP exposure and the development of prediabetes, with 95% confidence intervals (CI) and hazard ratio (HR) estimated for the associations. For the development of prediabetes, we used a one-to-one method to split the data into binary datasets for each category [28, 29]. Considering collinearity (Supplementary Table 4), we included baseline covariates related to atherosclerosis and the development of diabetes in the multivariable models that incorporated time, including age, gender, education, registered residence, CVD, stroke, hypertension, smoking status, drinking status, height, systolic blood pressure (SBP), diastolic blood pressure (DBP), LDL-C, HbA1c, UA, Cr, BUN, and hs-CRP. Additionally, we constructed and visualized RCS regressions (with 4 knots) to test the dose-response relationship between CumAIP exposure and the development of prediabetes. Stratified analyses were also performed to explore whether the association between CumAIP exposure and the development of prediabetes varied by gender, age, education, marital status, registered residence, comorbidities (hypertension, CVD, stroke), and physical exercise or drug treatment during follow-up.
In sensitivity analyses, we evaluated the association between CumAIP exposure and the development of prediabetes based on the diagnostic criteria recommended by the World Health Organization (WHO) [30]. Secondly, given the adequate sample size in the current study, we followed the recommendations in “Regression Modeling Strategies” to apply an RCS analysis strategy with 5 knots to assess the dose-response relationship between CumAIP exposure and the development of prediabetes. Thirdly, considering the potential impact of unmeasured confounders, we calculated the minimum E-value needed to quantify the effect of potential confounders on the results based on the final model [31].
Data analysis in the current study was conducted using R statistical software (version 4.2.1) and Empower(R) (version 2.0). All statistical tests were two-sided, and a P-value less than 0.05 was considered significant.
Results
Study participants
In the current analysis, we included 1,357 male and 1,582 female prediabetic participants with an average age of 60 years. Table 1 presents the baseline characteristics of the study participants grouped by CumAIP quartiles. Participants with high CumAIP exposure were more likely to have adverse diabetes risk factors at baseline, and in further analysis, we also found that this unfavorable metabolic profile persisted during follow-up (Supplementary Table 5); including elevated SBP, DBP, weight, TC, TG, hs-CRP, glucose, UA, BUN, and lower HDL-C levels. Additionally, participants with high CumAIP exposure generally had lower education levels, a higher proportion were married, fewer had smoking or drinking habits and a higher proportion of subjects with comorbidities, including hypertension, CVD, stroke.
Table 1.
Summary of baseline characteristics of the study population according to CumAIP quartile group
| CumAIP quartile | P-value | ||||
|---|---|---|---|---|---|
| Q1(-0.14-0.06) | Q2(0.06–0.12) | Q3(0.12–0.18) | Q4(0.18–0.45) | ||
| No. of subjects | 735 | 734 | 735 | 735 | |
| Age, years | 60.72 (9.06) | 60.20 (8.70) | 58.98 (8.67) | 57.61 (8.42) | < 0.001 |
| SBP, mmHg | 128.67 (21.22) | 129.69 (20.29) | 131.21 (21.55) | 133.14 (20.06) | < 0.001 |
| DBP, mmHg | 73.65 (11.67) | 75.48 (11.54) | 77.15 (12.01) | 78.95 (11.97) | < 0.001 |
| Height, m | 1.58 (0.08) | 1.57 (0.09) | 1.58 (0.08) | 1.58 (0.09) | 0.045 |
| Weight, kg | 55.30 (10.37) | 58.33 (11.14) | 61.98 (11.21) | 65.28 (11.58) | < 0.001 |
| TC, mg/dL | 193.84 (34.61) | 195.23 (39.91) | 197.63 (37.63) | 203.75 (39.66) | < 0.001 |
| LDL-C, mg/dL | 115.21 (95.10-132.41) | 120.43 (100.90-144.97) | 122.17 (101.68–147.10) | 114.43 (91.43-139.95) | < 0.001 |
| TG, mg/dL | 66.38 (54.87–82.31) | 92.92 (77.88-115.71) | 126.56 (103.55-157.09) | 206.21 (163.72-279.66) | < 0.001 |
| HDL-C, mg/dL | 64.95 (56.44-75.00) | 52.96 (45.62–60.70) | 46.39 (40.98–52.19) | 36.73 (32.09–42.53) | < 0.001 |
| hs-CRP, mg/L | 0.75 (0.45–1.71) | 0.85 (0.51–1.78) | 1.16 (0.63–2.18) | 1.31 (0.71–2.79) | 0.091 |
| Glucose, mmol/L | 107.50 (7.02) | 107.71 (8.04) | 108.40 (7.93) | 110.03 (8.16) | < 0.001 |
| HbA1c, % | 5.22 (0.41) | 5.23 (0.42) | 5.24 (0.42) | 5.22 (0.44) | 0.800 |
| UA, mg/dL | 4.13 (3.45–4.99) | 4.16 (3.55–5.03) | 4.40 (3.71–5.21) | 4.62 (3.87–5.49) | < 0.001 |
| Cr, mg/dL | 0.75 (0.65–0.86) | 0.75 (0.64–0.87) | 0.76 (0.66–0.88) | 0.76 (0.66–0.88) | 0.405 |
| BUN, mg/dL | 15.80 (13.11–19.12) | 15.08 (12.69–18.42) | 14.96 (12.63–18.23) | 14.82 (12.46–17.59) | < 0.001 |
| Gender | < 0.001 | ||||
| Male | 400 (54.42%) | 344 (46.87%) | 309 (42.04%) | 304 (41.36%) | |
| Female | 335 (45.58%) | 390 (53.13%) | 426 (57.96%) | 431 (58.64%) | |
| Education, n (%) | 0.031 | ||||
| Below primary | 364 (49.52%) | 371 (50.54%) | 361 (49.12%) | 321 (43.67%) | |
| Primary schools | 176 (23.95%) | 157 (21.39%) | 160 (21.77%) | 165 (22.45%) | |
| Middle school | 150 (20.41%) | 148 (20.16%) | 143 (19.46%) | 173 (23.54%) | |
| High school and above | 45 (6.12%) | 58 (7.90%) | 71 (9.66%) | 76 (10.34%) | |
| Marital status | 0.012 | ||||
| Married | 638 (86.80%) | 628 (85.56%) | 637 (86.67%) | 668 (90.88%) | |
| Other | 97 (13.20%) | 106 (14.44%) | 98 (13.33%) | 67 (9.12%) | |
| Registered residence | < 0.001 | ||||
| Village | 641 (87.21%) | 619 (84.33%) | 606 (82.45%) | 578 (78.64%) | |
| City | 94 (12.79%) | 115 (15.67%) | 129 (17.55%) | 157 (21.36%) | |
| CVD | 0.004 | ||||
| Yes | 72 (9.80%) | 95 (12.94%) | 116 (15.78%) | 109 (14.83%) | |
| No | 663 (90.20%) | 639 (87.06%) | 619 (84.22%) | 626 (85.17%) | |
| Stroke | 0.033 | ||||
| Yes | 9 (1.22%) | 19 (2.59%) | 16 (2.18%) | 26 (3.54%) | |
| No | 726 (98.78%) | 715 (97.41%) | 719 (97.82%) | 709 (96.46%) | |
| Hypertension | < 0.001 | ||||
| No | 480 (65.31%) | 448 (61.04%) | 430 (58.50%) | 359 (48.84%) | |
| Yes | 255 (34.69%) | 286 (38.96%) | 305 (41.50%) | 376 (51.16%) | |
| Smoking status | 0.007 | ||||
| Never | 426 (57.96%) | 466 (63.49%) | 492 (66.94%) | 473 (64.35%) | |
| Current | 244 (33.20%) | 213 (29.02%) | 178 (24.22%) | 196 (26.67%) | |
| Quit | 65 (8.84%) | 55 (7.49%) | 65 (8.84%) | 66 (8.98%) | |
| Drinking status | < 0.001 | ||||
| Current | 235 (31.97%) | 182 (24.80%) | 160 (21.77%) | 168 (22.86%) | |
| Never | 454 (61.77%) | 512 (69.75%) | 533 (72.52%) | 509 (69.25%) | |
| Quit | 46 (6.26%) | 40 (5.45%) | 42 (5.71%) | 58 (7.89%) | |
Values were expressed as mean (standard deviation) or medians (quartile interval) or n (%)
SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, haemoglobin A1c; HDL-C, high‐density lipoprotein‐cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein‐cholesterol; TC, total cholesterol; TG, triglycerides; UA, uric acid; BUN, blood urea nitrogen; Cr, creatinine; CumAIP, cumulative atherogenic index of plasma
Summary of baseline characteristics according to follow-up outcomes
During a median follow-up period of 3 years, 15.21% of prediabetic patients progressed to diabetes, and 22.12% regressed to NFG. Table 2 summarizes the baseline characteristics of the study population according to the outcomes of prediabetes. Compared to participants who remained in a prediabetic state, those who progressed to diabetes had a higher proportion of lower education, were more likely to have CVD, hypertension and stroke, and had higher levels of DBP, weight, TG, hs-CRP, glucose, HbA1c, CumAIP, and lower levels of HDL-C and BUN. In addition, compared with subjects who remained prediabetic, participants who regressed to NFG were generally younger, had a higher proportion of higher education, and significantly lower levels of TC, TG, LDL-C, hs-CRP, HbA1c, and CumAIP at baseline.
Table 2.
Baseline characteristics summarized according to subjects’ glycemic status during follow-up
| Glucose status during follow-up | P-value | G1 vs. G2 comparison | G1 vs. G3 comparison | G2 vs. G3 comparison | |||
|---|---|---|---|---|---|---|---|
| Prediabetes to prediabetes (G1) | Prediabetes to diabetes (G2) | Prediabetes to NFG (G3) | P-value | P-value | P-value | ||
| No. of subjects | 1842 | 447 | 650 | ||||
| Age, years | 59.59 (8.71) | 60.39 (8.97) | 58.10 (8.78) | < 0.001 | 0.194 | < 0.001 | < 0.001 |
| SBP, mmHg | 130.67 (21.16) | 132.98 (19.39) | 129.13 (20.83) | 0.011 | 0.090 | 0.235 | 0.007 |
| DBP, mmHg | 76.09 (11.95) | 77.63 (11.89) | 76.02 (11.97) | 0.040 | 0.039 | 0.991 | 0.073 |
| Height, m | 1.58 (0.09) | 1.56 (0.08) | 1.59 (0.08) | < 0.001 | 0.006 | 0.010 | < 0.001 |
| Weight, kg | 59.79 (11.48) | 62.19 (12.28) | 60.10 (11.79) | < 0.001 | < 0.001 | 0.822 | 0.010 |
| TC, mg/dL | 199.80 (38.37) | 200.28 (37.23) | 189.59 (37.26) | < 0.001 | 0.969 | < 0.001 | < 0.001 |
| LDL-C, mg/dL | 120.43 (98.58-143.33) | 120.23 (99.74-142.85) | 109.79 (89.40-133.18) | < 0.001 | 0.961 | < 0.001 | < 0.001 |
| TG, mg/dL | 109.74 (77.88-161.96) | 115.94 (84.96-182.31) | 104.43 (73.45-155.76) | 0.016 | 0.008 | 0.243 | 0.001 |
| HDL-C, mg/dL | 49.87 (40.98–61.08) | 46.39 (37.50-57.99) | 49.29 (40.59–60.31) | < 0.001 | < 0.001 | 0.497 | 0.015 |
| hs-CRP, mg/L | 1.02 (0.56–2.18) | 1.23 (0.66–2.63) | 0.85 (0.50–1.73) | 0.006 | 0.001 | 0.002 | < 0.001 |
| Glucose, mmol/L | 108.07 (7.52) | 111.22 (9.41) | 107.44 (7.19) | < 0.001 | < 0.001 | 0.172 | < 0.001 |
| HbA1c, % | 5.25 (0.41) | 5.41 (0.43) | 5.04 (0.36) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| UA, mg/dL | 4.32 (3.61–5.18) | 4.43 (3.71–5.29) | 4.32 (3.58–5.15) | 0.251 | 0.415 | 0.732 | 0.229 |
| Cr, mg/dL | 0.75 (0.66–0.88) | 0.75 (0.63–0.87) | 0.77 (0.67–0.88) | 0.091 | 0.428 | 0.284 | 0.078 |
| BUN, mg/dL | 15.15 (12.72–18.37) | 14.57 (12.25–17.77) | 15.45 (12.91–18.57) | 0.015 | 0.031 | 0.648 | 0.013 |
| CumAIP | 0.13 (0.09) | 0.15 (0.09) | 0.12 (0.09) | < 0.001 | < 0.001 | 0.041 | < 0.001 |
| Gender | < 0.001 | ||||||
| Male | 838 (45.49%) | 176 (39.37%) | 343 (52.77%) | ||||
| Female | 1004 (54.51%) | 271 (60.63%) | 307 (47.23%) | ||||
| Education, n (%) | 0.090 | ||||||
| Below primary | 893 (48.48%) | 235 (52.57%) | 289 (44.46%) | ||||
| Primary schools | 413 (22.42%) | 98 (21.92%) | 147 (22.62%) | ||||
| Middle school | 390 (21.17%) | 76 (17.00%) | 148 (22.77%) | ||||
| High school and above | 146 (7.93%) | 38 (8.50%) | 66 (10.15%) | ||||
| Marital status | 0.641 | ||||||
| Married | 1615 (87.68%) | 385 (86.13%) | 571 (87.85%) | ||||
| Other | 227 (12.32%) | 62 (13.87%) | 79 (12.15%) | ||||
| Registered residence | 0.980 | ||||||
| Village | 1530 (83.06%) | 373 (83.45%) | 541 (83.23%) | ||||
| City | 312 (16.94%) | 74 (16.55%) | 109 (16.77%) | ||||
| CVD | < 0.001 | ||||||
| Yes | 232 (12.60%) | 87 (19.46%) | 73 (11.23%) | ||||
| No | 1610 (87.40%) | 360 (80.54%) | 577 (88.77%) | ||||
| Stroke | 0.002 | ||||||
| Yes | 35 (1.90%) | 21 (4.70%) | 14 (2.15%) | ||||
| No | 1807 (98.10%) | 426 (95.30%) | 636 (97.85%) | ||||
| Hypertension | < 0.001 | ||||||
| No | 1111 (60.31%) | 209 (46.76%) | 397 (61.08%) | ||||
| Yes | 731 (39.69%) | 238 (53.24%) | 253 (38.92%) | ||||
| Smoking status | 0.494 | ||||||
| Never | 1164 (63.19%) | 293 (65.55%) | 400 (61.54%) | ||||
| Current | 529 (28.72%) | 114 (25.50%) | 188 (28.92%) | ||||
| Quit | 149 (8.09%) | 40 (8.95%) | 62 (9.54%) | ||||
| Drinking status | 0.145 | ||||||
| Current | 481 (26.11%) | 93 (20.81%) | 171 (26.31%) | ||||
| Never | 1241 (67.37%) | 323 (72.26%) | 444 (68.31%) | ||||
| Quit | 120 (6.51%) | 31 (6.94%) | 35 (5.38%) | ||||
Quantitative variables were expressed as mean (standard deviation) or medians (quartile interval), and the differences among groups were evaluated by the Kruskal Wallis H test and Steel Dwass test or one-way ANOVA and Tukey’s HSD test; qualitative variables were presented as frequencies and percentages, and the chi-square test will be used to examine differences between groups;
SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, haemoglobin A1c; HDL-C, high‐density lipoprotein‐cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein‐cholesterol; TC, total cholesterol; TG, triglycerides; UA, uric acid; BUN, blood urea nitrogen; Cr, creatinine; CumAIP, cumulative atherogenic index of plasma
Association between CumAIP exposure and the development of prediabetes
Table 3 presents the results of the association analysis between CumAIP and the progression to diabetes and regression to NFG. A positive correlation between CumAIP and progression to diabetes and a negative correlation between CumAIP and regression to NFG were observed in all three progressively adjusted multivariable models (All P-trend < 0.05). According to the results of Model 3, after adjusting for age, gender, education, registered residence, CVD, stroke, hypertension, smoking status, drinking status, height, SBP, DBP, LDL-C, HbA1c, UA, Cr, BUN, and hs-CRP, the HR for CumAIP associated with progression to diabetes was 11.03 (3.76, 32.35), and the HR for regression to NFG was 0.40 (0.17, 0.96).
Table 3.
Multivariate Cox regression analysis of the role of CumAIP in assessing changes in glycemic status in patients with prediabetes
| HR (95% CI) | ||||
|---|---|---|---|---|
| No of case | Model 1 | Model 2 | Model 3 | |
| Prediabetes to diabetes | ||||
| CumAIP | 18.36 (6.80, 49.54) | 11.70 (4.21, 32.51) | 11.03 (3.76, 32.35) | |
| CumAIP (quartile) | ||||
| Q1 | 78 (10.61%) | 1.0 | 1.0 | 1.0 |
| Q2 | 100 (13.62%) | 1.29 (0.96, 1.74) | 1.22 (0.90, 1.64) | 1.18 (0.87, 1.59) |
| Q3 | 115 (15.65%) | 1.51 (1.13, 2.02) | 1.42 (1.06, 1.90) | 1.34 (1.00, 1.80) |
| Q4 | 154 (20.95%) | 2.09 (1.59, 2.75) | 1.86 (1.40, 2.46) | 1.76 (1.32, 2.34) |
| P-trend | < 0.0001 | < 0.0001 | < 0.0001 | |
| Prediabetes to NFG | ||||
| CumAIP | 0.38 (0.16, 0.91) | 0.39 (0.16, 0.96) | 0.40 (0.17, 0.96) | |
| CumAIP (quartile) | ||||
| Q1 | 173 (23.54%) | 1.0 | 1.0 | 1.0 |
| Q2 | 174 (23.71%) | 1.00 (0.81, 1.23) | 1.00 (0.81, 1.24) | 1.06 (0.85, 1.31) |
| Q3 | 163 (22.18%) | 0.91 (0.73, 1.13) | 0.91 (0.73, 1.13) | 0.98 (0.79, 1.23) |
| Q4 | 140 (19.05%) | 0.76 (0.61, 0.96) | 0.77 (0.61, 0.97) | 0.79 (0.62, 0.99) |
| P-trend | 0.0138 | 0.0190 | 0.0402 | |
HR: hazard ratios; CI: confidence interval; other abbreviations as in Table 1
Model 1 adjust for age, gender, education, registered residence;
Model 2 adjust for age, gender, education, registered residence, heart problem, stroke, hypertension, smoking status, drinking status, height, SBP, DBP
Model 3 adjust for age, gender, education, registered residence, heart problem, stroke, hypertension, smoking status, drinking status, height, SBP, DBP, LDL-C, HbA1c, UA, Cr, BUN, CRP
Dose-response relationship between CumAIP exposure and the development of prediabetes
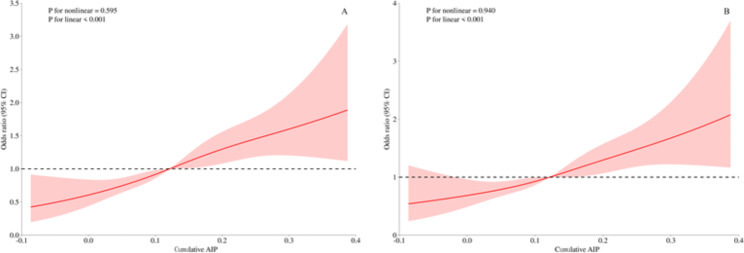
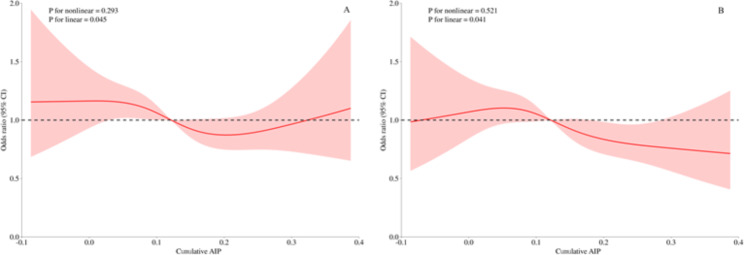
In Fig. 2, we used RCS curves with 4 knots to flexibly model and visualize the relationship between CumAIP and the progression to diabetes and regression to NFG. It can be seen that, before and after adjusting for covariates, CumAIP maintained a linear positive correlation with the progression to diabetes (Fig. 2) and a linear negative correlation with regression to NFG (Fig. 3).
Fig. 2.
Visualizing the relationship between CumAIP and progression from prediabetes to diabetes using 4-knots RCS (A: unadjusted; B: adjusted). CumAIP: cumulative atherogenic index of plasma; RCS: restricted cubic spline
Fig. 3.
Visualizing the relationship between CumAIP and regression of prediabetes to NFG using 4-knots RCS (A: unadjusted; B: adjusted). CumAIP: cumulative atherogenic index of plasma; RCS: restricted cubic spline
While there was an overall linear negative correlation between CumAIP and regression of prediabetes to NFG, there seem to be special associations between both low and high levels of CumAIP and regression to NFG. Specifically, in the unadjusted model, Fig. 3a suggests that participants with very high CumAIP were again likely to regress to NFG. However, after fully adjusting for covariates, Fig. 3b shows that lower levels of CumAIP did not appear to show a tendency to regress to NFG. It is also important to mention that the confidence intervals for the association between CumAIP and regression to NFG were wide, spanning over/under the OR of 1 though most of the CumAIP range both in the adjusted and unadjusted setting.
Subgroup analysis
The association between CumAIP exposure and the development of prediabetes did not change with age, gender, registered residence, comorbidities (hypertension, CVD, stroke), education, and physical exercise or drug treatment during follow-up (Table 4). We only observed a significant interaction effect in the marital status subgroup, which showed that compared to married individuals, those in an unmarried state (including separated, divorced, widowed, or never married) had a relatively higher risk of CumAIP-related diabetes. Furthermore, the stratified analysis indicated that the use of antidiabetic drugs, antihypertensive drugs, as well as engaging in physical exercise during the follow-up, influenced the transition to pre-diabetic glycemic status. However, the wide confidence intervals and results from further interaction tests showed that these effects were not statistically significant.
Table 4.
Exploratory subgroup analysis of the role and differences of CumAIP in assessing changes in glycemic status in prediabetes patients
| HR (95% CI) | ||
|---|---|---|
| Prediabetes to NFG | Prediabetes to diabetes | |
| Gender | ||
| Male | 0.51 (0.16, 1.68) | 7.06 (1.35, 36.84) |
| Female | 0.39 (0.11, 1.39) | 13.84 (3.39, 56.48) |
| P-interaction | 0.7473 | 0.5314 |
| Age, years | ||
| 45–59 | 0.77 (0.26, 2.27) | 6.62 (1.47, 29.94) |
| ≥ 60 | 0.15 (0.03, 0.64) | 17.03 (3.71, 78.11) |
| P-interaction | 0.0708 | 0.3846 |
| Education, n (%) | ||
| Below primary | 0.55 (0.15, 2.05) | 15.15 (3.52, 65.13) |
| Primary schools | 0.12 (0.02, 0.69) | 11.19 (1.33, 94.35) |
| Middle school | 1.01 (0.19, 5.44) | 5.40 (0.46, 62.97) |
| High school and above | 0.21 (0.01, 2.90) | 6.87 (0.20, 238.32) |
| P-interaction | 0.3088 | 0.8969 |
| Marital status | ||
| Married | 0.46 (0.18, 1.14) | 7.54 (2.40, 23.75) |
| Other | 0.13 (0.01, 1.89) | 184.83 (9.61, 3554.36) |
| P-interaction | 0.3809 | 0.0492 |
| Registered residence | ||
| Village | 0.42 (0.16, 1.08) | 9.47 (2.97, 30.22) |
| City | 0.33 (0.04, 2.70) | 26.39 (1.81, 384.91) |
| P-interaction | 0.8287 | 0.4861 |
| Antidiabetic drugs | ||
| Yes | inf (0.00, Inf) | 0.07 (0.00, 17.41) |
| No | inf (0.00, Inf) | 1.56 (0.03, 96.56) |
| P-interaction | 1.00 | 0.3806 |
| Antihypertensive drugs | ||
| Yes | 0.31 (0.05, 1.96) | 0.32 (0.01, 10.39) |
| No | 0.61 (0.04, 10.53) | 11.30 (1.99, 64.25) |
| P-interaction | 0.6930 | 0.6240 |
| Physical exercise | ||
| No activity | 0.74 (0.02, 29.07) | 21.38 (0.50, 913.76) |
| Light activity | 0.21 (0.02, 2.80) | 25.86 (1.04, 643.47) |
| Moderate activity | 0.35 (0.04, 2.72) | 9.45 (0.49, 181.91) |
| Intensive activity | 0.32 (0.04, 2.55) | 79.39 (6.63, 950.45) |
| P-interaction | 0.9593 | 0.7377 |
| Hypertension | ||
| No | 0.39 (0.13, 1.17) | 17.68 (3.85, 81.21) |
| Yes | 0.43 (0.11, 1.66) | 7.27 (1.71, 30.85) |
| P-interaction | 0.8988 | 0.3955 |
| Heart Problems | ||
| Yes | 1.02 (0.07, 14.57) | 12.83 (1.18, 139.25) |
| No | 0.36 (0.15, 0.91) | 10.66 (3.28, 34.62) |
| P-interaction | 0.4705 | 0.8893 |
| Stroke | ||
| Yes | 7.99 (0.03, 2078.50) | 1.01 (0.01, 103.16) |
| No | 0.38 (0.16, 0.91) | 12.62 (4.19, 38.04) |
| P-interaction | 0.4330 | 0.2893 |
Sensitivity analysis
When we analyzed the study population based on the WHO-recommended criteria, we observed similar results to those under the ADA criteria (Supplementary Table 6). The RCS with 5 knots showed similar linear association results to those with 4 knots (Supplementary Figs. 2 and 3). Finally, based on the results of Model 3, the E-values calculated were 9.03 (for progression to diabetes) and 3.16 (for regression to NFG).
Discussion
In this prospective cohort study involving middle-aged and elderly Chinese adults, we were the first to identify that high CumAIP exposure is a significant risk factor for the progression of prediabetes and is detrimental to the regression of prediabetes. Moreover, compared to unmarried individuals, being married significantly reduces the risk of prediabetes progression associated with high CumAIP exposure.
With the rapid global aging population [32], atherosclerosis is becoming increasingly prevalent [33, 34]. Previous studies have shown that AIP, representing atherosclerosis, is closely associated with prediabetes and diabetes [16–21], and further follow-up studies have indicated a nonlinear relationship between AIP and prediabetes and diabetes [17, 22]. Additionally, recent research has emphasized the clinical application potential of AIP in glycemic metabolic diseases. Studies have shown that AIP can be directly used for risk assessment of diabetes and prediabetes [17–20, 22] and for evaluating cardiovascular and metabolic complications in diabetic patients [35–38]. Despite the substantial evidence highlighting the importance of AIP in adult glycemic metabolic diseases, a limitation of previous studies is that they assessed AIP at a single time point, lacking repeated measurements of AIP. This has hindered a more comprehensive understanding of how changes in AIP affect disease progression. Notably, in a recent cohort study, Yi et al. conducted a bold design to investigate the impact of AIP transition patterns on diabetes [39]. They categorized baseline and follow-up AIP into low and high groups based on specific cutoff values and examined the effects of four transition patterns (maintaining high, maintaining low, high to low, and low to high) on diabetes. The study found that maintaining high AIP, high to low AIP, and low to high AIP transition patterns were positively associated with diabetes occurrence. However, the finding that the high to low AIP transition pattern was identified as a risk factor for diabetes warrants further verification, as it seems counterintuitive. We believe this particular result reported by Yi et al. is primarily related to the cutoff values used for AIP, where minor changes around the cutoff values could significantly affect the AIP transition patterns and further impact the study results. In the current study, we adopted an approach similar to some previous studies [24, 40, 41], combining baseline and repeated measurements of AIP with follow-up duration to calculate the continuous variable CumAIP. Our results showed that high CumAIP exposure was associated with a higher risk of diabetes, providing more direct evidence that monitoring and maintaining appropriate AIP levels is crucial for diabetes prevention.
Most previous studies on the development of prediabetes have focused on the progression to diabetes [42–44]. However, it is also important to note that the regression of prediabetes deserves attention as it is closely associated with reduced risks of diabetes and related complications [2, 7–9]. The progression and regression rates of prediabetes largely depend on the criteria used, which remains a significant challenge in this field [2]. The ADA criteria are the most inclusive, while the International Expert Committee and WHO criteria are more restrictive [45]. According to ADA criteria, a recent meta-analysis of 103 prospective studies reported that 18% of prediabetic patients progressed to diabetes within five years [6, 8]. Furthermore, another meta-analysis in 2022, based on 35 randomized controlled trials (RCTs) involving 10,164 prediabetic adults, showed that 31% of participants regressed to NFG within 1.6 years [9]. In the current national survey data based on CHARLS, 15.21% of prediabetic patients progressed to diabetes, and 22.12% regressed to NFG during a median follow-up of three years. Regarding diabetes progression, our study’s data are similar to international analysis results. However, the regression rate to NFG in our analysis is slightly lower than in the meta-analysis data [9]. We believe this is due to several reasons: (1) Compared to RCTs [9], our study is observational and did not include interventions specific to the study population. Instead, our findings are more reflective of the real-world situation in China. (2) Our study primarily involved middle-aged and elderly individuals, who are generally older and may have poorer metabolic conditions [1]. (3) Our analysis did not include oral glucose tolerance test data, which could lead to the omission of some patients with impaired glucose tolerance [45]. In this study, we also investigated the association between CumAIP exposure and prediabetes regression. Multivariate Cox regression showed a negative correlation, which further RCS analyses confirmed to be linear. Notably, in the dose-response relationship plots, the RCS analyses with both knots 4 and 5 showed wide confidence intervals for the associations between CumAIP and regression to NFG. Most of the CumAIP-related HRs crossed the reference line both before and after adjustments, regardless of whether the levels of CumAIP were high or low. These findings suggest that the use of CumAIP in assessing regression to NFG involves some uncertainty, and the results are relatively unstable. Regarding the RCS analysis results of CumAIP and regression to NFG, we have the following considerations: (1) Compared with the diagnostic criteria for diabetes, the threshold for diagnosing prediabetes based on blood glucose parameters is relatively loose, which may lead to some subjects who actually have normal blood glucose metabolism to be inappropriately classified as prediabetic patiens; It is necessary to improve and unify the criteria for prediabetes diagnosis as soon as possible [45], and then verify the current research results according to the latest standards. (2) The exclusion of a larger number of subjects with missing blood measurement information in the current study may somewhat contribute to the relative lack of sample size leading to a decrease in test efficacy, and validation of the results in further large sample cohorts is needed.
After establishing the relationship between CumAIP exposure and the progression or reversal of prediabetes in middle-aged and elderly populations, we further investigated the differences in this association among various subgroups. The results of the study showed that no significant specific population dependencies in almost all subgroups, indicating that the current findings are relatively stable, which was further confirmed by sensitivity analyses. However, we did find some notable differences within the marital status subgroup. Specifically, compared to married individuals, those who were unmarried (including separated, divorced, widowed, or never married) had a relatively higher risk of CumAIP-related diabetes. Previous studies have shown that being unmarried or having a poor marital relationship significantly increases atherosclerotic burden [46, 47] and negatively impacts cardiovascular health [48]. Additionally, evidence from the United States and Korea suggests that being unmarried also significantly promotes glucose deterioration and adverse metabolic outcomes [49, 50]. These findings provide context for our results, indicating that being unmarried may influence diabetes progression through atherosclerosis and glucose metabolism. These results suggest that marital status should be considered in diabetes risk assessment, and further research into atherosclerosis-related diabetes outcomes based on marital status is warranted.
The high prevalence and rapid growth of prediabetes worldwide have imposed a significant burden on society [2, 3]. Despite numerous RCTs indicating the potential of pharmacological treatments for prediabetes [9], no drugs have been approved for prediabetes treatment by regulatory agencies. Current evidence and clinical policies favor lifestyle changes for prediabetes management [26, 51], underscoring the importance of effectively implementing diabetes prevention strategies. In the current study, we also investigated the relative impact of physical exercise on the evolution of prediabetes during the follow-up period. Although the final interaction analyses did not detect significant modulatory effects, results from stratified analyses suggest that moderate activity may help reduce the risk of diabetes associated with CumAIP, whereas intensive activity appears to hinder diabetes prevention. Additionally, our study found that any level of physical exercise promotes the regression of CumAIP-related prediabetes. Similar findings have been reported in several previously conducted RCTs [52–55]. Overall, physical exercise can improve the progression of various chronic diseases and has a beneficial effect on glycaemic control in prediabetic patients [56]. Although conclusive evidence is lacking on the impact of physical exercise on CumAIP-related glycemic metabolism, from a pathophysiological perspective, atherosclerosis results from the interaction between metabolic and inflammatory pathways. Exercise can beneficially modulate these pathways, including lipid, inflammatory, and glucose metabolism [56]. Therefore, findings of the current study can be interpreted as moderate physical exercise exerting a beneficial effect on atherosclerosis, which may, in turn, promote favourable changes in glycaemic metabolism. Of course, the impact of physical exercise is multifaceted, as it influences both atherosclerosis and glycaemic metabolism, creating a positive feedback loop that prevents the progression of various chronic diseases.
The significance of the current study lies in further clarifying the impact of changes in AIP over follow-up periods on the development of prediabetes, building on previous research. High exposure to CumAIP during the follow-up period may reflect an important factor indicating ongoing adverse metabolic conditions. Given the simplicity and convenience of calculating and obtaining CumAIP [16], along with its crucial role in assessing cardiovascular and cerebrovascular diseases risk [24, 40, 57] and its relevance to glycaemic metabolic disorders, CumAIP holds significant potential for clinical applications and prognostic evaluation. China currently bears one of the highest global burdens of atherosclerotic disease, making the use of simple tools to quantify cumulative atherosclerotic exposure particularly significant [58, 59]. We believe that incorporating CumAIP into clinical practice could be instrumental in reducing the burden of atherosclerotic diseases and could inform primary prevention strategies for the regression of prediabetes. It is important to note that calculating CumAIP is a straightforward task for clinicians, with the main challenge being the repeated measurement of AIP. This is due to the relatively low rate of annual physical examination (APE) among middle-aged and elderly populations in China, which ranges from approximately 35–65%, with significant regional and urban-rural disparities [60–62]. Despite free APE services being available to older adults in China, a considerable proportion of them do not utilize these services, highlighting the importance of identifying barriers to APE uptake. It is recommended that government agencies establish health consultation centers for middle-aged and elderly individuals in communities and rural areas, enhance health education, and raise awareness of the importance of APE. In addition to the difficulty in obtaining repeated AIP measurements, there are currently no clear recommendations for maintaining appropriate CumAIP levels, as related studies remain limited and further research is needed.
Strengths and limitations
This study has several strengths, including its prospective design and dynamic assessment methods, which elucidate the association between atherosclerosis fluctuations and the development of prediabetes, thus enhancing its clinical significance. Additionally, the design involving repeated measurements of AIP adds a novel aspect to the study.
However, there are several limitations to consider when interpreting our results: (i) The diagnosis of prediabetes in the current analysis did not include oral glucose tolerance test data, potentially missing some patients with impaired glucose tolerance [45], which could lead to an underestimation of both the incidence of diabetes and the regression rate to NFG [45]. (ii) A substantial number of participants were excluded due to missing blood glucose data, which may introduce sampling bias. (iii) Considering the increasing trend of atherosclerosis in younger populations, our findings may also apply to younger adults, adolescents, and children [11, 63], but caution is needed when extrapolating our results based on middle-aged and elderly populations.
(iv) The study participants were Chinese, so caution is required when applying these findings to other racial or ethnic groups. (v) As with all observational studies, despite our efforts to account for relevant confounders, some residual confounding factors may still be present. Nonetheless, sensitivity analyses estimating the minimum E-value needed to account for unmeasured confounders indicated that our findings are relatively robust. (vi) Since this is a non-interventional study, we cannot infer the specific impact of lifestyle interventions on the progression and regression of prediabetes associated with cumulative CumAIP. (vii) Since the Wave 3 survey did not include information on lipid-lowering drug use, the impact of these drugs on the development of CumAIP-associated prediabetes during follow-up could not be assessed in this analysis. In addition, we only obtained information on the use of antihypertensive drugs, antidiabetic drugs, and physical exercise information during follow-up for no more than half of the subjects, which brought certain obstacles to the production of meaningful results in subgroup analysis and also further research is needed. (viii) The first wave of CHARLS national data was applied as the baseline information in the current study. Since prediabetes was diagnosed based on blood glucose measurement parameters and the questionnaire lacked detailed data on the history of prediabetes, the duration of prediabetes in the study population could not be confirmed in the current study for the time being, which is an important help for further interpretation of the results [9], and further research is needed. (ix) Estimates of CumAIP for the current study were calculated using AIP data from waves 1 and 3. Although this cumulative exposure assessment method has been widely used in many studies [24, 40, 41], it should be noted that the calculation of CumAIP includes measurement data at the time of the study outcome, which may influence the results and necessitates further prospective research.
Conclusion
This prospective cohort study of middle-aged and elderly Chinese adults demonstrates that CumAIP is closely associated with the progression and regression of prediabetes. High CumAIP exposure increases the risk of prediabetes progression and hinders its regression. These findings suggest that monitoring and maintaining appropriate AIP levels may help prevent the deterioration of blood glucose levels.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the CHARLS project team for their great efforts throughout the project, and also thank our colleagues at the Jiangxi Cardiovascular Research Institute for their suggestions on the writing of this paper.
Abbreviations
- CumAIP
Cumulative atherogenic index of plasma
- CHARLS
China Health and Retirement Longitudinal Study
- RCS
Restricted cubic spline
- NFG
Normal fasting glucose
- HR
Hazard ratio
- CI
Confidence interval
- ADA
American Diabetes Association
- TG
Triglycerides
- vLDL-C
Very low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- Hb
Hemoglobin
- CVD
Cardiovascular disease
- FPG
Fasting plasma glucose
- RPG
Random plasma glucose
- TC
Total cholesterol (TC)
- BUN
Blood urea nitrogen
- hs-CRP
High-sensitivity C-reactive protein
- UA
Uric acid
- Cr
Creatinine
- SBP
Systolic blood pressure
- DBP
diastolic blood pressure
- WHO
World Health Organization
- RCTs
Randomized controlled trials
- APE
Annual physical examination
Author contributions
LH-D and HY-Y: Conceptualization, methodology, supervision, and project administration.YZ, SL, and DD-L: writing-original draft preparation.XH, CW, LH-D, HY-Y and GB-X: writing-reviewing and editing.YZ, SL and DD-L: Software.YZ, LH-D and GB-X: formal analysis and validation.YZ and HY-Y: data curation.All authors read and approved the final manuscript.
Funding
This work was supported by Natural Science Foundation of Jiangxi Province [No. 20232BAB216004], Jiangxi Provincial Health Commission Science and Technology Plan Project (SKJP_1320240983) and Jiangxi Province Key Laboratory of Immunity and Inflammation (No. 2024SSY06251).
Data availability
CHARLS datasets are available for download at the CHARLS home website (http://charls.pku.edu.cn/en).
Declarations
Ethics approval and consent to participate
The entire study process adhered to the Declaration of Helsinki, and the study results were reported following the STROBE guidelines. The protocol for the CHARLS cohort was authorized by the Ethics Review Committee of Peking University (IRB00001052–11015), and all participants provided written informed consent at the time of participation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Zou, Song Lu and Dongdong Li contributed equally to this work.
Contributor Information
Lihua Duan, Email: lh-duan@163.com.
Hongyi Yang, Email: yanghyzy@outlook.com.
References
- 1.Al-Sofiani ME, Asiri A, Alajmi S, Alkeridy W. Perspectives on prediabetes and aging. Endocrinol Metab Clin North Am. 2023;52:377–88. 10.1016/j.ecl.2022.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Echouffo-Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2021;42:59–77. 10.1146/annurev-publhealth-090419-102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. 10.1136/bmj.m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallace AS, Rooney MR, Fang M, Echouffo-Tcheugui JB, Grams M, Selvin E. Natural history of prediabetes and long-term risk of clinical outcomes in middle-aged adults: the atherosclerosis risk in communities (ARIC) Study. Diabetes Care. 2023;46:e67–8. 10.2337/dc22-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shang Y, Marseglia A, Fratiglioni L, Welmer AK, Wang R, Wang HX, et al. Natural history of prediabetes in older adults from a population-based longitudinal study. J Intern Med. 2019;286:326–40. 10.1111/joim.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10:CD012661. 10.1002/14651858.CD012661.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vistisen D, Kivimäki M, Perreault L, Hulman A, Witte DR, Brunner EJ, et al. Reversion from prediabetes to normoglycaemia and risk of cardiovascular disease and mortality: the Whitehall II cohort study. Diabetologia. 2019;62:1385–90. 10.1007/s00125-019-4895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakkalakal RJ, Galaviz KI, Thirunavukkarasu S, Shah MK, Narayan KMV. Test and treat for Prediabetes: a review of the Health effects of prediabetes and the role of screening and prevention. Annu Rev Public Health. 2024;45:151–67. 10.1146/annurev-publhealth-060222-023417. [DOI] [PubMed] [Google Scholar]
- 9.Galaviz KI, Weber MB, Suvada KBS, Gujral UP, Wei J, Merchant R, et al. Interventions for reversing prediabetes: a systematic review and meta-analysis. Am J Prev Med. 2022;62:614–25. 10.1016/j.amepre.2021.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Açar B, Ozeke O, Karakurt M, Ozen Y, Özbay MB, Unal S, et al. Association of prediabetes with higher coronary atherosclerotic burden among patients with first diagnosed acute coronary syndrome. Angiology. 2019;70:174–80. 10.1177/0003319718772420. [DOI] [PubMed] [Google Scholar]
- 11.Liang Y, Wang M, Wang C, Liu Y, Naruse K, Takahashi K. The mechanisms of the development of atherosclerosis in prediabetes. Int J Mol Sci. 2021;22(8):4108. 10.3390/ijms22084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergman M. Pathophysiology of prediabetes and treatment implications for the prevention of type 2 diabetes mellitus. Endocrine. 2013;43:504–13. 10.1007/s12020-012-9830-9. [DOI] [PubMed] [Google Scholar]
- 13.Ferrannini E, Gastaldelli A, Iozzo P. Pathophysiology of prediabetes. Med Clin North Am. 2011;95:327–39, vii–viii. 10.1016/j.mcna.2010.11.005 [DOI] [PubMed]
- 14.VergèsB. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58:886–99. 10.1007/s00125-015-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Östgren CJ, Otten J, Festin K, Angerås O, Bergström G, Cederlund K, et al. Prevalence of atherosclerosis in individuals with prediabetes and diabetes compared to normoglycaemic individuals-a Swedish population-based study. Cardiovasc Diabetol. 2023;22:261. 10.1186/s12933-023-01982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Y, Wen M. Sex-specific differences in the effect of the atherogenic index of plasma on prediabetes and diabetes in the NHANES 2011–2018 population. Cardiovasc Diabetol. 2023;22:19. 10.1186/s12933-023-01740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin B, Wu Z, Xia Y, Xiao S, Chen L, Li Y. Non-linear association of atherogenic index of plasma with insulin resistance and type 2 diabetes: a cross-sectional study. Cardiovasc Diabetol. 2023;22:157. 10.1186/s12933-023-01886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li YW, Kao TW, Chang PK, Chen WL, Wu LW. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: a 9-year longitudinal study. Sci Rep. 2021;11:9900. 10.1038/s41598-021-89307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Suo Y, Wang L, Liu D, Jia Y, Fu Y, et al. Association between atherogenic index of plasma and gestational diabetes mellitus: a prospective cohort study based on the Korean population. Cardiovasc Diabetol. 2024;23:237. 10.1186/s12933-024-02341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang L, Li L, Xu Z, Tang Y, Zhai Y, Fu X, et al. Non-linear associations of atherogenic index of plasma with prediabetes and type 2 diabetes mellitus among Chinese adults aged 45 years and above: a cross-sectional study from CHARLS. Front Endocrinol (Lausanne). 2024;15:1360874. 10.3389/fendo.2024.1360874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onat A, Can G, Kaya H, Hergenç G. Atherogenic index of plasma (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 2010;4:89–98. 10.1016/j.jacl.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Kuang M, Yang R, Xie G, Sheng G, Zou Y. Evaluation of the role of atherogenic index of plasma in the reversion from Prediabetes to normoglycemia or progression to diabetes: a multi-center retrospective cohort study. Cardiovasc Diabetol. 2024;23:17. 10.1186/s12933-023-02108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah AS, Black S, Wadwa RP, Schmiege SJ, Fino NF, Talton JW, et al. Insulin sensitivity and arterial stiffness in youth with type 1 diabetes: the SEARCH CVD study. J Diabetes Complic. 2015;29:512–6. 10.1016/j.jdiacomp.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43:61–8. 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Min Q, Wu Z, Yao J, Wang S, Duan L, Liu S, et al. Association between atherogenic index of plasma control level and incident cardiovascular disease in middle-aged and elderly Chinese individuals with abnormal glucose metabolism. Cardiovasc Diabetol. 2024;23(1):54. 10.1186/s12933-024-02144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15–33. 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 27.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–42. 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 28.Gharavi E, Gu A, Zheng G, Smith JP, Cho HJ, Zhang A, et al. Embeddings of genomic region sets capture rich biological associations in lower dimensions. Bioinformatics. 2021;37:4299–306. 10.1093/bioinformatics/btab439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mugler EM, Tate MC, Livescu K, Templer JW, Goldrick MA, Slutzky MW. Differential representation of Articulatory gestures and phonemes in Precentral and Inferior Frontal Gyri. J Neurosci. 2018;38:9803–13. 10.1523/JNEUROSCI.1206-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- 31.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 32.Chang AY, Skirbekk VF, Tyrovolas S, Kassebaum NJ, Dieleman JL. Measuring population ageing: an analysis of the global burden of Disease Study 2017. Lancet Public Health. 2019;4:e159–67. 10.1016/S2468-2667(19)30019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyrrell DJ, Goldstein DR. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat Rev Cardiol. 2021;18:58–68. 10.1038/s41569-020-0431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–59. 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 35.Fu L, Zhou Y, Sun J, Zhu Z, Xing Z, Zhou S, et al. Atherogenic index of plasma is associated with major adverse cardiovascular events in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20:201. 10.1186/s12933-021-01393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samimi S, Rajabzadeh S, Rabizadeh S, Nakhjavani M, Nakhaei P, Avanaki FA, et al. Atherogenic index of plasma is an independent predictor of metabolic-associated fatty liver disease in patients with type 2 diabetes. Eur J Med Res. 2022;27:112. 10.1186/s40001-022-00731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma X, Sun Y, Cheng Y, Shen H, Gao F, Qi J, et al. Prognostic impact of the atherogenic index of plasma in type 2 diabetes mellitus patients with acute coronary syndrome undergoing percutaneous coronary intervention. Lipids Health Dis. 2020;19:240. 10.1186/s12944-020-01418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou K, Qin Z, Tian J, Cui K, Yan Y, Lyu S. The atherogenic index of plasma: a powerful and reliable predictor for coronary artery disease in patients with type 2 diabetes. Angiology. 2021;72:934–41. 10.1177/00033197211012129. [DOI] [PubMed] [Google Scholar]
- 39.Yi Q, Ren Z, Bai G, Zhu S, Li S, Li C, et al. The longitudinal effect of the atherogenic index of plasma on type 2 diabetes in middle-aged and older Chinese. Acta Diabetol. 2022;59:269–79. 10.1007/s00592-021-01801-y. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Chen S, Tian X, Wang P, Xu Q, Xia X, et al. Association between cumulative atherogenic index of plasma exposure and risk of myocardial infarction in the general population. Cardiovasc Diabetol. 2023;22:210. 10.1186/s12933-023-01936-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X, Wen S, Huang Y, Huang Z. Gender differences in the association between changes in the atherogenic index of plasma and cardiometabolic diseases: a cohort study. Lipids Health Dis. 2024;23:135. 10.1186/s12944-024-02117-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CDC; CDC. National Diabetes Prevention Program, Atlanta. GA: 2012. www.cdc.gov/diabetes/prevention/index.htm
- 43.Chan JC, Zhang Y, Ning G. Diabetes in China: a societal solution for a personal challenge. Lancet Diabetes Endocrinol. 2014;2:969–79. 10.1016/S2213-8587(14)70144-5. [DOI] [PubMed] [Google Scholar]
- 44.Ackermann RT, Kenrik Duru O, Albu JB, Schmittdiel JA, Soumerai SB, Wharam JF, et al. Evaluating diabetes health policies using natural experiments: the natural experiments for translation in diabetes study. Am J Prev Med. 2015;48:747–54. 10.1016/j.amepre.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim SH. Reframing prediabetes: a call for better risk stratification and intervention. J Intern Med. 2024;295:735–47. 10.1111/joim.13786. [DOI] [PubMed] [Google Scholar]
- 46.Gallo LC, Troxel WM, Kuller LH, Sutton-Tyrrell K, Edmundowicz D, Matthews KA. Marital status, marital quality, and atherosclerotic burden in postmenopausal women. Psychosom Med. 2003;65:952–62. 10.1097/01.psy.0000097350.95305.fe. [DOI] [PubMed] [Google Scholar]
- 47.Smith TW. Intimate relationships and coronary heart disease: implications for risk, prevention, and patient management. Curr Cardiol Rep. 2022;24:761–74. 10.1007/s11886-022-01695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong CW, Kwok CS, Narain A, Gulati M, Mihalidou AS, Wu P, et al. Marital status and risk of cardiovascular diseases: a systematic review and meta-analysis. Heart. 2018;104:1937–48. 10.1136/heartjnl-2018-313005. [DOI] [PubMed] [Google Scholar]
- 49.Schwandt HM, Coresh J, Hindin MJ. Marital status, hypertension, coronary heart disease, diabetes, and death among African American women and men: incidence and prevalence in the atherosclerosis risk in communities (ARIC) Study participants. J Fam Issues. 2010;31:1211–29. 10.1177/0192513X10365487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jung YA, Kang LL, Kim HN, Park HK, Hwang HS, Park KY. Relationship between Marital Status and metabolic syndrome in Korean middle-aged women: the Sixth Korea National Health and Nutrition Examination Survey (2013–2014). Korean J Fam Med. 2018;39:307–12. 10.4082/kjfm.17.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boltri JM, Tracer H, Strogatz D, Idzik S, Schumacher P, Fukagawa N, et al. The national clinical care commission report to congress: leveraging federal policies and programs to prevent diabetes in people with prediabetes. Diabetes Care. 2023;46:e39–50. 10.2337/dc22-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai X, Zhai L, Chen Q, Miller JD, Lu L, Hsue C, et al. Two-year-supervised resistance training prevented diabetes incidence in people with prediabetes: a randomised control trial. Diabetes Metab Res Rev. 2019;35:e3143. 10.1002/dmrr.3143. [DOI] [PubMed] [Google Scholar]
- 53.Yan J, Dai X, Feng J, Yuan X, Li J, Yang L, et al. Effect of 12-month resistance training on changes in abdominal adipose tissue and metabolic variables in patients with prediabetes: a randomized controlled trial. J Diabetes Res. 2019;2019:8469739. 10.1155/2019/8469739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shamizadeh T, Jahangiry L, Sarbakhsh P, Ponnet K. Social cognitive theory-based intervention to promote physical activity among prediabetic rural people: a cluster randomized controlled trial. Trials. 2019;20:98. 10.1186/s13063-019-3220-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H, Guo Y, Hua G, Guo C, Gong S, Li M, et al. Exercise training modalities in prediabetes: a systematic review and network meta-analysis. Front Endocrinol (Lausanne). 2024;15:1308959. 10.3389/fendo.2024.1308959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer-Lindemann U, Moggio A, Dutsch A, Kessler T, Sager HB. The impact of exercise on immunity, metabolism, and atherosclerosis. Int J Mol Sci. 2023;24:3394. 10.3390/ijms24043394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng H, Wu K, Wu W, Chen G, Chen Z, Cai Z, et al. Relationship between the cumulative exposure to atherogenic index of plasma and ischemic stroke: a retrospective cohort study. Cardiovasc Diabetol. 2023;22:313. 10.1186/s12933-023-02044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li S, Liu HH, Guo YL, Zhu CG, Wu NQ, Xu RX, et al. Improvement of evaluation in Chinese patients with atherosclerotic cardiovascular disease using the very-high-risk refinement: a population-based study. Lancet Reg Health West Pac. 2021;17:100286. 10.1016/j.lanwpc.2021.100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18:394–405. 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 60.Dong W, Gao J, Wu Y, Shen C, Bai R. How different is the annual physical examination of older migrants than that of older nonmigrants? A coarsened exact matching study from China. Healthc (Basel). 2022;10:815. 10.3390/healthcare10050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ge D, Chu J, Zhou C, Qian Y, Zhang L, Sun L. Rural-urban difference in the use of annual physical examination among seniors in Shandong, China: a cross-sectional study. Int J Equity Health. 2017;16:86. 10.1186/s12939-017-0585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun X, Chen Y, Tong X, Feng Z, Wei L, Zhou D, et al. The use of annual physical examinations among the elderly in rural China: a cross-sectional study. BMC Health Serv Res. 2014;14:16. 10.1186/1472-6963-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barraclough JY, Skilton MR, Garden FL, Toelle BG, Marks GB, Celermajer DS. Early and late childhood telomere length predict subclinical atherosclerosis at age 14 yrs.—the CardioCAPS study. Int J Cardiol. 2019;278:250–3. 10.1016/j.ijcard.2018.12.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CHARLS datasets are available for download at the CHARLS home website (http://charls.pku.edu.cn/en).