Abstract
Virus infection of neurons leads to different outcomes ranging from latent and noncytolytic infection to cell death. Viruses kill neurons directly by inducing either apoptosis or necrosis or indirectly as a result of the host immune response. Sindbis virus (SV) is an alphavirus that induces apoptotic cell death both in vitro and in vivo. However, apoptotic changes are not always evident in neurons induced to die by alphavirus infection. Time lapse imaging revealed that SV-infected primary cortical neurons exhibited both apoptotic and necrotic morphological features and that uninfected neurons in the cultures also died. Antagonists of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors protected neurons from SV-induced death without affecting virus replication or SV-induced apoptotic cell death. These results provide evidence that SV infection activates neurotoxic pathways that result in aberrant NMDA receptor stimulation and damage to infected and uninfected neurons.
Neuronal death is a tightly regulated process that is necessary for proper development of the nervous system. However, neuronal death that is inappropriate, either in timing or extent, is also involved in production of disease associated with neurodegeneration, stroke, and trauma (50). Similar to cell death in other tissues, neuronal death can be characterized as either apoptotic or necrotic. Apoptotic cell death, a caspase-dependent programmed cell death, is important for the elimination of unnecessary or potentially harmful cells and involves nuclear and cytoplasmic condensation, intranucleosomal DNA cleavage, and blebbing of the cell into membrane-bound apoptotic bodies. Necrotic, or lytic, cell death occurs following intense cellular injury and is associated with swelling of the cell body, increases in cellular volume, changes in plasma membrane permeability, and release of cellular contents into the extracellular space. The types of morphological changes that occur during neuronal death depend on the developmental state of the neuron and on the cell death stimulus (34, 50).
Sindbis virus (SV) is an enveloped, single-stranded, positive-sense RNA alphavirus related to eastern, western, and Venezuelan equine encephalitis viruses, important causes of acute mosquito-borne encephalitis in the Americas (55). SV causes fever, rash, and arthritis in humans but causes an age-dependent encephalitis in mice and serves as a model for studying viral encephalitis and neuronal damage caused by the encephalitic alphaviruses (18). SV induces apoptotic cell death in vitro and in vivo (35–37, 45, 60), but characteristic apoptotic changes are not always evident in neurons induced to die by alphavirus infection (17, 19, 24, 51). As determined by caspase-3 activation, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling positivity, and morphological changes, apoptotic neurons are present in the hippocampi of infected animals (24; T. Kimura and D. E. Griffin, submitted for publication). However, swollen neurons without condensed apoptotic nuclei can also be detected in this region, and SV-induced motor neuron death does not appear to be apoptotic (24). Therefore, the mechanism of SV-induced death appears to differ according to the type and maturity of the infected neuron (24, 36).
Neuronal excitotoxicity is mediated by excessive or prolonged activation of excitatory amino acid receptors and is involved in the pathogeneses of ischemic brain injury, epilepsy, and neurodegenerative diseases. Glutamate is an excitatory amino acid neurotransmitter that triggers neuronal death when it is present in excess quantities (11, 49). Excess glutamate overstimulates α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)-, kainate-, and N-methyl-d-aspartate (NMDA)-type glutamate receptors, resulting in an influx of Ca2+, Na+, and Zn2+ ions through channels gated by these receptors. The resulting elevation in intracellular Ca2+ activates phospholipases, oxidases, nitric oxide synthase, proteases, and phosphatases and leads to lethal metabolic derangements (references 34 and 47 and references therein). In many types of mature neurons, glutamate-induced Ca2+ influx is mediated predominantly by NMDA receptors, and the treatment of primary neuronal cultures with NMDA receptor antagonists protects the cells from glutamate-induced death (10). Furthermore, the ischemic release of glutamate can cause lethal excitation of surrounding neurons. Frequently, a mix of both apoptotic and necrotic morphological changes follow ischemic injury (34).
Excitotoxicity has been implicated in the pathogeneses of some virus-induced diseases of the central nervous system. Lentivirus- and measles virus-induced central nervous system damage may result, at least in part, from excitotoxic neuronal death (4, 15, 16, 20, 39–41, 54, 57, 58). Interestingly, virus antigen-negative apoptotic neurons can be detected following alphavirus infection, suggesting that uninfected cells also die during the infection process (2; Kimura and Griffin, submitted). Using SV infection of primary cortical neurons as an in vitro system of SV-induced neuronal death, the morphological changes that occur in infected and uninfected neurons in the same culture were examined. Treatment with NMDA receptor antagonists revealed that the excitatory amino acid neurotransmitter glutamate contributes to SV-induced neuronal death.
MATERIALS AND METHODS
Primary cortical-cell cultures.
Primary cortical cells were prepared from gestational day 18 Long-Evans rats as previously described (56). Briefly, the cortex was dissected in Hanks balanced salt solution and digested in 10 U of papain (Worthington Biochemical, Lakewood, N.J.)/ml in dissociation medium (80 mM Na2SO4, 30 mM K2SO4, 6 mM MgCl2, 250 μM CaCl2, 1 mM HEPES, 20 mM glucose, and 0.001% phenol red adjusted to pH 7.4 with 0.1 N NaOH). Dissociated cortical cells were plated on poly-d-lysine- and laminin-coated 35-mm-diameter glass-bottom dishes (MatTek Corporation, Ashland, Mass.) at 1.5 × 106 cells per dish in glutamine-free basal medium Eagle, 2 mM glutaMAX-I supplement, 1% N-2 supplement, 10% fetal bovine serum, 5% horse serum, 100 U of penicillin/ml, and 100 μg of streptomycin (GIBCO, Grand Island, N.Y.)/ml and maintained at 37°C in 5% CO2–95% room air for 5 days prior to use. Based on morphology and immunohistochemical staining for F4/80 antigen (macrophages and microglia) and glial fibrillary acidic protein (astrocytes), >95% of the cells were neuronal at the time of infection (D. Irani, unpublished data). Infection with recombinant SV expressing green fluorescent protein (SV-GFP) allowed visualization of distinct cell types. Many cells had a pyramidal cell body, a gradually tapering major apical dendrite that terminated in a branched tuft and a number of basal dendrites typical of pyramidal neurons. Other neurons were multipolar and had several equivalent primary branches (56).
Virus infection, viability assays, and time lapse imaging.
SV-GFP was generated by cloning the cDNA encoding GFP (CLONTECH) into the SV clone TE12Q genetically modified to contain a duplicate subgenomic mRNA promoter and a unique BstEII cloning site downstream of the genes for the structural proteins (9, 35). SV strain AR339 (referred to here as SV; American Type Culture Collection, Manassas, Va.) and SV-GFP were produced and assayed by plaque formation on BHK-21 cells. Cortical cells were infected at a multiplicity of infection (MOI) of 5 with virus diluted in basal medium Eagle, 2 mM glutaMAX-I supplement, 0.5% fetal bovine serum, and N-2 supplement (infection medium) or in infection medium containing either 300 μM d(−)-2-amino-5-phosphonopentanoic acid (APV) (TOCRIS, Ballwin, Mo.), 3 μM MK-801 hydrogen maleate (Research Biochemicals International, Natick, Mass.), or 5 μM tetrodotoxin (TTX) (Alexis Biochemicals, San Diego, Calif.). Control cells were treated with appropriately diluted supernatant fluid from uninfected BHK cells. After 1 h at 37°C, the medium was replaced with conditioned medium or conditioned medium containing D-APV, MK-801, or TTX. Staurosporine (Sigma, St. Louis, Mo.) was used at a concentration of 1.0 μM. Viability was assayed 24, 48, and 72 h postinfection (p.i.) by microscopic examination with computer-assisted cell counting (IPLabSpectrum version 3.2) following staining of all nuclei with 10 μg of Hoescht 33342/ml and staining of dead cell nuclei with 5 μg of propidium iodide (PI)/ml in phosphate-buffered saline containing 25 mM glucose. Digital imaging of Hoescht-stained nuclei was performed 24 and 48 h p.i. Time lapse imaging was performed in a temperature-controlled environment 16 to 26 h p.i. with SV-GFP. PI (5 μg/ml) and a mineral oil overlay were added to the culture media. Images for GFP and PI were acquired every 5 and 25 min, respectively.
LDH and histone release assays.
Cell death was assessed by measuring levels of lactate dehydrogenase (LDH; Boehringer Mannheim, Indianapolis, Ind.) released into the culture supernatant. Cortical cells (1.5 × 106) were infected at an MOI of 5 and maintained for 24 or 48 h in 1.0 ml of infection medium or infection medium with 300 μM APV. LDH activity was measured according to the manufacturer's instructions. Apoptotic cell death was assessed by measuring cytoplasmic histone-associated DNA fragments (22, 28, 30). Uninfected, SV-infected, APV-treated, or SV-infected and APV-treated cortical cells (1.5 × 106) were lysed 24 and 48 h p.i. The lysate was centrifuged at 200 × g for 10 min to separate the cytoplasmic fraction from the cell nuclei. The presence of histone-associated DNA fragments in cytoplasmic fractions was determined with antibodies against both DNA and histone in a cell death detection enzyme-linked immunosorbent assay (Boehringer Mannheim) according to the manufacturer's instructions. The results shown are from three independent experiments, each done in triplicate, and are presented as the mean ratio of DNA-histone released in infected wells to that released in uninfected wells (percent of control) ± the standard deviation (SD).
Calcium imaging.
Measurement of the intracellular Ca2+ concentration was performed using the Ca2+-sensitive indicator fura-2 AM (Molecular Probes, Eugene, Oreg.). At various times p.i., cells were loaded for 1 h with 5 μM fura-2 AM that had been sonicated for 30 s in conditioned cortical culture medium. The cells were washed twice with a solution containing (in mM) NaCl, 140; KCl, 5; CaCl2, 2; MgCl2, 0.8; HEPES, 10; and glucose, 10. Imaging was performed at room temperature as previously described (29, 44). Fura-2 AM ratio imaging of intracellular free Ca2+ was accomplished by measuring the background-corrected fluorescence ratio at 340- and 380-nm excitation with a cooled charge-coupled device camera system. A galvanometer-driven mirror assembly was used to switch light from a 100-W mercury burner through two optical paths containing 340- and 380-nm excitation filters. The light was then recombined in a liquid light guide coupled to the epifluorescence train of a Zeiss Axiovert 100 with an 40× 1.3-aperture oil immersion objective. Emission at 505 nm was passed through a dichroic mirror and focused on the chip of a slow-scan cooled charge-coupled device camera. Digitized images were acquired on disk using custom software (kindly provided by David Linden, Johns Hopkins University). The intracellular Ca2+ concentration per cell was derived from the ratio of the average emission at 505 nm from both excitation wavelengths (340/380 ratio) (21). For each timepoint, the intracellular Ca2+ concentration was determined for 120 to 200 cells, and the average concentration was plotted versus time.
RESULTS
SV infection is lethal for cortical neurons.
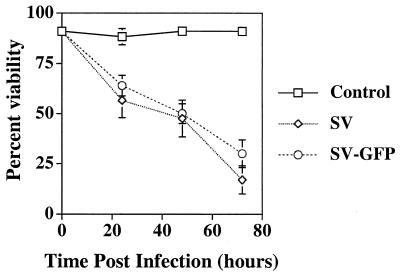
SV infection is rapidly lethal in freshly explanted dorsal root ganglion neurons, whereas neurons differentiated for 6 weeks survive for more than 2 weeks after infection (36). To determine if cultured cortical neurons were susceptible to SV-induced death, the viability of cortical neurons infected at an MOI of 5 was determined by PI exclusion (Fig. 1). Cortical neurons died rapidly after infection: by 72 h p.i., only 17% of the neurons were viable. To visualize infected cells, a recombinant SV expressing GFP (SV-GFP) was constructed. The virulence of SV-GFP in cortical neurons was equivalent to that of SV (Fig. 1).
FIG. 1.
Cortical neurons are susceptible to SV-induced death. Cortical cells were infected at an MOI of 5 with SV or SV-GFP. Viability was assayed by PI exclusion. The results from four independent experiments, each done in triplicate, are shown and are presented as the mean percent viability ± SD.
SV induces both necrotic and apoptotic cell death in primary neuronal cultures.
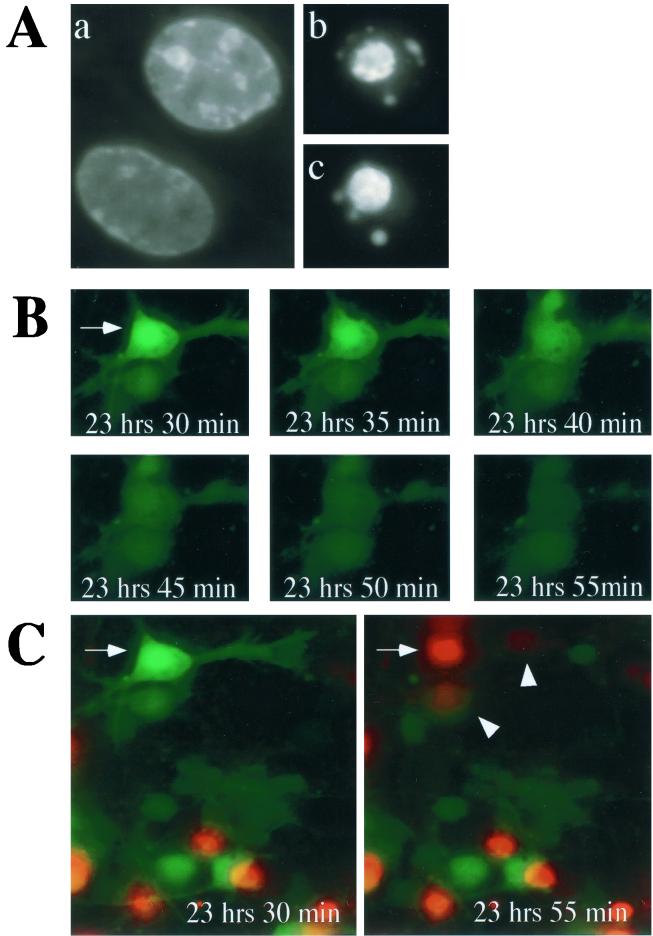
To determine the morphological changes that occurred in SV-infected primary cortical neurons, digital imaging of SV-GFP-infected cortical neurons was performed 16 to 26 h p.i. By 24 h p.i., Hoescht staining revealed condensed fragmented nuclei in approximately 5% of infected neurons, suggesting that SV induced apoptotic cell death in cortical neurons (Fig. 2A). The frequency with which apoptotic nuclei were observed increased with the length of time after infection (data not shown). Additionally, time lapse imaging revealed that approximately 2% of the cortical neurons lysed following infection with SV (Fig. 2B). Images for GFP were digitally acquired at 5-min intervals and revealed that GFP, a small cytoplasmic protein, disappeared from lysed cells. Imaging for PI staining of nuclei, a marker of plasma membrane integrity, was performed every 25 min (Fig. 2C). The inability to exclude PI coincided with the loss of GFP detection, suggesting that GFP leaked out of lysed cells after plasma membrane integrity was lost. Infected as well as uninfected cells adjacent to lysed cells often became PI positive immediately following cell lysis (Fig. 2C). By 16 h p.i., 49% of neurons were infected, as indicated by GFP positivity. During 8 h of imaging (16 to 24 h p.i.), 4.5% of infected cells became PI positive. Of the 51% of neurons that were GFP negative (i.e., uninfected), 7.1% became PI positive during these 8 h of imaging (data not shown). Two percent of mock-infected cells became PI positive during the same period. The viability in mock-infected cultures at 24 h was 91%.
FIG. 2.
SV infection induces apoptosis and lysis of cortical neurons. Cortical cells were infected at an MOI of 5 with SV-GFP. (A) Digital images of Hoescht-stained nuclei of uninfected (a) and infected (b and c) cells. The nuclei of infected cells appear condensed and fragmented. (B) Digital images of SV-GFP-infected cortical cells were acquired every 5 min. The arrow indicates an SV-infected cortical neuron that lysed during the time interval shown. GFP, a small cytoplasmic protein, is released from the cell after loss of plasma membrane integrity. (C) Digital images for PI were acquired every 25 min. PI (5 μg/ml) was included in the imaging medium. The cell indicated by the arrow is the same cell shown in panel B and became PI positive and GFP negative in the 25-min interval shown. The arrowheads indicate cells adjacent to a lysed cell which became PI positive.
SV-induced death of cortical neurons is ameliorated by NMDA receptor antagonists.
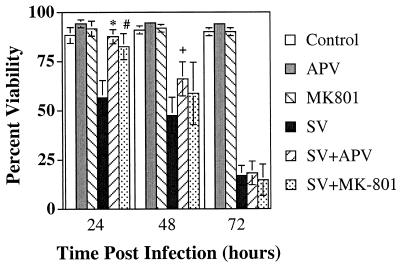
To determine whether the death of uninfected neurons resulted from the release of glutamate and excitotoxic death mediated by Ca2+ influx through NMDA receptor-gated ion channels, infected cortical cells were treated with either APV, a competitive NMDA receptor antagonist, or MK-801, a noncompetitive NMDA receptor antagonist (Fig. 3). Treatment of uninfected cortical cells with NMDA receptor antagonists did not affect viability (control, 88.4%; APV, 94.3%; MK-801, 91.7%). Twenty-four hours p.i., cortical-cell viability was reduced to 56.0%. Treatment of infected cells with either APV or MK-801 improved survival to levels similar to that of the control (SV plus APV, 87.7%; APV, 94.2%; SV plus MK-801, 82.6%; MK-801, 91.7%). APV and MK-801-mediated protection of SV-infected cortical cells was transient, and by 72 h p.i., the viabilities were similar (SV, 17.0%; SV plus APV, 18.3%; SV plus MK-801, 14.7%). APV did not improve the viability of cortical neurons treated for 24 h with 1 μM staurosporine, a potent inducer of apoptotic cell death (staurosporine, 49.3%; staurosporine plus APV, 46.1%). Thus, NMDA receptor blockade delayed SV-induced death.
FIG. 3.
SV-induced death of cortical neurons is ameliorated by NMDA receptor antagonists. Cortical cells were infected at an MOI of 5 with SV with or without 300 μM APV or 3 μM MK-801. Viability was assayed by PI exclusion. The results from four independent experiments, each done in triplicate, are shown and are presented as the mean percent viability ± SD (∗, P = 0.0005 for APV + SV versus SV; #, P = 0.004 for MK-801 + SV versus SV; +, P = 0.018 for APV + SV versus SV by Student's t test).
In vitro NMDA receptor blockade does not affect SV replication.
To determine if NMDA receptor blockade improves the survival of SV-infected cortical cells by inhibiting virus replication, virus growth rates in the presence and absence of APV were compared. NMDA receptor blockade did not alter SV replication at either time point (24 h p.i., SV, 3.6 × 108 ± 7.6 × 107 PFU/ml; SV plus APV, 8.2 × 108 ± 1.9 × 108 PFU/ml; 48 h p.i., SV, 3.3 × 108 ± 5.1 × 107 PFU/ml; SV plus APV, 3.6 × 108 ± 2.0 × 108 PFU/ml). Additionally, MK-801 did not limit SV replication in vivo (J. L. Nargi-Aizenman, unpublished results).
SV-induced neuronal death is not affected by treatment with TTX.
Primary cortical neurons are synaptically active (13, 14, 27), and toxic levels of the neurotransmitter glutamate can be released from depolarized neurons. To determine if neuronal death following SV infection resulted from the synaptic release of glutamate, action potentials were blocked by treating infected cells with the voltage-gated Na+ channel inhibitor TTX. TTX treatment did not affect the viability of SV-infected cells as assessed by PI exclusion (data not shown).
In vitro treatment of SV-infected cells with NMDA receptor antagonists decreases cell death but does not affect apoptotic cell death.
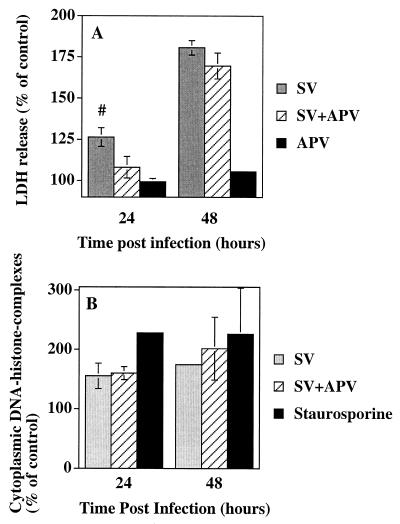
To quantitate the protection conferred by NMDA receptor blockade, LDH activity in the supernatant fluid of SV-infected cell cultures was measured (Fig. 4A). LDH is a stable cytoplasmic enzyme, and measurement of LDH release was originally used to measure neuronal cell death occurring via necrosis. However, because disintegration of late-stage apoptotic cells contributes to LDH release, this assay has also been used to measure apoptosis in cortical-neuron cultures (23, 31, 32, 38, 42). Therefore, quantitation of LDH release reflects the total amount of cell death that occurs following SV infection. APV treatment blocked 69% of LDH release 24 h p.i. (P = 0.06). However, by 48 h p.i., NMDA receptor blockade no longer prevented cell death. Excitotoxic cell death can be either apoptotic or necrotic depending on the intensity of the injury and levels of intracellular Ca2+ (34). To determine whether NMDA receptor blockade affects SV-induced apoptotic cell death, cytoplasmic histone-associated DNA fragments were measured (22, 28, 30) (Fig. 4B). During apoptotic cell death, cellular DNA is cleaved into internucleosomal fragments, and NMDA receptor blockade did not affect SV-induced DNA fragmentation 24 or 48 h p.i. Thus, 24 h p.i., APV improved the viability of infected cells without affecting apoptotic cell death (Fig. 3 and 4).
FIG. 4.
APV treatment of SV-infected cortical cells decreases cell death without affecting DNA fragmentation. Cortical cells were infected at an MOI of 5 with SV with or without 300 μM APV. (A) LDH activity in the supernatant fluid 24 and 48 h p.i. The results from three independent experiments, each done in triplicate, are shown and are presented as the mean percent change in LDH release compared to that of uninfected wells ± standard error of the mean (#, P = 0.06 for SV versus SV plus APV by Student's t test). (B) Apoptotic cell death assessed by measuring cytoplasmic histone-associated DNA fragments by enzyme-linked immunosorbent assay. Staurosporine was used at a concentration of 1 μM for 24 h as a control for the induction of apoptotic cell death. The results from three independent experiments, each done in triplicate are shown. The percent of control was determined by dividing the absorbance reading from SV-infected cells by the absorbance reading from mock-infected cells and multiplying this ratio by 100. The results are presented as the mean percent of control ± SD.
SV infection increases intracellular calcium concentrations.
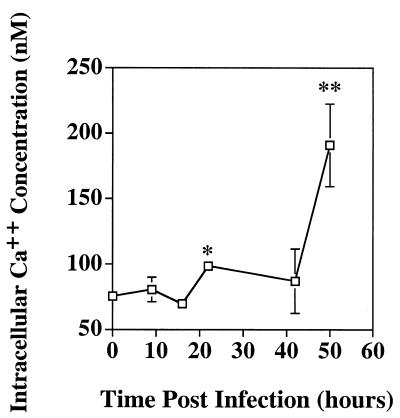
To determine if intracellular Ca2+ concentrations increased during SV infection, control and infected cells were loaded with the Ca2+-sensitive indicator fura-2 AM and fluorescent signals were acquired. By 22 h p.i., the average intracellular Ca2+ concentration was 131% that of the control, and by 50 h p.i., it was 253% that of the control (Fig. 5).
FIG. 5.
SV infection increases intracellular calcium concentrations. At various times p.i., control and infected cells were loaded with the Ca2+-sensitive indicator fura-2 AM and fluorescent images were acquired. The results are presented as the average intracellular Ca2+ concentration per cell ± the standard error of the mean (SEM) (∗, P = 0.0006 for 22 versus 0 h; ∗∗, P < 0.000006 for 50 versus 0 h by Student's t test). The SEMs at 16 and 22 h p.i. are too small to appear on a graph at this scale.
DISCUSSION
Using primary cortical neurons as an in vitro model of SV infection of neurons, we have shown that both apoptotic and necrotic cell death occurred in infected neurons and that bystander death occurred in uninfected neurons. Glutamate excitotoxicity was an important mediator of early virus-induced neuronal death in these cultures. NMDA receptor antagonists delayed virus-induced death without affecting virus growth but did not prevent apoptotic cell death. Thus, neuronotropic viruses can induce neuronal cell death both directly and indirectly and through more than one pathway.
SV was one of the first viruses shown to induce apoptosis in infected cells (36), and subsequent studies have indicated that SV induces apoptotic cell death both in vitro and in vivo (35–37, 45, 60). In continuous lines of proliferating cells, SV triggers apoptosis at the cell membrane during the process of virus fusion and entry (25, 26). Neuronal susceptibility to apoptosis is determined in part by neuronal maturity (36) and is influenced by the levels of expression of cellular regulators of the death process, the amount of virus initiating infection, and the neurovirulence of the infecting virus (19). However, characteristic apoptotic changes are not always detected in neurons induced to die by alphavirus infection (17, 19, 24, 51). For instance, the motor neurons of paralyzed SV-infected mice do not exhibit apoptotic changes, although apoptotic neurons are present in the hippocampi of the same mice (24). The reasons for these site-specific differences are not clear, but such differences indicate that SV can induce neuronal death by more than one pathway. Both apoptotic and necrotic changes have also been detected in cells infected with Semliki Forest virus, poliovirus, human herpesvirus 7, and murine polyomavirus (1, 3, 17, 52). For polyomavirus-infected cells, necrotic death predominates early in infection, and apoptosis is detected only at later time points (3). This is similar to our observations of SV-infected primary cortical neurons, which had both apoptotic and necrotic changes. Therefore, even neurons from the same region of the brain can respond in different ways to virus infection. This may reflect an inherent heterogeneity in the cortical-neuron population or differential virus exposure.
However, at least half of the dying neurons in our cortical-cell cultures were not infected with SV. Bystander neuronal-cell death occurs in other paradigms of neuronal injury. Neurotoxic insults, such as ischemia or stroke, trigger excitotoxic cell death that is rapidly necrotic in the most severely affected areas, but apoptosis subsequently occurs in areas distal to the ischemic focus (34). The severity of injury, neuronal maturity, the availability of trophic support, and the concentration of intracellular free Ca2+ may, in part, determine which process predominates (5, 34). As NMDA receptor blockade delayed virus-induced death and reduced LDH release early after SV infection but did not affect SV-induced apoptotic cell death, it is possible that NMDA receptor blockade prevented SV-induced necrotic cell death. By 72 h p.i., APV and MK-801 no longer conferred protection, suggesting that other cell death pathways, such as overstimulation of the AMPA subtype of glutamate receptors (8, 43, 46, 53, 63) or virus-induced ceramide release (25), contributed to SV-induced cell death in vitro. These results provide evidence that SV infection activates neurotoxic pathways that result in aberrant NMDA receptor stimulation and neuronal damage.
The death of uninfected neurons and oligodendrocytes located close to infected cells has been observed in the brains of mice infected with dengue and Theiler's viruses (12, 59). Reovirus infection also induces apoptosis in both infected and uninfected cells (48). Postulated mechanisms include the release of toxic factors, loss of trophic support from infected connecting neurons, and binding of virus particles to cell surface receptors. Our results suggest that glutamate-induced excitotoxicity is an additional mechanism. SV induces apoptosis at an early step of virus entry, so virus replication may not be required to induce cell death (26), but release of glutamate from necrotic SV-infected cells could cause excitotoxic damage to adjacent neurons, regardless of their infection status. The type of neuron death would depend on the amount of glutamate released.
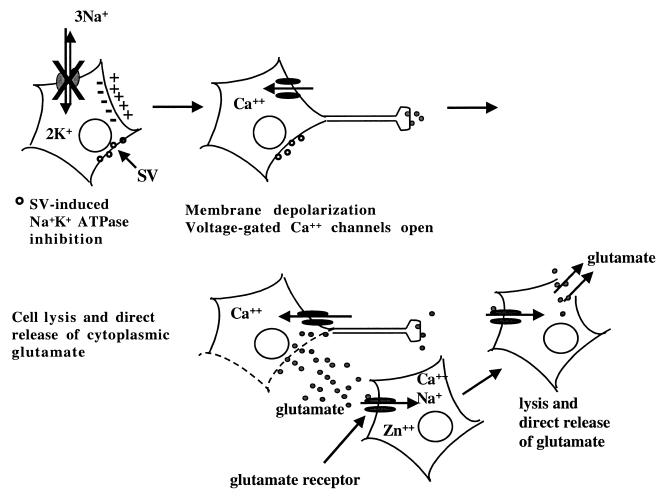
Toxic levels of glutamate can be synaptically released from depolarized neurons. However, TTX treatment did not affect the viability of SV-infected cells, suggesting that synaptic transmission was not responsible for excess glutamate release. Alphavirus infection decreases cellular Na+K+-ATPase activity, resulting in increased intracellular Na+ concentrations, membrane depolarization, and opening of voltage-dependent ion channels (7, 61, 62). Entry of toxic levels of Ca2+ through voltage-gated Ca2+ channels could cause cellular lysis with release of glutamate and trigger cell death in adjacent neurons regardless of their infection status (Fig. 6). Intracellular Ca2+ levels were elevated only late in infection. In contrast to that of nonneuronal cells, neuronal apoptosis can be attenuated by increasing intracellular Ca2+ levels, suggesting that decreased intracellular Ca2+ contributes to apoptotic neuronal death (6, 33). As both apoptotic and necrotic cell death pathways are activated during in vitro SV infection, decreases in intracellular Ca2+ concentrations in apoptotic cells may have hindered detection of increased Ca2+ levels in necrotic neurons at all of the time points studied. Since NMDA receptor blockade did not affect apoptotic cell death, glutamate-induced excitotoxic death of infected, as well as bystander, cells was most likely necrotic. Excitotoxic death of uninfected cells represents a novel mechanism by which neuronal loss occurs during SV infection.
FIG. 6.
Model of SV-induced glutamate release. SV infection decreases cellular Na+K+-ATPase activity, increases intracellular Na+ concentrations, and causes membrane depolarization. The opening of voltage-dependent Ca2+ channels may allow entry of toxic levels of Ca2+ and induce cellular lysis with direct release of glutamate. Excess glutamate activates glutamate receptors on neighboring neurons, leading to the excitotoxic death of both infected and uninfected cells. Glutamate receptor blockade would protect both infected and uninfected cells from bystander death. The open circles at cell membranes indicate SV-infected cells.
ACKNOWLEDGMENTS
We thank Anirvan Ghosh, Marie Hardwick, Jeffrey Rothstein, and Carlos Aizenman for helpful discussions, David Linden for help with calcium imaging, Doug Murphy for assistance with microscopy, and Dzung Thach for help with statistical analysis.
This work was funded by grants T32 ES07141 (J.L.N.) and RO1 NS18596 (D.E.G.) from the National Institutes of Health.
REFERENCES
- 1.Agol V I, Belov G A, Bienz K, Egger D, Kolesnikova M S, Raikhlin N T, Romanova L I, Smirnova E A, Tolskaya E A. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology. 1998;252:343–353. doi: 10.1006/viro.1998.9438. [DOI] [PubMed] [Google Scholar]
- 2.Allsopp T E, Scallan M F, Williams A, Fazakerley J K. Virus infection induces neuronal apoptosis: a comparison with trophic factor withdrawal. Cell Death Differ. 1998;5:50–59. doi: 10.1038/sj.cdd.4400298. [DOI] [PubMed] [Google Scholar]
- 3.An K, Fattaey H K, Paulsen A Q, Consigli R A. Murine polyomavirus infection of 3T6 mouse cells shows evidence of predominant necrosis as well as limited apoptosis. Virus Res. 2000;67:81–90. doi: 10.1016/s0168-1702(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 4.Andersson T, Schultzberg M, Schwarcz R, Love A, Wickman C, Kristensson K. NMDA-receptor antagonist prevents measles virus-induced neurodegeneration. Eur J Neurosci. 1991;3:66–71. doi: 10.1111/j.1460-9568.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 5.Ankarcrona M, Dypbukt J M, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton S A, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 6.Balazs R, Jorgensen O S, Hack N. N-Methyl-d-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience. 1988;27:437–451. doi: 10.1016/0306-4522(88)90279-5. [DOI] [PubMed] [Google Scholar]
- 7.Bashford C L, Alder G M, Gray M A, Micklem K J, Taylor C C, Turek P J, Pasternak C A. Oxonol dyes as monitors of membrane potential: the effect of viruses and toxins on the plasma membrane potential of animal cells in monolayer culture and in suspension. J Cell Physiol. 1985;123:326–336. doi: 10.1002/jcp.1041230306. [DOI] [PubMed] [Google Scholar]
- 8.Buchan A M, Li H, Cho S, Pulsinelli W A. Blockade of the AMPA receptor prevents CA1 hippocampal injury following severe but transient forebrain ischemia in adult rats. Neurosci Lett. 1991;132:255–258. doi: 10.1016/0304-3940(91)90314-j. [DOI] [PubMed] [Google Scholar]
- 9.Cheng E H-Y, Levine B, Boise L H, Thompson C B, Hardwick J M. Bax-independent inhibition of apoptosis by bcl-xL. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 10.Choi D W, Koh J, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi D W, Maulucci-Gedde M A, Kriegstein A R. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Despres P, Frenkiel M-P, Ceccaldi P-E, Duarte Dos Santos C, Deubel V. Apoptosis in the mouse central nervous system in response to infection with mouse-neurovirulent dengue viruses. J Virol. 1998;72:823–829. doi: 10.1128/jvi.72.1.823-829.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dichter M A. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978;149:279–293. doi: 10.1016/0006-8993(78)90476-6. [DOI] [PubMed] [Google Scholar]
- 14.Dichter M A, Lisak J, Biales B. Action potential mechanism of mammalian cortical neurons in cell culture. Brain Res. 1983;289:99–107. doi: 10.1016/0006-8993(83)90010-0. [DOI] [PubMed] [Google Scholar]
- 15.Giulian D, Vaca K, Noonan C A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 16.Giulian D, Wendt E, Vaca K, Noonan C A. The envelope glycoprotein of human immunodeficiency virus type-1 stimulates release of neurotoxins from monocytes. Proc Natl Acad Sci USA. 1993;90:2769–2773. doi: 10.1073/pnas.90.7.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasgow G M, McGee M M, Sheahan B J, Atkins G J. Death mechanisms in cultured cells infected by Semliki Forest virus. J Gen Virol. 1997;78:1559–1563. doi: 10.1099/0022-1317-78-7-1559. [DOI] [PubMed] [Google Scholar]
- 18.Griffin D E. Alphavirus pathogenesis and immunity. In: Schlesinger S, Schlesinger M J, editors. The Togaviridae and Flaviviridae. New York, N.Y: Plenum Publishing Corp.; 1986. pp. 209–249. [Google Scholar]
- 19.Griffin D E, Hardwick J M. Perspective: virus infections and the death of neurons. Trends Microbiol. 1999;7:155–160. doi: 10.1016/s0966-842x(99)01470-5. [DOI] [PubMed] [Google Scholar]
- 20.Gruol D L, Yu N, Parsons K L, Billaud J N, Elder J H, Phillips T R. Neurotoxic effects of feline immunodeficiency virus, FIV-PPR. J Neurovirol. 1998;4:415–425. doi: 10.3109/13550289809114540. [DOI] [PubMed] [Google Scholar]
- 21.Grynkiewicz G, Poenie M, Tsien R Y. A new generation of Ca indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 22.Gummuluru S, Novembre F J, Lewis M, Gelbard H A, Dewhurst S. Apoptosis correlates with immune activation in intestinal lymphoid tissue from macaques acutely infected by a highly enteropathic simian immunodeficiency virus, SIVsmmPBj14. Virology. 1996;225:21–32. doi: 10.1006/viro.1996.0571. [DOI] [PubMed] [Google Scholar]
- 23.Gwag B J, Lobner D, Koh J-Y, Wie M B, Choi D W. Blockade of glutamate receptors unmasks neuronal apoptosis after oxygen-glucose deprivation in vitro. Neuroscience. 1995;68:615–619. doi: 10.1016/0306-4522(95)00232-8. [DOI] [PubMed] [Google Scholar]
- 24.Havert M B, Schofield B, Griffin D E, Irani D N. Divergent neuronal cell death pathways activated in different target cell populations during neuroadapted Sindbis virus infection of mice. J Virol. 2000;74:5352–5356. doi: 10.1128/jvi.74.11.5352-5356.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jan J-T, Chatterjee S, Griffin D E. Sindbis virus entry into cells triggers apoptosis by activating sphingomyelinase, leading to the release of ceramide. J Virol. 2000;74:6425–6432. doi: 10.1128/jvi.74.14.6425-6432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jan J T, Griffin D E. Induction of apoptosis by Sindbis virus occurs at cell entry and does not require virus replication. J Virol. 1999;73:10296–10302. doi: 10.1128/jvi.73.12.10296-10302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones K A, Baughman R W. Both NMDA and non-NMDA subtypes of glutamate receptors are concentrated at synapses on cerebral cortical neurons in culture. Neuron. 1991;7:593–603. doi: 10.1016/0896-6273(91)90372-7. [DOI] [PubMed] [Google Scholar]
- 28.Jones M M, Xu C, Ladd P A. Selenite suppression of cadmium-induced testicular apoptosis. Toxicology. 1997;116:169–175. doi: 10.1016/s0300-483x(96)03541-x. [DOI] [PubMed] [Google Scholar]
- 29.Kaplin A I, Snyder S H, Linden D J. Reduced nicotinamide adenine dinucleotide-selective stimulation of inositol 1,4,5-triphosphate receptors mediates hypoxic mobilization of calcium. J Neurosci. 1996;16:2002–2011. doi: 10.1523/JNEUROSCI.16-06-02002.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kikuchi S, Hiraide H, Tamakuma S, Yamamoto M. Expression of wild-type p53 tumor suppressor gene and its possible involvement in the apoptosis of thyroid tumors. Surg Today. 1997;27:226–233. doi: 10.1007/BF00941650. [DOI] [PubMed] [Google Scholar]
- 31.Koh J-Y, Cotman C W. Programmed cell death: its possible role in calcium channel antagonist neurotoxicity. Brain Res. 1992;587:233–240. doi: 10.1016/0006-8993(92)91002-v. [DOI] [PubMed] [Google Scholar]
- 32.Koh J-Y, Gwag B J, Lobner D, Choi D W. Potentiated necrosis of cultured cortical neurons by neurotrophins. Science. 1995;268:573–575. doi: 10.1126/science.7725105. [DOI] [PubMed] [Google Scholar]
- 33.Lampe P A, Cornbrooks E B, Juhasz A, Johnson E M J, Franklin J L. Suppression of programmed neuronal death by a thapsigargin-induced Ca++ influx. J Neurobiol. 1995;26:205–212. doi: 10.1002/neu.480260205. [DOI] [PubMed] [Google Scholar]
- 34.Lee J-M, Zipfel G, Choi D W. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 35.Levine B, Goldman J E, Jiang H H, Griffin D E, Hardwick J M. Bcl-2 protects mice against alphavirus encephalitis. Proc Natl Acad Sci USA. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine B, Hardwick J M, Trapp B D, Crawford T O, Bollinger R C, Griffin D E. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 37.Lewis J, Wesselingh S L, Griffin D E, Hardwick J M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Zhang J. Inhibition of apoptosis by ginsenoside Rg1 in cultured cortical neurons. Chin Med J. 1997;110:535–539. [PubMed] [Google Scholar]
- 39.Lipton S A. Models of neuronal injury in AIDS: another role for the NMDA receptor? Trends Neurosci. 1992;15:75–79. doi: 10.1016/0166-2236(92)90013-x. [DOI] [PubMed] [Google Scholar]
- 40.Lipton S A. Requirement for macrophages in neuronal injury induced by HIV envelope protein gp120. Neuroreport. 1992;3:913–915. doi: 10.1097/00001756-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Lipton S A, Sucher N J, Kaiser P K, Dreyer E B. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- 42.Lobner D. Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis? J Neurosci Methods. 2000;96:147–152. doi: 10.1016/s0165-0270(99)00193-4. [DOI] [PubMed] [Google Scholar]
- 43.McDonald J W, Althomsons S P, Hyrc K L, Choi D W, Goldberg M P. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- 44.Narasimhan K, Pessah I N, Linden D J. Inositol-1,4,5-trisphosphate receptor-mediated Ca mobilization is not required for cerebellar long-term depression in reduced preparations. J Neurophysiol. 1998;80:2963–2974. doi: 10.1152/jn.1998.80.6.2963. [DOI] [PubMed] [Google Scholar]
- 45.Nava V E, Rosen A, Veliuona M A, Clem R J, Levine B, Hardwick J M. Sindbis virus induces apoptosis through a caspase-dependent, CrmA-sensitive pathway. J Virol. 1998;72:452–459. doi: 10.1128/jvi.72.1.452-459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nellgard B, Wieloch T. Postischemic blockade of AMPA but not NMDA receptors mitigates neuronal damage in the rat brain following transient severe cerebral ischemia. J Cereb Blood Flow Metab. 1992;12:2–11. doi: 10.1038/jcbfm.1992.2. [DOI] [PubMed] [Google Scholar]
- 47.Nicotera P, Orrenius S. The role of calcium in apoptosis. Cell Calcium. 1998;23:173–180. doi: 10.1016/s0143-4160(98)90116-6. [DOI] [PubMed] [Google Scholar]
- 48.Oberhaus S M, Smith R L, Clayton G H, Dermody T S, Tyler K L. Reovirus infection and tissue injury in the mouse central nervous system are associated with apoptosis. J Virol. 1997;71:2100–2106. doi: 10.1128/jvi.71.3.2100-2106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olney J W. Brain lesion, obesity and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 50.Pettmann B, Henderson C E. Neuronal cell death. Neuron. 1998;20:633–647. doi: 10.1016/s0896-6273(00)81004-1. [DOI] [PubMed] [Google Scholar]
- 51.Sammin D J, Butler D, Atkins G J, Sheahan B J. Cell death mechanisms in the olfactory bulb of rats infected intranasally with Semliki Forest virus. Neuropathol Appl Neurobiol. 1999;25:236–243. doi: 10.1046/j.1365-2990.1999.00170.x. [DOI] [PubMed] [Google Scholar]
- 52.Secchiero P, Flamand L, Gibellini D, Falcieri E, Robuffo I, Capitani S, Gallo R C, Zauli G. Human herpesvirus 7 induces CD4+ T-cell death by two distinct mechanisms: necrotic lysis in productively infected cells and apoptosis in uninfected or nonproductively infected cells. Blood. 1997;90:4502–4512. [PubMed] [Google Scholar]
- 53.Sheardown M J, Suzdak P D, Nordholm L. AMPA, but not NMDA, receptor antagonism is neuroprotective in gerbil global ischaemia, even when delayed 24 h. Eur J Pharmacol. 1993;236:347–353. doi: 10.1016/0014-2999(93)90470-3. [DOI] [PubMed] [Google Scholar]
- 54.Starling I, Wright A, Arbuthnott G, Harkiss G. Acute in vivo neurotoxicity of peptides from Maedi Visna virus transactivating protein Tat. Brain Res. 1999;830:285–291. doi: 10.1016/s0006-8993(99)01407-9. [DOI] [PubMed] [Google Scholar]
- 55.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
- 57.Toggas S M, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res. 1996;706:303–307. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- 58.Toggas S M, Masliah E, Rockenstein E M, Rall G F, Abraham C R, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 59.Tsunoda I, Kurtz C I B, Fujinami R S. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]
- 60.Ubol S, Park S, Budihardjo I, Desnoyers S, Montrose M H, Poirier G G, Kaufmann S H, Griffin D E. Temporal changes in chromatin, intracellular calcium, and poly(ADP-ribose) polymerase during Sindbis virus-induced apoptosis of neuroblastoma cells. J Virol. 1996;70:2215–2220. doi: 10.1128/jvi.70.4.2215-2220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulug E T, Garry R F, Bose H R., Jr The role of monovalent cation transport in Sindbis virus maturation and release. Virology. 1989;172:42–50. doi: 10.1016/0042-6822(89)90105-0. [DOI] [PubMed] [Google Scholar]
- 62.Ulug E T, Garry R F, Waite M R F, Bose H R J. Alterations in monovalent cation transport in Sindbis virus-infected chick cells. Virology. 1984;132:118–130. doi: 10.1016/0042-6822(84)90096-5. [DOI] [PubMed] [Google Scholar]
- 63.Xue D, Huang Z G, Barnes K, Lesiuk H J, Smith K E, Buchan A M. Delayed treatment with AMPA, but not NMDA, antagonists reduces neocortical infarction. J Cereb Blood Flow Metab. 1994;13:251–261. doi: 10.1038/jcbfm.1994.32. [DOI] [PubMed] [Google Scholar]