Abstract
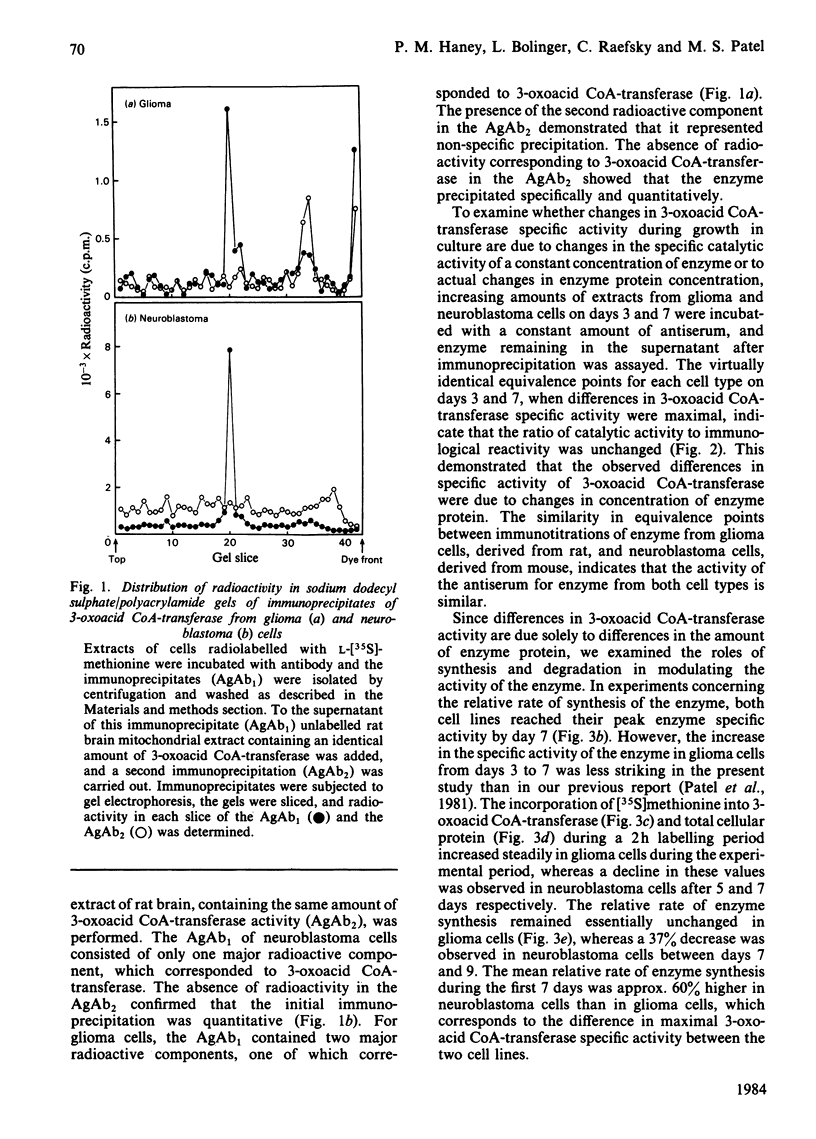
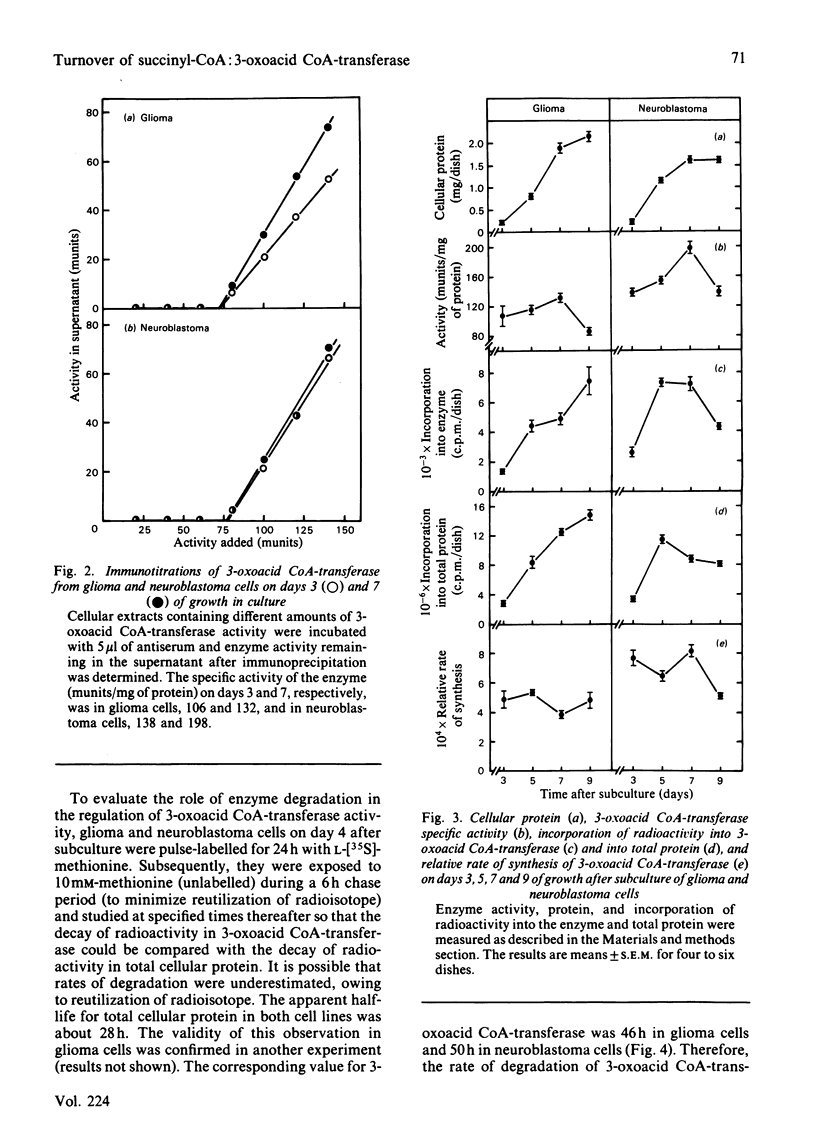
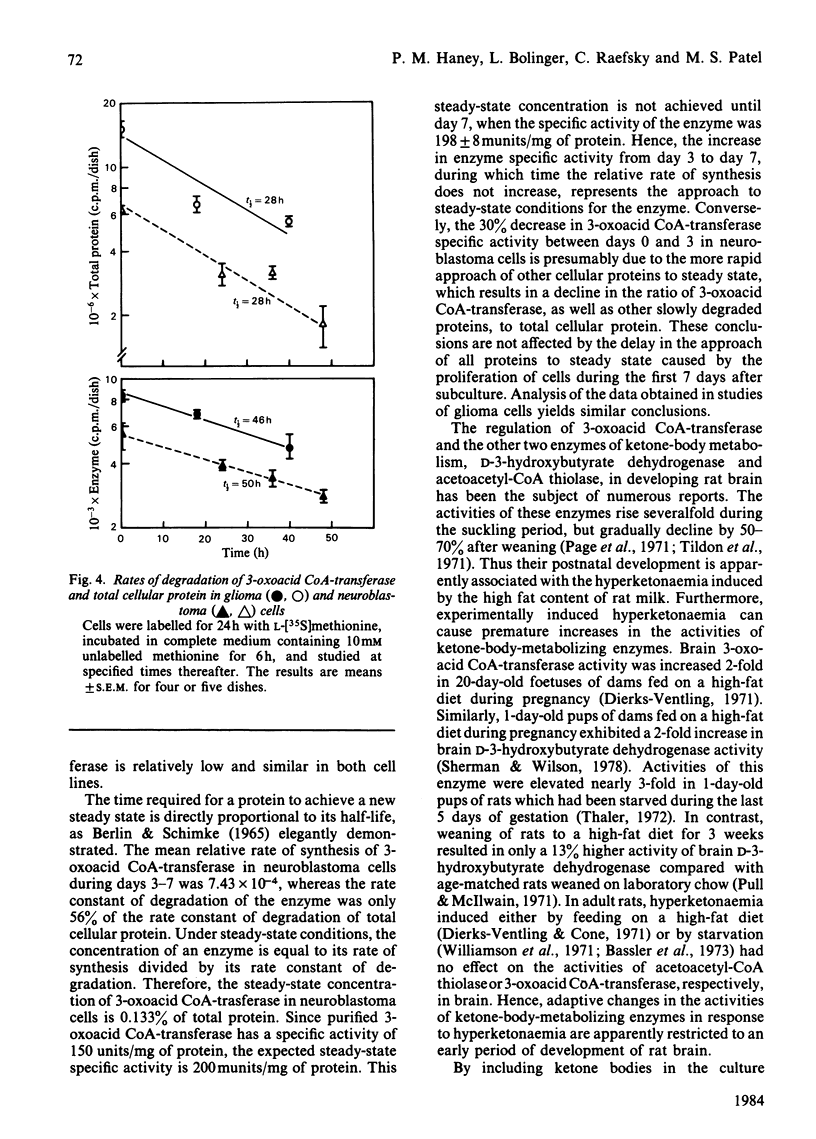
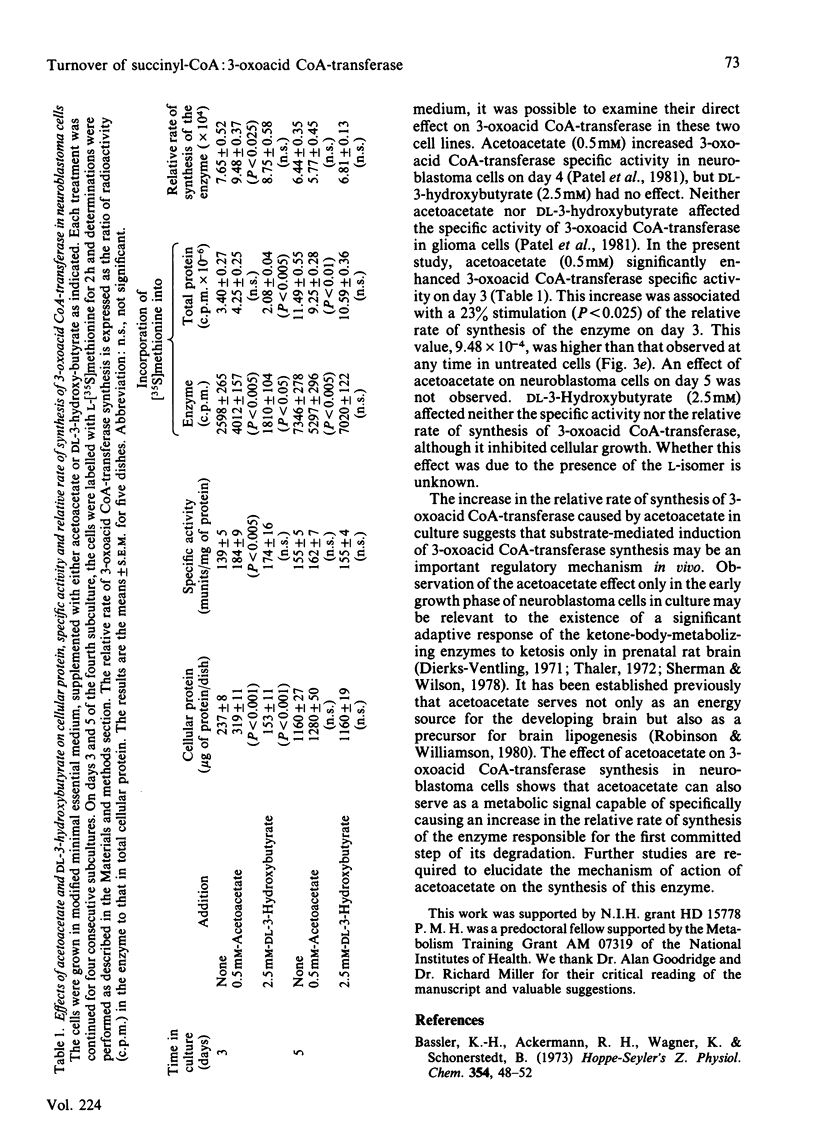
The specific activity of succinyl-CoA:3-oxo-acid CoA-transferase (3-oxoacid CoA-transferase, EC 2.8.3.5) increases significantly during growth in culture in both mouse neuroblastoma N2a and rat glioma C6 cells. To investigate the mechanism(s) responsible for this, antibody specific for rat brain 3-oxoacid CoA-transferase was raised in rabbits. Immunotitrations of 3-oxoacid CoA-transferase from neuroblastoma and glioma cells on days 3 and 7 of growth after subculture showed that the ratio of 3-oxoacid CoA-transferase activity to immunoprecipitable enzyme protein remained constant, indicating that differences in specific activity of the enzyme at these times in both cell types reflect differences in concentration of enzyme protein. In glioma cells, the relative rate of 3-oxoacid CoA-transferase synthesis was about 0.04-0.05% throughout 9 days in culture. In contrast, the relative rate of synthesis of 3-oxo-acid CoA-transferase in neuroblastoma cells was about 0.07-0.08% on days 3, 5 and 7 after subculture, but fell to 0.052% on day 9. The degradation rates of total cellular protein (t1/2 = 28 h) and 3-oxoacid CoA-transferase (t1/2 = 46-50 h) were similar in both cell lines. The rise in specific activity of the enzyme in both cell lines from days 3 to 7 without a significant increase in the relative rate of synthesis reflects a slow approach to steady-state conditions for the enzyme secondary to its slow degradation. Differences in 3-oxoacid CoA-transferase specific activity between the two cell lines are apparently due to a difference of about 60% in relative rates of enzyme synthesis. The presence of 0.5 mM-acetoacetate in the medium significantly increased the specific activity of 3-oxoacid CoA-transferase in neuroblastoma cells during the early exponential growth phase. This treatment increased the relative rate of synthesis of 3-oxoacid CoA-transferase by 23% (P less than 0.025) in these cells on day 3, suggesting that substrate-mediated induction of enzyme synthesis is a mechanism of regulation of 3-oxoacid CoA-transferase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin C. M., Schimke R. T. Influence of turnover rates on the responses of enzymes to cortisone. Mol Pharmacol. 1965 Sep;1(2):149–156. [PubMed] [Google Scholar]
- Bässler K. H., Ackermann R. H., Wagner K., Schönerstedt B. Enzymatic changes associated with ketosis in long standing diabetes and prolonged starvation of rats. Hoppe Seylers Z Physiol Chem. 1973 Jan;354(1):48–52. doi: 10.1515/bchm2.1973.354.1.48. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks-Ventling C., Cone A. L. Ketone body enzymes in mammalian tissues. Effect of high fat diet. J Biol Chem. 1971 Sep 10;246(17):5533–5534. [PubMed] [Google Scholar]
- Dierks-Ventling C. Prenatal induction of ketone-body enzymes in the rat. Biol Neonate. 1971;19(4):426–433. doi: 10.1159/000240435. [DOI] [PubMed] [Google Scholar]
- Freytag S. O., Utter M. F. Induction of pyruvate carboxylase apoenzyme and holoenzyme in 3T3-L1 cells during differentiation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1321–1325. doi: 10.1073/pnas.77.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moore T. J., Lione A. P., Sugden M. C., Regen D. M. Beta-hydroxybutyrate transport in rat brain: developmental and dietary modulations. Am J Physiol. 1976 Mar;230(3):619–630. doi: 10.1152/ajplegacy.1976.230.3.619. [DOI] [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. S., Russell J. J., Gershman H. Ketone-body metabolism in glioma and neuroblastoma cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7214–7218. doi: 10.1073/pnas.78.11.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull I McIlwain H. 3-Hydroxybutyrate dehydrogenase of rat brain on dietary change and during maturation. J Neurochem. 1971 Jun;18(6):1163–1165. doi: 10.1111/j.1471-4159.1971.tb12046.x. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980 Jan;60(1):143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- Russell J. J., Patel M. S. Purification and properties of succinyl-CoA:3-oxo-acid CoA-transferase from rat brain. J Neurochem. 1982 May;38(5):1446–1452. doi: 10.1111/j.1471-4159.1982.tb07924.x. [DOI] [PubMed] [Google Scholar]
- Sherman T. G., Wilson J. E. Effect of prenatally-induced and postnatally-maintained ketosis on beta-hydroxybutyrate dehydrogenase and hexokinase levels in the developing rat brain. J Neurochem. 1978 Mar;30(3):639–641. doi: 10.1111/j.1471-4159.1978.tb07820.x. [DOI] [PubMed] [Google Scholar]
- Thaler M. M. Effects of starvation on normal development of -hydroxybutyrate dehydrogenase activity in foetal and newborn rat brain. Nat New Biol. 1972 Apr 5;236(66):140–141. doi: 10.1038/newbio236140a0. [DOI] [PubMed] [Google Scholar]
- Tildon J. T., Cone A. L., Cornblath M. Coenzyme A transferase activity in rat brain. Biochem Biophys Res Commun. 1971 Apr 2;43(1):225–231. doi: 10.1016/s0006-291x(71)80111-0. [DOI] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]


