Abstract
The adverse outcome pathway (AOP) framework has gained significant international traction as a systematic approach for capturing toxicological knowledge to transparently link mechanistic data to apical endpoints that inform research and regulatory decision-making. While the framework has evolved significantly since its introduction in 2010, it was recognized that a survey of the broader scientific community would be useful in identifying shortcomings and guiding further development. In 2016 we reached out to this community through an international horizon scanning exercise to gather information on key outstanding challenges that must be addressed in order to realize the full potential of the AOP framework. Four key themes emerged from this exercise, which were then addressed by international experts representing industry, government, academia, and non-governmental organizations at a 2017 Society of Environmental Toxicology & Chemistry (SETAC) Pellston™ Workshop. These themes were 1) AOP networks and their applications; 2) quantitative AOPs (qAOPs) and their applications; 3) regulatory use of the AOP framework; and 4) expanding awareness and acceptance of AOPs to support aspects of predictive toxicology and regulatory decision-making. Herein we provide an overview of the workshop discussions and describe the outcomes and recommendations that emerged to advance the AOP framework. Common themes that spanned the main topics are also presented, and outstanding questions and future needs are discussed. In short, the current momentum of the AOP framework, driven by increased AOP development, publication and interest by regulatory communities, provides an unique opportunity to advance the AOP framework both technically (e.g., via networks and qAOPs) and socially (e.g., by strategic engagement of various stakeholder communities and applications). Such advances were collectively deemed essential for the overall sustainabilitiy of the AOP framework, and are addressed by six companion papers repesenting the products of the Pellston™ Workshop.
SETTING THE STAGE FOR AN AOP-FOCUSED PELLSTON™ WORKSHOP
Legislative mandates world-wide require chemical safety assessments to increase consumer confidence and protect human health and wildlife. Many of these mandates necessitate that regulators evaluate large numbers of chemicals within short timeframes to make informed regulatory decisions. To address this challenge and reduce costs of toxicity testing, the US National Research Council (NRC) laid out a vision and strategy for toxicity testing in the 21st century, which shifted focus from historical whole-organism testing to mechanistic studies (NRC 2007). This strategy aspired to transform toxicity testing by making greater use of recent scientific advances in cell-based and computational methods. A key concept aligning with this vision is the adverse outcome pathway (AOP) framework (Ankley et al. 2010). In brief, AOPs organize available toxicological knowledge and describe the causal linkages between a molecular initiating event (MIE; the first interaction of a chemical with a biological macromolecule such as an enzyme or a receptor) and subsequent measurable responses (termed key events [KEs]) across biological levels of organization, which culminate in an adverse outcome (AO) of regulatory significance — typically at the individual or population levels (BOX 1). Over the past decade, the AOP framework has matured significantly and has increasingly been recognized as a powerful approach for organizing biological information into a format applicable for chemical safety evaluation in both human health and ecological contexts (Ankley et al. 2010). Further, critical advances in AOP development and technology, including the development of a web-based AOP Knowledgebase (AOP-KB, https://aopkb.oecd.org/; BOX 2), provided the opportunity to engage stakeholders (users and developers AOPs) from industry, government, and academia to both evaluate the state-of-the-science and identify next steps in advancing the AOP framework and its uses.
BOX 1-. AOP Framework.
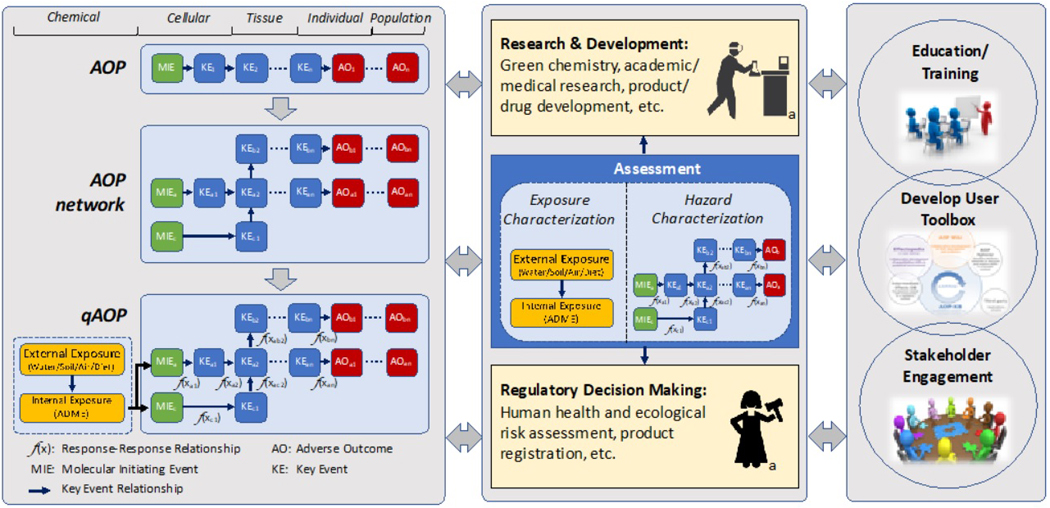
BOX 2 – AOP Knowledge Base.
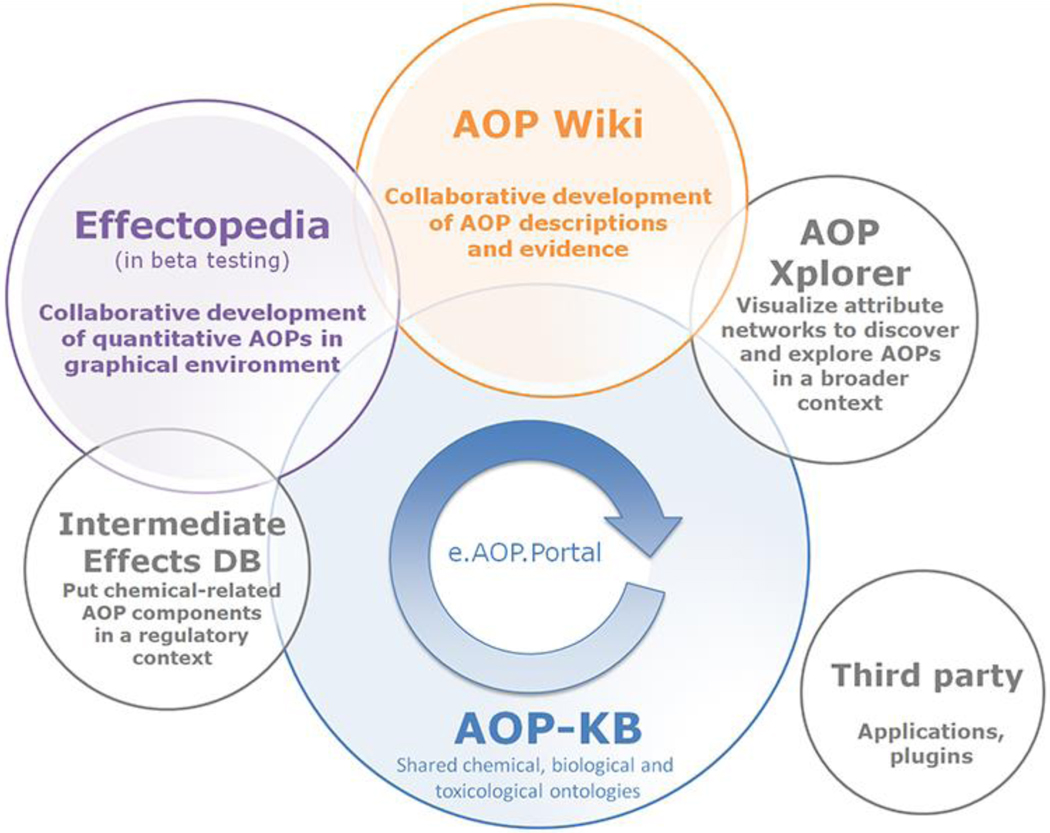
The AOP knowledge base (AOP KB; Source: https://aopkb.oecd.org/index.html) is the web-based infrastructure that has been envisioned, and continues to evolve, to support the collaborative development, distribution, visualization, and use of adverse outcome pathway knowledge, with the aspiration of serving as the search-engine and integration point for the AOP community. The AOP-KB captures effects data from toxicity studies (i.e., integration with OECD Harmonized Template 201; http://www.oecd.org/ehs/templates/), and assembles the data to produce quantitative relationships between KEs (i.e., through integration with Effectopedia; www.effectopedia.org). The ultimate goal of this KB is to form a comprehensive collection of accessible resources for disseminating AOP knowledge captured by internationally introduced standards.
The AOP KB consists of four separate AOP related modules. The furthest developed and most widely used module is the AOP-Wiki (https://aopwiki.org/), developed initially by the US Environmental Protection Agency. The other three modules include the Effectopedia (https://www.effectopedia.org/) developed by the Organisation for Economic Cooperation and Development and the European Commission Joint Research Centre, the AOPXplorer (http://www.aopxplorer.org/) developed by the US Army, and the Intermediate Effects Data Base, which is still under development. Because each module was initially created by different organizations as standalone tools, which exist in various stages of development, to-date the envisioned completed and well-integrated AOP KB has not yet been fully realized. Recently, the OECD launched the e.AOP.Portal, which currently serves as a search engine to mine information from the AOP Wiki and Effectopedia, bringing the integration of AOP modules a step closer.
AOP Wiki
The AOP Wiki captures manually entered AOP knowledge in a standardized format through the use of crowd-sourcing. It constitutes the main source for AOPs to be accessed and evaluated, and it has been instrumental in capturing AOP knowledge and associated weight of evidence over the past years. The AOP Wiki has expanded significantly since its initial public release in 2014 with a total of 216 AOP entries, consisting of 6 AOPs that have are endorsed by OECD after formal review. As the most advanced module of the AOP KB, the AOP Wiki was the primary focal point for discussions surrounding advances to the AOP framework throughout the Pellston Workshop. Particularly, workgroups explored the strengths and weaknesses of the Wiki in its current state and made recommendations for future iterations as a means to better accommodate multiple AOP stakeholders, which include researchers, risk assessors, risk managers, with diverse needs and uses for AOP knowledge. A general theme to these discussions included the desire to manipulate the level of detail displayed based on the intended use of the AOP knowledge to address the needs of the stakeholder.
Presented herein is a summary and synthesis of the discussions and outcomes from a Society of Environmental Toxicology and Chemistry (SETAC) Pellston™ Workshop, “Advancing the Adverse Outcome Pathway Concept – An International Horizon Scanning Approach,” that was held in Cornwall, ON, Canada between April 2–6, 2017. The main purpose of this workshop was to begin addressing recognized issues relevant to the development and application of AOPs for chemical risk assessment for both human and ecological health. Through engagement of the international scientific community via an international horizon scanning effort, as described by LaLone et al. (2017a), the Pellston™ Workshop aimed to develop solutions, strategies, and recommendations to effectively address current challenges and identify critical next steps in realizing the full potential of the AOP framework. The specific aim of this article is to summarize the overall format, discussions, and cross-cutting themes of this Pellston™ Workshop, which have culminated into a series of companion journal articles representing the dilberations and outcomes of four workgroups (i.e., Carusi et al. 2018; Coady et al. 2018; Knapen et al. 2018; Perkins et al. 2018; Villeneuve et al. 2018).
A horizon scanning, or question solicitation, approach was used to set the stage for the 2017 Pellston™ Workshop, reaching out to global scientific and regulatory communities (LaLone et al. 2017a; Figure 1). Briefly, an online survey was developed asking participants to propose questions that consider key outstanding challenges or limitations that must be addressed to realize the full potential of the AOP framework. From this, ~340 valid questions were collected from countries in all continents, and across diverse sectors (LaLone et al. 2017a). Questions were subjected to an expert ranking exercise and used to develop the themes and charge questions to be addressed at the Pellston™ Workshop (LaLone et al. 2017a). The Pellston™ Workshop discussions were centered around four core themes from this horizon scanning exercise (BOX 3). Four work groups, consisting of 8–11 experts each, were assigned to address each of the four themes and associated questions. In total, 41 international invitees participated in the Pellston™ Workshop (http://www.saaop.org/workshops/pellston2017.html accessed Feb. 15, 2018). These experts were from nine countries with backgrounds in academia (35%), government (40%), industry (20%) and non-government organizations (5%).
Figure 1:
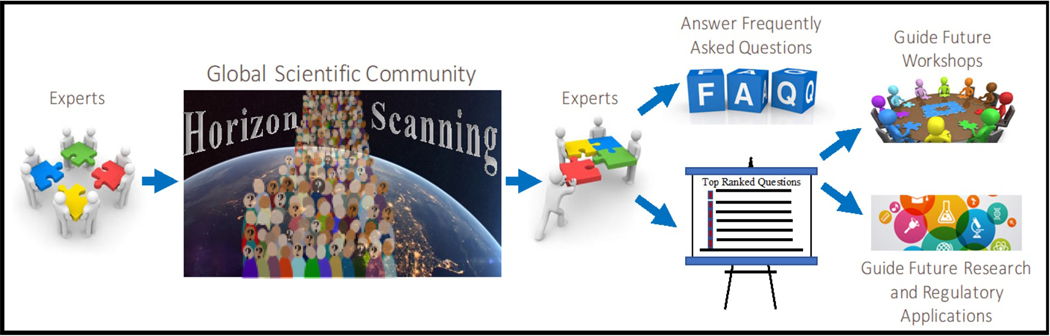
Concept and structure of the Horizon Scanning exercise conducted to guide the SETAC PellstonTM Workshop on “Advancing the Adverse Outcome Pathway Concept – An International Horizon Scanning Approach”, held in Cornwall, ON between April 2 and 6, 2017 (LaLone et al. 2017a).
BOX 3: Themes and Charge Questions to Workgroups.
| Theme 1 | AOP networks and their applications |
|---|---|
| Charge Questions | • Definition of an AOP network • Identification of key considerations in designing and evaluating AOP networks, including appropriate experimental designs or structurual considerations for development based on anticipated application • Determine how AOPs could better capture changes in toxicity due to timing of exposure or biological changes over time (e.g., acute versus chronic toxicity, delayed toxicity, epigenetics, i.e., heritable changes that do not involve modifications to the DNA sequence, repeat exposures, feedback loops and compensatory mechanisms) • Define for which species the AOP network is most applicable to; • Describe how AOP networks can be used to understand complex chemical mixtures |
| Theme 2 | Quantitative AOPs (qAOPs) and their applications |
| Charge Questions | • Define exactly what consistutes a qAOP • Identify experimental design considerations appropriate for developing models for and applying qAOPs, including what level of detail is necessary based on the application • Determine how uncertainty should be captured for transparency when developing models to support qAOPs • Describe how qAOPs can be used in conjunction with exposure information, such as absorption, distributuion, metabolism and elimination data in risk assessment |
| Theme 3 | Regulatory use of the AOP framework |
| Charge Questions | • Describe to what regulatory scenarios AOPs could be applied, including consideration of geographic location and regulatory authority similarities and differences • Consider how one would evaluate fit-for-purpose of an AOP • Determine how regulatory needs may inform AOP development |
| Theme 4 | Expanding awareness of, involvement in, and acceptance of AOPs to support aspects of predictive toxicology and regulatory decision-making |
| Charge Questions | • How can education and outreach related to the AOP framework be effectively and efficiently improved • Encourage/incentivize coordination and development of AOPs • Increase recognition of AOPs, and enhanced uptake across stakeholder communities • Facilitate adoption of the AOP framework for regulatory application |
ADDRESSING THE WORKSHOP CHARGES
Workgroups were asked to review the state-of-the-science of their respective theme, and to focus on demonstrating the application of AOPs in a research and regulatory context (BOX 3). Charge questions were provided from the top ranked questions obtained during horizon scanning and the expert ranking exercise to guide deliberations (Reviewed by LaLone et al. 2017a). During these deliberations it was requested that, when possible, Worksgroups 1–3 use case studies to illustrate the current state of AOP science and how relevant tools for AOP development and evaluation can be applied. Workgroups also were asked to consider current technologies available for capturing, sharing, evaluating, reviewing, and using AOPs, namely the AOP-KB, (BOX 2). Finally, to integrate identified issues surrounding communication, collaborative development, and adoption of the AOP framework, a primary task for each workgroup was to provide feedback to Workgroup 4, who focused on exploring how awareness of, involvement in, and acceptance of AOPs could be expanded among the broader global scientific and regulatory/policy community.
Enhanced Communication of Global and Multi-sector Issues
The horizon scanning effort that preceded the Pellston™ Workshop highlighted the global interest in the AOP framework and its applications for chemical research and regulation (LaLone et al. 2017a). Therefore, workgroups addressed the themes from a cross-sector and global perspective, using collaborative discussions with participants across workgroups to ensure issues due to governance/geographic location and entity mission were captured. For example, this involved the integration of risk assessors from other workgroups to join in discussions surrounding the use of qAOPs in regulatory decision-making. Additionally, participants that represented different countries (e.g., Belgium, Canada, China, Germany, Italy, U.K., U.S.A.) were included in discussions of regulatory use of AOPs, as regulations and regulatory practices can differ significantly from country to country.
ILLUSTRATION OF CONCEPTS THROUGH CASE STUDIES
Demonstrating application of the AOP concepts discussed during the Pellston™ Workshop was an emphasis. It was recognized that case studies would help illustrate the “readiness” of AOP science to address key questions that emerged from the horizon scanning exercise. Further, case studies help enable the evaluation of whether a particular AOP or AOP network is “fit-for-purpose” (i.e., appropriate for use based on a specific scenario) in addressing regulatory challenges or research questions. Concurrently, workgroups were also asked to developed strategies and recommendations for future initiatives to advance the AOP framework such that it addresses the needs of a broader global stakeholder community (Figure 2). From this, with the exception of Workgroup 4, each workgroup identified AOP case studies which varied in degree of development and review status (Table 1). Some of the case studies, such as hepatic steatosis or inihibition of aromatase, were used by multiple workgroups because they illustrated diverse characteristics and challenges associated with development of AOP networks and qAOPs, as well as the application of AOPs in different regulatory contexts (Knapen et al. 2018; Perkins et al. 2018; Coady et al. 2018). Other examples, such as the “nAChR activation leading to colony death/failure in honeybees” AOP (AOP-Wiki ID 88), were used to make more specific points (e.g., the application of AOPs to inform and support ecological risk assessments) (LaLone et al. 2017b; Coady et al. 2018). Depending on workgroup objectives, a variety of case studies were used ranging from individual AOPs illustrating the application to specific questions (e.g., protein alkylation leading to liver fibrosis to demonstrate the application of AOPs to support chemical read-across, or targeted ecological risk assessment of nAChR activators in honeybees; Coady et al. 2018) to using the AOP-Wiki as a repository for bioactivity profiling of complex environmental mixture for prediction of apical hazards by constructing an AOP network (e.g., Knapen et al. 2018). Overall, the successful application and illustrative power of the case studies used by the different workgroups demonstrated the utility of the AOP-KB in diverse applications, ranging from chemical design and development to ecological risk assessments of complex environmental mixtures. It also was recognized that case studies represent powerful tools for engaging stakeholders, so there is a continual need for further development of case examples that speak to the specific needs of a broader stakeholder community, including those involved in risk management, medicine/health, and policy (Carusi et al. 2018). Furthermore, successful demonstration of relevant case studies is important to gain an overall acceptance of this framework.
Figure 2:
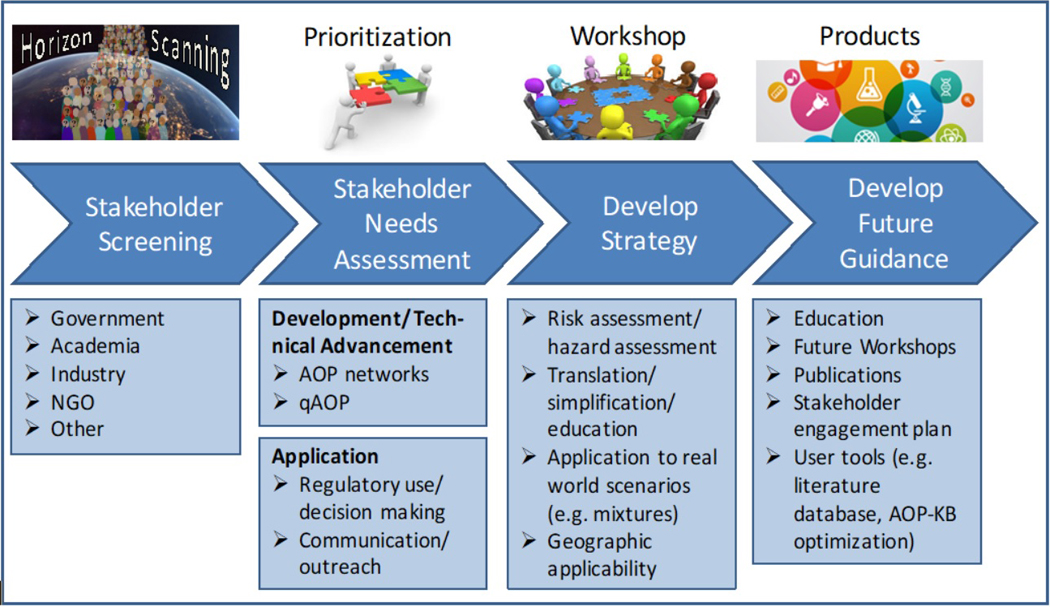
Advancing the AOP framework using a horizon scanning approach.
Table 1:
Summary of case studies used by each work group and associated AOPs, their stages of development and prior use in regulatory decision making processes.
| Case Studies of AOPs or AOP Networks | AOP-Wiki IDs | Stage of Development | Use in Regulatory Decision making | Workgroup |
|---|---|---|---|---|
| Hepatic Steatosis | 8, 34, 57–62, 213 | • Various stages of development • AOP-Wiki pages developed • No OECDa review |
No | 1 2 3 |
| Decreased serum thyroid hormone | 8, 42, 54, 134, 155-158, 175, 176, 188-194 | • Various stages of development • AOP-Wiki pages developed • Under EAGMSTb Review |
Endocrine Disruptor Screening Program | 1 |
| Hazard assessment for a complex mixture in the South Platte River | 11, 14, 21, 29, 52, 53, 57, 60, 61, 131, 150, 167 | • Various stages of development • AOP-Wiki pages developed • Under EAGMST Review |
No | 1 |
| Polypharmacology of beclomethasone dipropionate (BDP) using the fathead minnow | 14, 23 | • Various stages of development • AOP-Wiki pages developed • EAGMST Review or Approved |
No | 1 |
| Inhibition of aromatase and fecundity driven population-level decreases in the fathead minnow | 7,23, 25, 29, 30, 100, 122, 123, 155–159, 216 | • Various stages of development • AOP-Wiki pages developed • EAGMST Review or Approved or TFHA/WNTc Endorsed |
Endocrine Disruptor Screening Program | 1, 2 |
| nAChR activation leading to colony death/failure in honeybees | 88 | • Formal AOP • AOP-Wiki pages developed • No OECD review |
No | 3 |
OECD, Organisation for Economic Cooperation and Developemnt
EAGMST, Extended Advisory Group on Molecular Screening and Toxigenomics
TFHA/WNT, OECD Task Force on Hazard Assessment/Working Group of the National Coordinators of the Test Guidelines Programme
WORKGROUP OBJECTIVES AND DISCUSSIONS
To date, the majority of activities with regard to AOPs have focused on their development. This is because out of necessessity populating the AOP-KB was the pragmatic first step in laying the foundation for regulatory application. However, with increasing population of the AOP-KB (BOX 2), this initial focus has rapidly turned towards application of the AOP framework in a regulatory context, both to maintain support from current stakeholders and expand usefulness to new stakeholders. This is reflected in the number of recent workshops that have explored how the AOP concept could be applied to improve regulatory decision making (Whittwehr et al. 2017; Brockmeier et al. 2017; Tollefsen et al. 2014; Villeneuve et al 2014a,b), and which have identified a number of technical challenges that needed to be addressed. Since these initial workshops, significant progress has been made with regard to developing technical solutions and improvements to address many of these challenges, including improved modeling approaches in support of qAOP development (e.g., Conolly et al. 2017; Perkins et al. 2018), optimizing the AOP-Wiki to enable building of AOP networks (Knapen et al. 2018; Villeneuve et al. 2018), and establishing online tools (e.g., US EPA SeqAPASS; https://seqapass.epa.gov/seqapass/) to facilitate cross species extrapolation (LaLone et al., 2016; LaLone et al., 2018; Doering et al., 2018a; Hecker 2018), among others. However, the development and application of this expanding toolbox for AOP development and understanding has been the foundational work of a core group of scientists from government, industry, non-government organizations, and academics in North America and Europe with the specific needs of the scientific community involved with chemical risk assessment in mind. For the AOP framework to expand its applicability, it must be accepted by a broader global stakeholder community, including risk managers and other decision makers. This requires an inclusive strategey that reaches out to, and considers the needs of, diverse stakeholder groups across geographic regions (Carusi et al., 2017; Coady et al., 2018). Therefore, the objectives of the 2017 SETAC Pellston™ Workshop were not only to make progress in addressing technical challenges and in application of the AOP framework in both regulatory and research contexts, but importantly to explore how the framework can gain acceptance by, and addresses the needs of, a broader global stakeholder community that includes risk managers and perhaps even those in the biomedical field.
Pellston™ Workgroup 1: Adverse outome pathway networks and their applications
A fundamental principle underlying the AOP framework is that while individual AOPs are a pragmatic unit for development and evaluation, AOP networks composed of multiple AOPs often will be the functional unit for prediction, representing real-world scenarios of pathway perturbation (Villeneuve et al., 2014a). International efforts to populate the AOP-Wiki have lead to the creation of 216 AOPs over the last four years, providing the basis to begin formally developing and further probing the AOP network concept. The objectives of Workgroup 1 were to 1) evaluate the state-of-the-science concerning AOP networks, and 2) recommend best practices to guide future development to ensure meaningful use in research and regulatory decision-making (BOX 3). Discussions surrounding these topics led to the development of three manuscripts. The first publication (Part I) by Knapen et al. (2018) discussed AOP network development including new recommendations for implementing filters and layers, similar to those used in Geographic Information Systems, depending on intended application to aid in maneuvering complex networks and to efficiently extract relevant information depending on endusers’ needs. This work used case studies (e.g., hepatic steatosis, thryroid axis disruption, environmental mixtures of chemicals, and polypharmacology) to demonstrate how these features of networks would be advantageous under specific scenarios. Also described in this publication are approaches for including greater biological detail in AOP networks that may be necessary for applications that require quantitative pathway evaluations. For example, it may be necessary to include greater detail in descriptions of feedback loops, where the product or intermediate in a biological system increases (positive feedback) or decreases (negative feedback) the system as a means to maintain homeostasis, and for modulating factors (e.g., external environmental factors or nutritional status) that may influence the biology. In contrast, simplified or less detailed AOP network descriptions may be equally necessary for rapid qualitative evaluations. Therefore, the authors illustrated how filters and layers could be implemented in the AOP-KB, allowing users to reduce or increase the level of detail extracted for customization of the output based on data needs for specific applications. The publication goes on to introduce the concepts of AOP network topology analysis, which provides a means to evaluate the structure, size and shape of the network to glean information on pathway intereactions for example. Additionally, critical path identification, where a stakeholder focuses attention on a specific pathway due to the research or regulatory question being addressed, was introduced along with descriptions for characterization of interactions among AOP networks. These concepts were then expanded upon in the second publication (Part II) by Villeneuve et al. (2018) that focused on network analytics, or more simply, the analysis of the AOP network to identify tendencies or patterns that can inform the context or utility of AOPs for a specific enduser need. Specifically this work integrated discussions of how graph theory, which includes mathematical descriptions of biological pathways, can be employed to understand interactions among AOPs and identify pathways of importance (Villeneuve et al., 2018). This publication describes in detail and through examples how network structure can inform assay or model development in considering pathways that meet at a particural KE (i.e., convergent pathway) or alternatively separate after a KE (i.e., divergent pathways). Additionally, descriptions of how critical path identification could be used in selection of the relevant pathways for risk assessment and how interactions between AOP networks can be useful for identification of additive, synergistic or antagonistic responses were included. The third manuscript (SAAOP, 2018) builds upon concepts from the first two publications and demonstrates the utility of graph theory and network analysis, using the AOP-Wiki as a case study, for evaluating large AOP networks and identifying individual AOPs that emerge because of the network analyses (Pollesch et al., 2018). Overall, the work presented in the series of papers developed from Workgroup 1 discussions at the Pellston™ Workshop addressed several questions submitted to the horizon scanning exercise and expanded the ability to more consistently develop, describe, and evaluate AOP networks (Figure 3; Supplemental Table S1).
Figure 3:
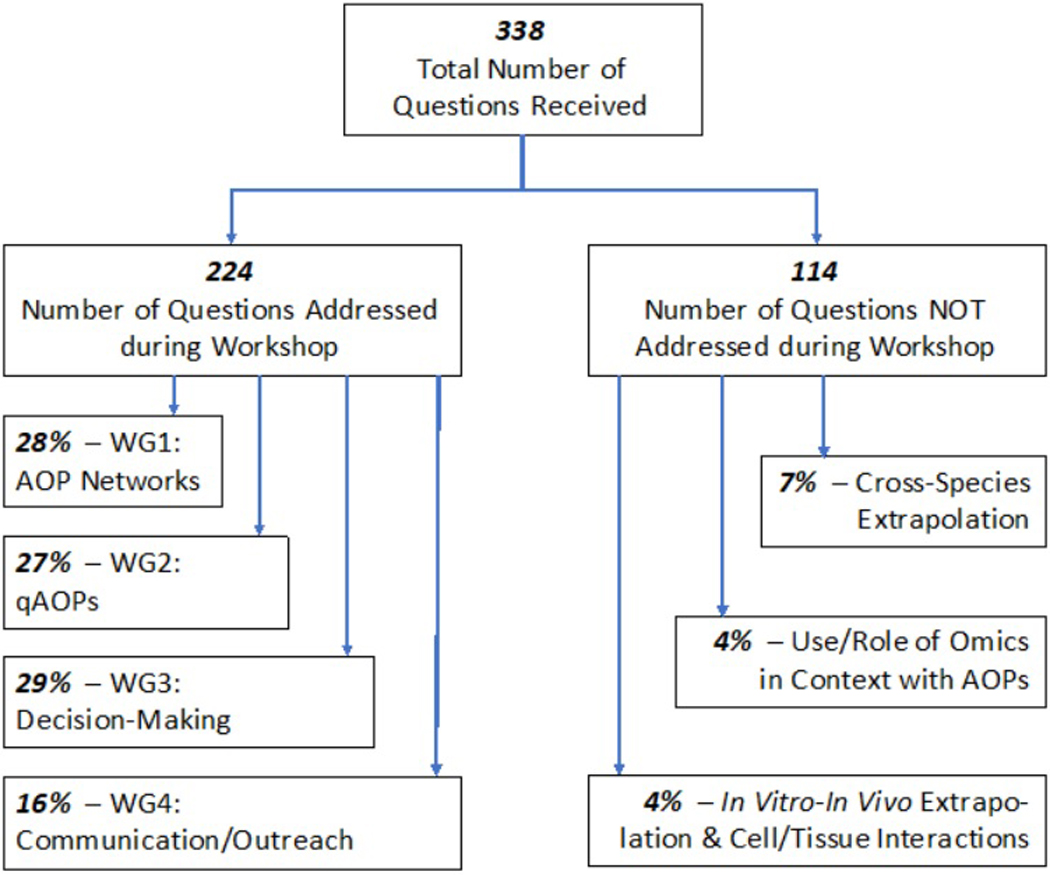
Flow chart of the number of questions collected during the horizon scanning exercise that were addressed during the PellstonTM workshop, and main themes associated with remaining questions to be addressed by future activities. Abbreviation - WG: Workgroup.
Pellston™ Workgroup 2: Quantitative AOPs and their applications
At its most basic, an AOP is a qualitative description of measurable biological endpoints and the relationstips between those endpoints. Qualitative AOPs, though appropriate for certain applications, pose a challenge for risk assessment as their qualitative nature precludes them from directly inferring risk of chemicals. While qAOPs remain chemical agnostic, they include quantitative features that depict response-response information essential in understanding the probability or severity of the resulting adverse outcome (i.e. how much of an effect at an upstream KE has to occur to result in a significant change at a downstream KE). Therefore, this quantitative understanding of the biology can be used to support regulatory decision-making if the potency of the target compound at an early KE or the MIE is known. The objective of Workgroup 2 was to explore how the AOP framework and knowledgebase can be used to develop qAOPs to assess and predict hazards and risks of chemicals or mixtures in support of regulatory decision-making (BOX 3). The manuscript published by Workgroup 2 (Perkins et al. 2018) focused on characterizing best practices for choosing certain modeling approaches, model building and the necessity for transparent and comprehensive documentation in order to gain confidence for the development of qAOP models. Different types of quantitative relationships in a qAOP were discussed including correlations and response-response relationships (i.e., relationship between an upstream and downstream KE). These relationships may take the form of simple mathematical equations or sophisticated biologically-based computational models that consider other modulating factors such as compensatory responses, or interactions with other biological or environmental variables. Perkins et al. (2018) further explore how chemical agnostic qAOP models can be combined with exposure models or models that aim to understand how chemicals enter the body (e.g., toxicokinetic models) that, by nature, include chemical specific information. Examples are provided that coupled qAOP models with extrapolation from concentrations within the cell of an organism to effects on the whole organisms (i.e., in vitro to in vivo extrapolation). Additionally, the manuscript explores and demonstrate application of these concepts by developing a qAOP network case study for hepatic steatosis using probability-based models (e.g., Baysian models) of varying complexity (Perkins et al., 2018). It was shown that probability-based networks allow for rapid modeling of interactions among AOPs and enable integration of multiple data types collected from cell-based or whole-organism experimentation. The manuscript illustrates the diverse applications of qAOPs ranging from simple qAOP networks that are useful for screening level decision-making (e.g., to identify and prioritize chemicals for more rigorous testing) to more computationally complex models in support of complex research questions (e.g., to understanding the potential hazards of chemical mixtures). The cases presented by Workgroup 2 clearly demonstrated the potential of qAOP models to support regulatory risk assessment bridging available mechanistic knowledge to application in predictive assessments. However, examples of mature qAOP models are scarce to date, and based on the successful examples provided by Perkins et al. (2018) development of similar case studies is strongly encouraged. Furthermore, it will be crucial to properly define the applicability domain of specific qAOP models, avoiding mis-use and poor regulatory uptake that would confound confidence and application of such models.
Pellston™ Workgroup 3: Regulatory use of the AOP framework
The applicability of AOPs to regulatory decision-making processes has been the focus of several recent publications (reviewed in Coady et al. 2018). While these prior publications concluded that the AOP framework represents a promising and potentially useful approach for this purpose, there remains a need for increased validation of AOPs, relative to the current practices, for regulatory contexts such as prioritization, categorization, application to integrated testing strategies, and quantitative risk assessment of chemicals (Becker et al. 2015; Kleinstreuer et al., 2016). In recognition of these needs, the objectives of Workgroup 3 were to 1) characterize the regulatory decision-making scenarios to which the AOP framework can be applied, 2) establish guidance for decision-makers in determining if/when an AOP is fit-for-purpose, and 3) identify how regulatory needs can inform future AOP development (BOX 3). The manuscript by Coady et al. (2018), capturing the discussions by Workgroup 3, focused on the needs of different stakeholder groups including both the regulated community and those involved in regulatory decision-making in context with chemical safety assessments. The workgroup used the concept of the “life-cycle” of a chemical to illustrate the applicability of the AOP framework across chemical assessment scenarios, moving from research and development (R & D) through chemical registration, and out to post-registration activities as a framework for guiding their discussions. Specifically, Workgroup 3 explored how developmental status of a given AOP, from a hypothesized pathway to a quantitative network, could be applied to regulatory scenarios requiring different levels of detail/complexity. It was acknowledged that during each of these stages of the chemical life-cycle both stakeholder groups and decision processes can differ significantly. For example, chemical R & D is mostly driven by the regulated community whereas chemical registration and post-registration processes involve both the regulated and regulatory communities. Accordingly, depending on the stage of a chemical during its life-cycle, fit-for-purpose considerations for AOPs would differ. Several factors were identified that could inform the fit-for-purpose of an AOP depending on the intended application (Coady et al. 2018). For example, a high degree of confidence and strong weight-of-evidence for KEs informing regulatory endpoints would be needed if AOPs were to be used in chemical decision-making. In contrast, less stringent requirements would apply during early stages of R & D with new chemistries or for priorization and screening level assessments. Other scenarios to which AOPs could be applied include emergency situations and bioassay development as described in the context of the US Endocrine Disruptor Screening Program (EDSP) by Browne et al. (2017), which is mandated to use validated testing methods representing specific KEs to identify and evaluate the potential of chemicals to disrupt the endocrine system of vertebrates including humans. In conclusion, each AOP use scenario has unique requirements with regard to robustness of and confidence in the respective AOPs or AOP networks of interest. Based on these discussions Workgroup 3 developed criteria for evaluating fit-for-purpose of an AOP as a function of the chemical life-cycle. Case studies were used to illustrate how AOPs have been, or could be, used in support of regulatory decision-making (Table 1). It was concluded that the AOP framework represents a usful tool in chemical decision-making across the different life-stages of a chemical, but that requirements differ depending on the purpose of the safety assessment. Furthermore, Coady et al. (2018) identified several implementation challenges, including 1) the need for an increased understanding of the AOP framework among regulatory communities, 2) a need for increased demonstration of successful application of AOPs in regulatory decision-making and chemical testing, and 3) the development of validated tools that capture relevant KEs in support of chemical screening and prioritization efforts.
Pellston™ Workgroup 4: Expanding awareness of, involvement in, and acceptance of AOPs to support aspects of predictive toxicology and regulatory decision-making
The AOP framework has been largely developed by a core group of technical and regulatory experts with the specific needs of the chemical risk assessment community in mind. Despite the momentum gained within the existing AOP community, it became clear during the horizon scanning exercise that there was limited awareness of the AOP framework throughout the broader scientific and regulatory/environmental policy communities. In fact, one of the common themes discussed by all workgroups was the need to better communicate the AOP framework beyond the currently recognized stakeholder groups as a means to gain broader acceptance and use of the framework across multiple stakeholder groups. The main objectives of Workgroup 4 were to 1) explore how the AOP framework is useful to a broader stakeholder community, 2) develop strategies to more efficiently communicate the AOP framework to diverse stakeholder groups, and 3) identify strategies whereby stakeholder engagement can be secured and by which current challenges regarding the long-term sustainability of the AOP framework and the associated knowledgebase can be secured (BOX 3). Discussions from Workgroup 4 on these topics lead to a publication by Carusi et al. (2018) that incorporated the identification of unique features and qualities of the AOP framework that make it useful to a broad stakeholder community. Aditionally, Carusi et al. (2018) highlighted the need for consistency in presentation of the AOP descriptions with unambiguous terminology and the availability of such knowledge in the AOP-KB, the assemblage and review of the knowledge, and the potential applications for this knowledge. In support of these discussions a variety of stakeholders were profiled/ characterized, describing how they could benefit from further development of the AOP framework and its application. These stakeholders included regulatory toxicologists and chemical risk assessors, risk managers, the chemical industry, pharmaceutical and agrochemical industries, clinicians, academics, and non-government organizations (Carusi et al. 2018). Carusi et al. (2018) then continue to describe a number of key challenges in advancing the AOP framework. Examples include 1) challenges in publishing AOPs for career advancement, 2) risks and burdens for stakeholders to support the AOP framework due to reputational concerns or risks due to AOP use for decision-making and accountability to consumers, and 3) overall the challenge in governance of the AOP framework, particularly in maintaining the AOP-KB. The deliberations of Workgroup 4 concluded with a set of recommendations regarding the steps that need to be taken in order to resolve existing challenges. For example, strategies were outlined for engaging a variety of stakeholder communities with different communication and training approaches relevant to the needs of specific stakeholders. Furthermore, approaches related to mulit-stakeholder governance and the publication and review process for AOPs, were identified as areas for improvements to ensure further progression of the AOP framework as a useful, sustainable tool, that will ultimately transform the way we apply our scientific knowledge of living systems in numerous decision-making contexts.
Workshop Conclusions
The Pellston™ Workshop, “Advancing the Adverse Outcome Pathway Concept – An International Horizon Scanning Approach,” provided the unique opportunity to address key challenges and identify approaches and solutions to progress the AOP framework to the next level. To that end, discussions that took place during the workshop reviewed and addressed key questions raised during an international horizon scanning exercise that set the stage for Pellston™ Workshop (LaLone et al. 2017a; Supplemental Table S1). Of the 338 questions that were collected during the horizon scanning exercise, over 220 were addressed by workgroups and presented in the resulting manuscripts (Figure 3). Of particular significance was the unique contribution of Workgroup 4 (Carusi et al., 2018) in addressing social science topics related to public outreach and communication that traditionally are rarely covered through expert-driven SETAC science initiatives. The fact that a significant percentage of questions from the horizon scanning exercise fell into this category, cleary demonstrated the importance of expanding the historically science-based discussions to social science questions pertaining to societal relevance of, sustainability of, and broader stakeholder engagement in the AOP framework (Carusi et al. 2018).
One of the common observations resulting from discussion among and within workgroups was the need to strategically simplify the amount of biology presented to the most essential information that could readily be translated by the stakeholder for the intended application (Carusi et al. 2018). For example, the AOP-Wiki was built by scientist and has served as the central repository for AOP development and collaboration for scientists; however, AOP information is not necessarily presented in a manner that would allow it to be directly applied in regulatory decision-making scenarios, where risk assessors/managers need to make informed decisions quickly. Specifically, to be useful to risk managers, the AOP-KB must allow for user-friendly searching and extraction of information through an intuitive interface. Examples illustrating the complex nature of extracting relevant information from the AOP-Wiki are the AOPs associated with thyroid-or estrogen-related processes. When searching the AOP-Wiki for “thyroid” or “estrogen” 30 and 26 AOPs are retrieved, respectively (https://aopwiki.org/aops). From a decision-making perspective, it is daunting to identify and extract information from this large body of AOP information. Thus, the AOP-KB, particularly the AOP-Wiki, needs to be enhanced to include search functions that allow stakeholders to sort through and retrieve information relevant to their specific needs. In fact, three of the four workgroups (Workgroups 2, 3 and 4) highlighted that proper education of stakeholders is of particular importance considering that improper use of the AOP-KB could result in erroneous decision-making yielding unintended consequences to the environment or human health. Further, if information from AOPs are misused or misunderstood, economic damage could incur due to over protection (Carusi et al. 2018; Coady et al. 2018; Perkins et al. 2018).
It was also highlighted that the AOP framework represents a versatile tool that can be applied to a wide range of processes and questions requiring different levels of AOP development, stringency and validation. In contrast to the highly stringent requirements associated with applying AOPs to regulatory decision-making due to the significant consequences that erroneous application can have in this context, less complete development and validation status are needed for the application of AOPs to a range of scenarios. For example, AOPs during early stages of development that have not undergone rigorous validation may provide information highly useful to the R & D of new chemicals or the initial characterization stages in pharmaceutical drug development (Coady et al. 2018). In some cases, even partial AOPs (e.g. AOP 21) have been shown to provide strong evidence that selected early MIEs or KEs can be reliable predictors of apical outcomes of regulatory relevance, and thus, are directly applicable to the risk assessment of chemicals. In the specific case of AOP21, quantitative understanding is largely limited to the indirect KER between the MIE (AhR activation by dioxin-like chemicals), and the AO (early life stage mortality in oviparous vertebrates) with little knowledge regarding intermediate KEs. However, given the very strong direct quantitative relationship between the MIE and the AO (Doering et al. 2018b) this AOP has direct utility towards risk assessment of agonists of the AhR with regard to cross-chemical, cross-species, and cross-taxa extrapolations.
Another main observation that was made across workgroups during workshop discussions was that the idea of a pathway-driven approach to toxicity assessment has been equally adopted by both the human and ecological risk assessment communities (https://humantoxicologyproject.org/tox-101/pathway-based-toxicology/; Sonich-Mullin et al. 2001; Boobis et al. 2008; Ankley et al. 2010). Thus, the AOP framework has facilitated a change in thinking that previously separated human toxicology and ecotoxicology. This is due, in part, to the fact that biological pathways, particularly at the molecular level, are often conserved among taxonomically related groups (e.g., vertebrates or invertebrates). Such pathway-based thinking is reflected in current regulatory programs including the US-EPA EDSP, which has evolved to utilize mammalian-based in vitro high-throughput screening assays to evaluate chemicals for their endocrine disrupting potential relative to both humans and wildlife (Ankley et al., 2016; Browne et al. 2017; LaLone et al., 2018). While it is acknowledged that there is still need for significant expansion of research into the conservation of toxicity pathways across taxonomic groups and species, recent advances in evolutionary biology and next generation high-throughput approaches are increasingly facilitating reliable extrapolation across receptors of interest (LaLone et al., 2018; Doering et al., 2018a; Hecker, 2018; LaLone et al. 2016). These recent advances and the development of novel cross species extrapolation tools provide the opportunity for a unified approach to human health and ecological risk assessment that is facilitated by the AOP framework.
Finally, in addition to the large proportion of questions that were addressed by the four workgroups, a number of topics emerged during the question solicitation exercise that were not directly discussed during the workshop (Figure 3). In particular, questions pertaining to three additional themes emerged, including the use/utility of AOPs in support of species extrapolation, the use/role of omics in informing AOPs, and the ability of AOPs to support in vitro – in vivo extrapolations. Since concluding the horizon scanning exercise, a number of manuscripts have been published begin to address these issues specifically (LaLone et al. 2018), and an upcoming SETAC Pellston™ workshop is being held in winter 2018/19 that will focus on the role omics in context with the AOP framework and regulatory science. As such, the horizon scanning exercise identified a number of areas that needed greater focus, and which already have begun to be taken up by different groups that are taking the initiative to move that science forward.
Supplementary Material
Aknowledgements
We gratefully acknowledge the Society of Environmental Toxicology and Chemistry (SETAC) North America staff, in particular Greg Schiefer, Nikki Mayo, and Tamar Schlekat who provided support to the workshop co-chairs, steering committee, and workshop participants before, during, and after the Pellston™ workshop. We appreciate the funding support from the Society of Environmental Toxicology and Chemistry, United States Environmental Protection Agency, American Cleaning Institute, Cefic-LRI (European Chemical Industry Council Long-Range Research Initiative), Chevron-Environmental, ECETOC (European Center for Ecotoxicology and Toxicology of Chemicals), European Commission Joint Research Centre, European Crop Protection, ExxonMobil, Humane Society International, The Humane Society of the United States, Human Toxicology Project Consortium, Syngenta, and Unilever. In addition, we thank the groups from academia, industry, and government who supported participants’ travel. Dr. Hecker has been supported by the Canada Research Chairs program. This manuscript has been reviewed in accordance with the requirements of the US Environmental Protection Agency (EPA) Office of Research and Development. The views expressed in this work are those of the authors and do not necessarily reflect the views or policies of the US EPA, nor does the mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741. [DOI] [PubMed] [Google Scholar]
- Ankley GT, LaLone CA, Gray LE, Villeneuve DL, Hornung MW. 2016. Evaluation of the scientific underpinnings for identifying estrogenic chemicals in nonmammalian taxa using mammalian test systems. Environ Toxicol Chem 35: 2806–2816. [DOI] [PubMed] [Google Scholar]
- Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, Watanabe H, Barton-Maclaren TS. 2015. Increasing Scientific Confidence in Adverse Outcome Pathways: Application of Tailored Bradford-Hill Considerations for Evaluating Weight of Evidence. Regul Toxicol Pharmacol 72:514–537. [DOI] [PubMed] [Google Scholar]
- Boobis AR, Doe JE, Heinrich-Hirsch B, Meek ME, Munn S, Ruchirawat M, Schlatter J, See J, Vickers C. 2008. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit Rev Toxicol 38:87–96. [DOI] [PubMed] [Google Scholar]
- Brockmeier EK, Hodges G, Hutchinson TH, Butler E, Hecker M, Tollefsen KE, Garcia-Reyero N, Kille P, Becker D, Chipman K, Colbourne J, Collette TW, Cossins A, Cronin M, Graystock P, Gutsell S, Knapen S, Katsiadaki I, Lange A, Marshall S, Owen SF, Perkins EJ, Plaistow S, Schroeder A, Taylor D, Viant M, Ankley G, Falciani F. 2017. The Role of Omics in the Application of Adverse Outcome Pathways for Chemical Risk Assessment. Toxicol Sci 158:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne PD, Noyes PM, Casey WM, Dix DJ. 2017. Application of adverse outcome pathways to US EPA’s endocrine disruptor screening program. Environ Health Perspect 10.1289/EHP1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carusi A, Davies MR, De Grandis G, Escher BI, Hodges G, Leung KMY, Whelan M, Willett C, Ankley GT. 2018. Harvesting the promise of AOPs: An assessment and recommendations. Environ Sci Technol 628–629:1542–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coady K, Browne P, Embry M, Hill III T, Leinala E, Steeger T, Maślankiewicz L, Hutchinson T. 2018. When are Adverse Outcome Pathways and Associated Biassays “Fit for Purpose” for Regulatory Decision-Making and Management of Chemicals? In prep [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conolly RB, Ankley GT, Cheng W, Mayo ML, Miller DH, Perkins EJ, Villeneuve DL, Watanabe KH. 2017. Quantitative Adverse Outcome Pathways and Their Application to Predictive Toxicology. Environ Sci Technol 51:4661–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering JA, Lee S, Kristiansen K, Evenseth L, Barron M, Sylte I, LaLone CA. 2018a. In silico site-directed mutagenesis informs species-specific predictions of chemical susceptibility derived from the Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS) tool. In review [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering J, Wiseman S, Giesy J, Hecker M. 2018b. A cross-species quantitative adverse outcome pathway for activation of the aryl hydrocarbon receptor leading to early life stage mortality in birds and fishes. Environ Sci Technol. In review [DOI] [PubMed] [Google Scholar]
- Hecker M. 2018. Non-Model Species in Ecological Risk Assessment. In: A Systems Biology Approach to Advancing Adverse Outcome Pathways for Risk Assessment. Murphy C. and Reyero N, Eds., Springer, New York, USA. pp. 107–148. [Google Scholar]
- Kleinstreuer NC, Sullivan K, Allen D, Edwards S, Mendrick DL, Embry M, Matheson J, Rowlands JC, Munn S, Maull E, Casey W. 2016. Adverse Outcome Pathways: From Research to Regulation. Scientific workshop report. Reg Toxicol Pharmacol 76:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen D, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O’Brien JM, Pollesch N, Smith LC, Zhang X, Villeneuve DL. 2018. Adverse Outcome Pathway Networks I: Development 1 and Applications. Environ Toxicol Chem. doi: 10.1002/etc.4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLone CA, Villeneuve DL, Doering J, Blackwell B, Transue T, Simmons C, Swintek J, Degitz S, Williams A, Ankely GT. 2018. Defining the Taxonomic Domain of Applicability for Mammlian-Based High-Throughput Screening Assays. In Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLone CA, Ankley GT, Belanger SE, Embry MR, Hodges G, Knapen D, Munn S, Perkins EJ, Rudd MA, Villeneuve DL, Whelan M, Willett C, Zhang X, Hecker M. 2017a. Advancing the adverse outcome pathway framework—An international horizon scanning approach. Environ Toxicol Chem 36:1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLone CA, Villeneuve DL, Wu-Smart J, Milsk RY, Sappington K, Garber KV, Housenger J, Ankley GT. 2017b. Weight of evidence evaluation of a network of adverse outcome pathways linking activation of the nicotinic acetylcholine receptor in honey bees to colony death. Sci Total Environ. 584–585:751–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLone CA, Villeneuve DL, Lyons D, Helgen HW, Robinson SL, Swintek JA, Saari TW, Ankley GT. 2016. Editor’s Highlight: Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS): A Web-Based Tool for Addressing the Challenges of Cross-Species Extrapolation of Chemical Toxicity. Toxicol Sci 153:228–245. [DOI] [PubMed] [Google Scholar]
- National Research Council. 2007. Toxicity testing in the 21st century: a vision and a strategy. National Academies Press. [Google Scholar]
- Perkins EJ, Ashauer R, Burgoon L, Conolly R, Landesmann B, Mackay C, Murphy CA, Pollesch N, Wheeler JR, Zupanic A, Scholz S. 2018. Building and Applying Quantitative Adverse Outcoe Pathway Models for Chemical Hazard and Risk Assessment. In prep [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollesch N, Villeneuve D, O’Brien J. 2018. Extracting and benchmarking emerging adverse outcome pathway knowledge. In prep Society for the Advancement of Adverse Outcome Pathways (SAAOP). http://www.saaop.org/, accessed May 9th, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonich-Mullin C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp P, Grant D, Hartley M, Knaap A, Kroese D, Mangelsdorf I, Meek E, Rice JM, Younes M. 2001. IPCS Conceptual Framework for Evaluating a Mode of Action for Chemical Carcinogenesis. Reg Toxicol Pharmacol 34:146–152. [DOI] [PubMed] [Google Scholar]
- Tollefsen KE, Scholz S, Cronin MT, Edwards SW, de Knecht J, Crofton K, Garcia-Reyero N, Hartung T, Worth A, Patlewicz G. 2014. Applying Adverse Outcome Pathways (AOPs) to support Integrated Approaches to Testing and Assessment (IATA). Reg Toxicol Pharmacol 70:629–640. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O’Brien JM, Pollesch N, Smith LC, Zhang X, Knapen D. 2018. Adverse Outcome Pathway Networks II: Network Analytics. Environ Toxicol Chem. doi: 10.1002/etc.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson T, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. 2014a. Adverse Outcome Pathway (AOP) Development I: Strategies and Principles. Tox Sci 142:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson T, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. 2014b. Adverse Outcome Pathway (AOP) Development II: Best Practices. Tox Sci 142:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwehr C, Aladjov H, Ankley G, Byrne HJ, deKnecht J. 2017. How Adverse Outcome Pathways Can Aid the Development and Use of Computational Prediction Models for Regulatory Toxicology. Toxicol Sci 155:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


