Abstract
Purpose
Sulfur mustard (SM) is a potent blistering agent. This alkylating chemical agent has extremely toxic effects on the eye. MMP-2 and MMP-9 are the two most important matrix metalloproteinase enzymes involved in the pathology of chemical eye injuries. Curcumin is regarded as a natural anti-inflammatory agent. This study aims to compare the anti-inflammatory effects of curcumin versus doxycycline on chemically induced corneal injuries.
Methods
The HCE-2 cell line was used as a model for corneal cells. The effective concentrations of 2-chloroethyl ethyl sulfide (CEES) – as an analog of SM – doxycycline, and curcumin were determined using the MTT assay. The gene expression of MMP-2, MMP-9, and tissue inhibitors of metalloproteinase (TIMP-1) was evaluated by the real-time PCR method. Also, the activity of MMP-2 and MMP-9 enzymes was determined by zymography.
Results
The expression of the MMP-2 and MMP-9 genes increased 5- and 3.3-fold after exposure to CEES, respectively. Following the treatment with curcumin and doxycycline, MMP-2 expression decreased significantly. Also, after treatment with curcumin and doxycycline, the MMP-9 expression decreased 2.5- and 1.6-fold, respectively. The reduction in activity was 32% for MMP-2 and 56% for MMP-9 after treatment with curcumin. The corresponding values were 12% and 40% following doxycycline treatment. There was no significant difference between the effects of curcumin and doxycycline on reducing MMP-2 expression, but the difference was statistically significant in the case of MMP-9.
Conclusion
Doxycycline and curcumin can inhibit MMP expression and activity in chemically exposed corneal cells. Curcumin has a greater ability than doxycycline to inhibit MMP-2 and MMP-9 enzymes; however, the difference is statistically significant only in the case of MMP-9. After further validation, these substances can be introduced as anti- inflammatory agents to treat corneal chemical burns.
Keywords: 2-Chloroethyl Ethyl Sulfide, Doxycycline, Curcumin, Inflammation, MMP-2, MMP-9
INTRODUCTION
Sulfur mustard (SM), or bis [2-chloroethyl] sulfide, is a potent and extremely reactive blistering agent.[1] The use of SM as a chemical weapon in World War I and the Iran–Iraq War injured more than 210,000 people, 90% of whom developed chemical eye burns.[2,3] SM is an alkylating chemical agent, and the eyes are the most susceptible organs to the toxic effects of this agent.[3,4,5] Ocular damage occurs through various biochemical and molecular mechanisms. Studies have shown that the induction of severe inflammatory reactions in corneal layers plays an important role in the pathogenesis of SM.[3,6,7]
Another process that occurs during SM-induced eye damage is extracellular matrix (ECM) remodeling. ECM is mainly processed by matrix metalloproteinase (MMPs).[6,8] MMPs are a family of zinc-dependent endopeptidases that are involved in the pathological processes in which ECM regeneration occurs and play a major role in tissue destruction.[6,9] The two most important MMPs involved in the pathology of eye injuries are MMP-2 and MMP-9.[10,11,12] Since MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are components of the basement membrane and denatured collagen (gelatin), they play a key role in the destruction of the basement membrane.[13] ECM component degradation is controlled by the balance between MMPs and a group of tissue inhibitors of metalloproteinase (TIMPs).[9,13] MMP inhibitors have been considered as a treatment for these injuries, and the family of tetracycline antibiotics such as doxycycline are effective inhibitors for MMPs.[6] Doxycycline inhibits the activity of the MMPs through mechanisms such as inhibiting reactive oxygen species and restricting the expression of neutrophil collagenase and epithelial gelatinase genes.[6,10,14,15]
As the main phenolic and curcuminoid section of the Curcuma longa, curcumin is highly regarded as a natural anti-inflammatory agent.[16] Curcumin's most important medicinal properties include its high antioxidants activity and anti-inflammatory properties.[16] Because the FDA classifies curcumin as a GRAS substance (generally known as safe), it can be used to treat many ocular disorders such as dry eye syndrome and glaucoma.[17] Considering the role of inflammation in chemical eye burns, curcumin may be an effective treatment for such injuries because of its impact on inflammatory reactions. This study aims to compare the effect of curcumin and doxycycline, as potential treatments for chemical eye burns, on the synthesis and activity of MMP-2 and MMP-9 in human corneal epithelial cells (HCE-2).
METHODS
Cell Culture
The HCE-2 cell line was purchased from CELLnTEC Advanced Cell Systems AG (Switzerland). The culture medium consisted of keratinocyte serum-free medium (Gibco, UK), containing fetal bovine serum 10% (Sigma-Aldrich, Germany), insulin 0.005 mg/ml (Sigma-Aldrich, Germany), and penicillin 100 U/ml + streptomycin 100 µg/ml (Sigma-Aldrich, Germany). The cells were grown in a 10% CO atmosphere at 37ºC.
The study protocol was reviewed and approved by the Ethics Committee at Baqiyatallah University of Medical Sciences, Tehran, Iran (No. IR.BMSU.REC.1398.072.).
Cell Viability Assay and IC50 Calculation
The MTT assay was used to assess cell viability and IC50 calculations. In this method, 1 104 cells in 100 µl of culture medium were seeded in a 96-well plate. After overnight incubation, the wells were divided into four groups: group 1 with 24 wells for doxycycline treatment (0.75–80 µg/ml), group 2 with 24 wells for curcumin treatment (0.75–80 µM), group 3 with 18 wells for CEES treatment (0.75–20 mM), and group 4 with 6 wells as the control group (only HCE-2 cells). Each concentration was evaluated three times. Forty-eight hours after cell treatment, 20 l of MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium) was added and incubation was performed at 37ºC for 4 hours. Subsequently, the culture medium containing MTT was carefully removed, and 150 l of dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals.[8] Finally, optical density was measured at 570 nm with a microplate reader, and cell viability percentage was calculated. The IC50 calculation was performed by GraphPad Prism.
Determination of the Effective Dose of Doxycycline and Curcumin
After the IC50 was calculated, the corneal cells (HCE-2 cells) were seeded in 96-well plates to determine the effective dose of doxycycline and curcumin. After overnight incubation, the cells were treated according to Table 1. The considered concentration for CEES was equivalent to IC50 (3.42 mM). Also, three concentrations lower than IC50 were considered for curcumin and doxycycline. Each concentration was evaluated three times. Forty-eight hours after cell treatment, 20 l of MTT was added and incubation was conducted at 37ºC for 4 hours. Subsequently, the culture medium containing MTT was carefully removed, and 150 l of DMSO was added to dissolve the formazan crystals. Finally, optical density was measured at 570 nm, and cell viability percentage was calculated.
Preparation of Doxycycline Formulation
Having reviewed similar previous studies, we designed the formulation of the doxycycline product as a ready-to-dissolve powder at the time of use and a final product with 2% doxycycline. The ready-to-dissolve powder was considered to increase stability and maintain the maximum antioxidant power of the ophthalmic product [Table 2].
Evaluation of MMP-2, MMP-9, and TIMP-1 gene expression
In this section, the expression of MMP-2, MMP-9, and TIMP-1 genes in infected cells (by CEES) and treated cells (with doxycycline and curcumin) was measured using real-time PCR. For this purpose, 105 HCE-2 cells were seeded in six-well plates. After overnight incubation, the cells were treated according to Table 3.
The concentrations of CEES, curcumin, and doxycycline were considered at 3.42 mM, 3 µM, and 10.11 µg/ml, respectively. Then, incubation was done for 24 hours at 37ºC and 5% CO . Afterward, at first, total RNA was isolated from corneal epithelial cells through the acid-guanidinium thiocyanate-phenol-chloroform extraction method.[15,18,19] Next, total RNA (1 µg) was reverse transcribed into cDNA using MuLV reverse transcriptase (50 U), oligo dT (50 pmol) as a primer, and RNAse inhibitor (20 U) at 42ºC for 30 minutes. For cDNA amplification, 2 µl of cDNA product, 0.4 µl of the relevant primers, 10 µl of SYBR Premix Ex Taq II (Takara, Japan), and 7.2 µl of DDW were mixed according to the manufacturer's protocol. The PCR reaction was initiated at 95ºC for 30 seconds, followed by 40 cycles of 95ºC for 10 seconds and 60ºC for 1 minute. These reactions were performed in the Applied Biosystem 7500 real-time quantitative PCR system (ABI, USA). The 18s rRNA gene was used as a housekeeping internal control. All real-time PCR reactions were performed in triplicate. The PCR primers for MMP-2, MMP-9, TIMP-1, and 18s rDNA are shown in Table 4. The relative expression of mRNA was calculated in comparison to 18s rRNA using the 2 method.[20] The data are reported as the relative quantity of target mRNA compared to 18S rRNA mRNA.
Analysis of MMP Activity (Zymography)
The activity of MMP-2 and MMP-9 in corneal cell culture was evaluated by gelatin zymography. For this purpose, the cultures were treated with CEES, doxycycline, and curcumin, the same way as in the sub-section entitled “Preparation of Doxycycline Formulation”. After 24 hours, the cultured cells were centrifuged and the supernatants were used for gelatin zymography assays. The supernatant of the corneal epithelial cultures with different treatments was electrophoresed on an 8% polyacrylamide gel containing 1% gelatin (Sigma-Aldrich, Germany). First, 5 to 20 ml of each medium was used, and the volume was adjusted so that the samples could have equal amounts (5 mg) of protein. The supernatants were mixed with SDS-PAGE sample buffer and run at 100 V for 90 minutes at 4ºC. The gel was then soaked with 0.25% Triton X-100 (SDS removal buffer) 30 minutes at room temperature and then incubated in enzyme activation buffer (50 mM Tris-HCl, 150 mM NaCl, 10 mM CaCl , 0.02% Tween-20, and 5 mM PMSF) at 37ºC overnight to permit gelatinase (MMP-2 and MMP-9) to digest its substrate. Subsequently, the gel was rinsed in distilled water, stained with Coomassie Brilliant Blue R-250, and then destained (7% v/v acetic acid, 5% v/v methanol). After photography, densitometric analysis of the bands (intensity and area) was conducted using the Image Station 4000MM (Kodak Digital Science, USA).
Statistical Analysis
Data analysis was performed using the SPSS version 23 (IBM, USA), and the results were compared using the Student's t-test. The data are presented as mean SD, and a P-value 0.05 was considered statistically significant.
Table 1.
Treatment method to determine the effective dose of doxycycline and curcumin.
|
| |
| Well no. | Treatment method and the contents of the wells |
| 1 | 104 HCE-2 cells |
| 2 | 104 HCE-2 cells + 3.42 mM CEES |
| 3 | 104 HCE-2 cells + 3.42 mM CEES + 6.04 µM curcumin |
| 4 | 104 HCE-2 cells + 3.42 mM CEES + 3.02 µM curcumin |
| 5 | 104 HCE-2 cells + 3.42 mM CEES + 1.51 µM curcumin |
| 6 | 104 HCE-2 cells + 3.42 mM CEES + 20.22 µg/ml doxycycline |
| 7 | 104 HCE-2 cells + 3.42 mM CEES + 10.11 µg/ml doxycycline |
| 8 | 104 HCE-2 cells + 3.42 mM CEES + 5.05 µg/ml doxycycline |
| HCEC, human corneal epithelial cell, CEES, 2-chloroethyl ethyl sulfide | |
Table 2.
Doxycycline eye drop formulation.
|
| ||
| Substance | Role | Percentage |
| Doxycycline hyclate | Effective material | 2.3008 |
| HPMC | Covering polymer | 0.15 |
| NaCl | Isotonic factor | 0.112 |
| KCl | Isotonic factor | 0.093 |
| Sodium tetraborate | Buffer | 0.048 |
| Boric acid | Acidic agent | 0.048 |
| Water | Diluent | Up to 100 ml |
| HPMC, hydroxypropyl methylcellulose; NaCl, Sodium chloride; KCl, Potassium chloride 1.154 gram of doxycycline hyclate is equivalent to 1 gram of doxycycline | ||
Table 3.
Cell treatment method to evaluate the effect of curcumin and doxycycline on CEES cytotoxicity.
|
| |
| Well no. (Group) | Treatment method and the contents of the wells |
| 1 (Control group) | 105 HCE-2 cells |
| 2 (CEES group) | 105 HCE-2 cells + 3.42 mM CEES |
| 3 (Curcumin group) | 105 HCE-2 cells + 3.42 mM CEES + 3 µM curcumin |
| 4 (Doxycycline group) | 105 HCE-2 cells + 3.42 mM CEES + 10 µg/ml doxycycline |
| HCEC, Human corneal epithelial cell; CEES, 2-chloroethyl ethyl sulfide | |
Table 4.
The sequence of the primers used in qPCR.
|
| ||
| PCR product length | Primers Sequence | Primers |
| 200 bp | GCAACCTGTTTGTGCTGAAG | MMP-2 (Forward) |
| GTAGCCAATGATCCTGTATGTG | MMP-2 (Reverse) | |
| 114 bp | TCCAGTACCGAGAGAAAGCCTA | MMP-9 (Forward) |
| GCAGGATGTCATAGGTCACG | MMP-9 (Reverse) | |
| 174 bp | TGCGGATACTTCCACAGGTC | TIMP-1 (Forward) |
| GCATTCCTCACAGCCAACAG | TIMP-1 (Reverse) | |
| 63 bp | TGTGCCGCTAGAGGTGAAATT | 18s rRNA (Forward) |
| TGGCAAATGCTTTCGCTTT | 18s rRNA (Reverse) | |
| MMP, matrix metalloproteinase; TIMP, tissue inhibitors of metalloproteinase | ||
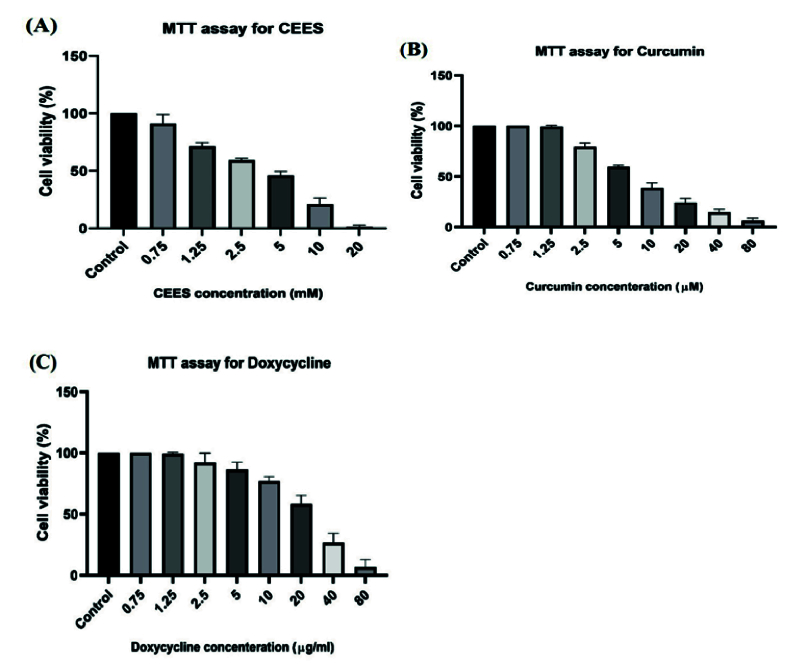
Figure 1.
Cell viability in different concentrations of CEES (A), doxycycline (B), and curcumin (C).
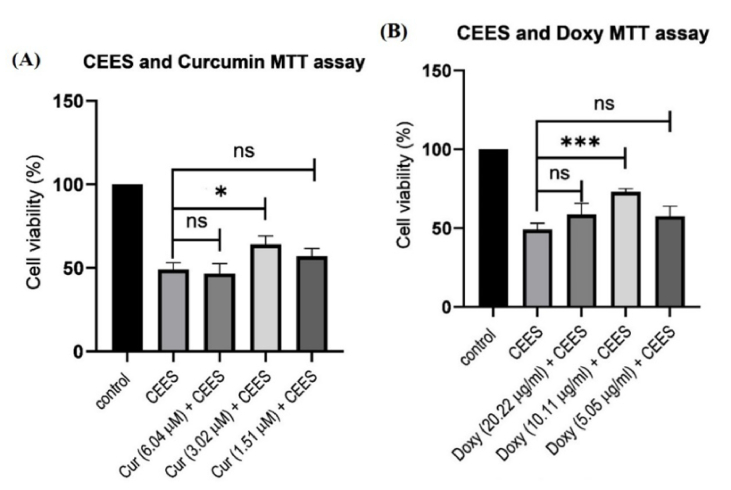
Figure 2.
The impact of different and optimal concentrations of curcumin (A) and doxycycline (B) in inhibiting CEES.
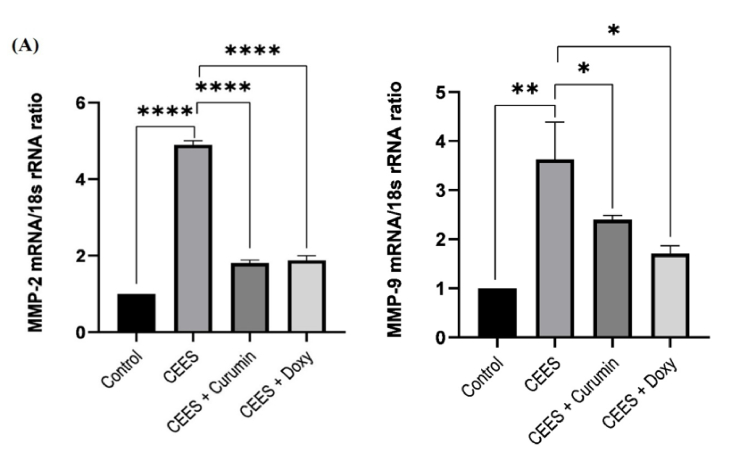
Figure 3.
The expression levels of MMP-2 (A) and MMP-9 (B) in the presence of CEES and after treatment with curcumin and doxycycline.
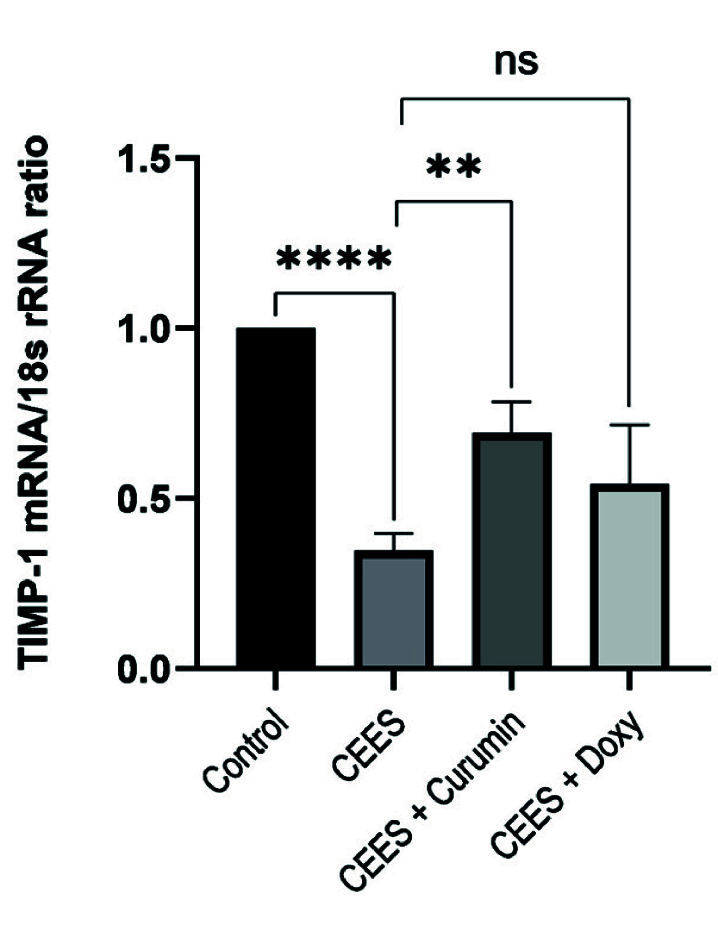
Figure 4.
The expression levels of TIMP-1 in the presence of CEES and after treatment with curcumin and doxycycline.
Figure 5.
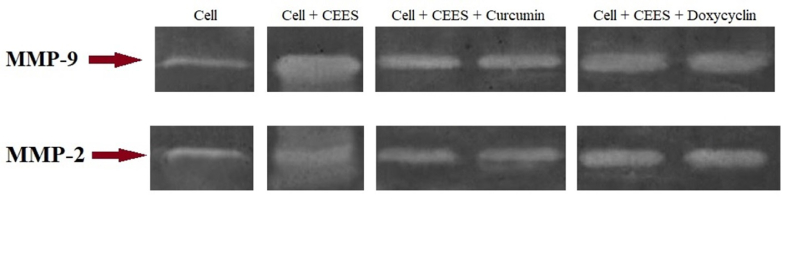
Activity of MMP-2 and MMP-9 in corneal epithelial cells in the presence of CEES and after treatment with curcumin and doxycycline, measured by zymography.
Figure 6.
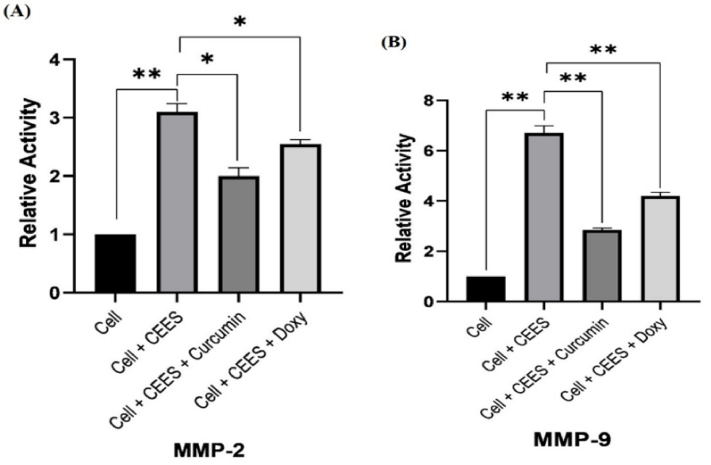
Relative activity of MMP-2 (A) and MMP-9 (B) in corneal epithelial cells in the presence of CEES and after treatment with curcumin and doxycycline, measured by zymography.
RESULTS
IC50 Calculation
To determine the appropriate concentration of doxycycline and curcumin for treating the cells, we evaluated cell viability and the IC50 dose by the MTT assay. Figure 1 depicts cell viability in different concentrations of CEES, doxycycline, and curcumin. The calculated IC50 values for doxycycline, curcumin, and CEES were 40.44 µg/ml, 12.08 µM, and 3.42 mM, respectively. To evaluate the therapeutic effects of curcumin and doxycycline on CEES-infected corneal cells, we first exposed the cells to the IC50 concentration of CEES (3.42 mM) and then treated them with 10 µg/ml and 3 µM (one-fourth of IC50) of doxycycline and curcumin, respectively.
Effective Doses of Doxycycline and Curcumin
To obtain the effective concentrations of curcumin and doxycycline for inhibiting CEES, we treated CEES-infected cells with different concentrations of these drugs. Next, the MTT test was performed to evaluate cell viability. (The results are shown in Figure 2.) The best concentrations of curcumin and doxycycline for CEES inhibition were 3.02 M and 10.11 g/ml, respectively.
Evaluation of MMP-2, MMP-9, and TIMP-1 gene expression
When cells are exposed to CEES, the amount of MMPs increases in the cell, leading to tissue destruction. On the other hand, treating these cells with curcumin or doxycycline will reduce the toxic effects of CEES. Therefore, it is helpful to examine the amounts of these enzymes (MMP-2 and MMP-9) and their inhibitor (TIMP-1). To this end, 24 hours after cell treatment, the expression levels of MMP-2, MMP-9, and TIMP-1 were assessed via qPCR. As seen in Figure 3A, the expression of MMP-2 in corneal epithelial cells increased approximately 5-fold after their infection with CEES. Conversely, the expression of MMP-2 declined 2.7-fold (P 0.001) and 2.6-fold (P 0.001) after treatment with curcumin and doxycycline, respectively. There was no significant difference between the effects of curcumin and doxycycline on reducing MMP-2 expression.
According to Figure 3B, MMP-9 showed a 3.3-fold higher expression in corneal epithelial cells after being infected with CEES, whereas the expression decreased 1.6-fold (P 0.05) and 2.5-fold (P 0.05) after treatment with curcumin and doxycycline, respectively. According to Figure 3B, the effect of doxycycline on reducing the activity of MMP-9 is greater than that of curcumin, but the difference is not statistically significant.
Also, the expression of TIMP-1 mRNA in the CEES group significantly decreased compared with the control group [Figure 4]. After treatment with curcumin, the expression of TIMP-1 was 2-fold higher (P 0.01) in corneal cells compared to the CEES group; however, no significant differences in the expression of TIMP-1 mRNA were observed between the doxycycline and CEES groups [Figure 4]. In other words, there was no significant difference between the effects of curcumin and doxycycline on increasing TIMP-1 expression.
Evaluation of MMP Activity (Zymography)
The activity of MMP-2 and MMP-9 in corneal epithelial cells was determined by zymography. Dried gels were photographed, and the intensity and area of the band were measured using the Image Station 4000MM (Kodak Digital Science, United States). As shown in Figure 5, CEES heightened the activity of MMP-2 and MMP-9. On the other hand, MMP-2 and MMP-9 activity decreased in the presence of 3.02 M of curcumin and 10.11 g/ml doxycycline. According to Figure 6, CEES increases the activity of MMP-2 and MMP-9 by about 3.1 and 6.9 fold, respectively. Treatment with curcumin reduced the activity of MMP-2 and MMP-9 by about 32% (P 0.05) and 56% (P 0.01), respectively, compared to the control group. Also, the activity of MMP-2 and MMP-9 decreased with doxycycline by about 12% (P 0.05) and 40% (P 0.01), respectively.
DISCUSSION
The eye, the most sensitive organ of the body, is often exposed to different injuries. SM causes a wide range of adverse effects when it comes into contact with the eye, highlighting the urgent need for treatment.[21] CEES has a chemical structure similar to SM and it can similarly damage the eye. For this reason, it was considered in the present research.[1]
Cost-effectiveness and ethical issues are two factors that reduce the efficiency of animal models in the early stages of pharmaceutical research; therefore, we used a cell culture model for this study. In this investigation, an in vitro model was successfully developed for drug screening. The HCE-2 culture system allowed us to economically test several concentrations of curcumin and doxycycline on the infected corneal cell and, thus, assess their ability to inhibit the adverse effects of CEES. Immortalized HCE-2 from healthy cornea were infected by CEES and then treated with curcumin and doxycycline. To determine the best concentration of curcumin and doxycycline for CEES inhibition, we treated CEES-infected cells with three concentrations of these compounds and selected the optimal concentration according to the results [Figure 2].
SM-induced ocular damage involves processes such as inflammation, neovascularization of corneas, and ECM remodeling, in which the ECM is mainly regenerated by MMPs.[22,23,24] Gelatinases (MMPs), mostly MMP-2 and MMP-9, play a key role in the destruction of basal membrane components that impair epidermal connectivity in the eye and skin.[1,25,26,27] Gordon et al showed that when ocular damage is caused by mustard toxin, MMP-2 and MMP-9 would play an important role in the separation of epithelial–stromal junctions in the ocular tissues.[21] Indeed, during the development of acute injury, the activity of corneal MMP-2 and MMP-9 increases in tandem with the clinical manifestation of inflammation; but under normal circumstances, these MMPs are involved in the short-term, strongly regulated ECM remodeling.[6,13,28] Also, TIMP-1 can inhibit the activity of MMPs.[29] Therefore, we also examined the expression of this enzyme in this study.
According to the results of previous studies as well as the current study, it can be argued that inflammation-signaling pathways play a major role in SM-induced ocular damage.[27] On the other hand, due to the involvement of MMP-2 and MMP-9 in the inflammatory process, we evaluated the impact of curcumin and doxycycline in treating ocular injuries.[6] In this investigation, we evaluated the effect of curcumin and doxycycline on the expression (qPCR) and activity (zymography) of TIMP-1, MMP-2, and MMP-9 in HCE-2 cells. According to the results, infection of cells with CEES heightens the expression and activity of MMP-2 and MMP-9. The results of qPCR and zymography revealed that curcumin and doxycycline significantly reduced MMP expression and activity compared to the control group.
There was no significant difference between the effects of curcumin and doxycycline on reducing MMP-2 expression, but the difference was significant in the case of MMP-9. According to the results, curcumin has a greater ability than doxycycline to inhibit MMP-2 and MMP-9 enzymes, yet the difference is statistically significant only in the case of MMP-9.
Marion et al tested four tetracyclines as a treatment against vesicants. Their results demonstrated that all tetracycline derivatives, including doxycycline, could inhibit MMP-9 expression. Therefore, doxycycline may be effective against SM exposure due to its anti-MMP activity.[21] In another study by Anumolu et al, an increase in MMP-9 activity and severity of the injury was observed in rabbit corneas exposed to SM analogs. The authors reported that topical doxycycline treatment diminished MMP-9 activity.[6] Horowitz et al also evaluated the effect of doxycycline (2 mg/ml) on SM injury in rabbit eyes. They collected corneal and tear samples at different times and measured MMP activity by zymography. The authors found that long-term treatment with doxycycline reduces the activity of MMP-9 in the tear fluid, leading to decreased severity of corneal damage during the acute phase.[6]
Banerji et al reported that 15 mM curcumin has a significant inhibitory effect on MMP-2 activity in metastatic murine melanoma cells B16F10. Interestingly, this inhibitory effect persisted for 28 days after curcumin removal.[30] In another study, Shao et al reported that curcumin has a strong anti-invasive effect on human breast carcinoma cells. Their result showed that this anti-invasive effect is related to downregulation of MMP-2 and upregulation of TIMP-1.[31] Also, Lin et al showed that curcumin reduced the levels of MMP-2 and MMP-9 in adenocarcinoma human epithelial cells (A549 cell line).
Chao et al explored the effects of curcumin on the secretion of interleukin 6 (IL-6) and interleukin 8 (IL-8) by human corneal limbal epithelial cells. The cells were irradiated by UVB at different dosages with or without curcumin. Next, the levels of IL-6 and IL-8 in the cells were measured by ELISA analysis. According to the results, curcumin (5-20 µmol/L) significantly inhibited UVB-induced secretion of IL-6 and IL-8 by limbal epithelial cells in a dose-dependent manner. Consequently, the authors suggested, curcumin may be a promising agent to be explored for the prevention and treatment of pterygium.[32]
In another study, Chen et al confirmed the anti-inflammatory effects of curcumin in dry eye disease. Specifically, the increase in osmolarity caused by the addition of sodium chloride to the culture medium of corneal epithelial cells enhances the production of IL-1 (a pro-inflammatory cytokine). The authors investigated the anti-inflammatory effects of curcumin in this cell model, and they observed that curcumin pretreatment (5 M) eliminated the increase in IL-1 production induced by the hyperosmotic medium. Accordingly, it appears that curcumin may have a therapeutic potential for dry eye disease.[33]
One of the most important factors that contribute to corneal hemostasis is the barrier function of the corneal epithelium, which is disturbed by inflammation. Whereas tight junctions and adherents junctions of the corneal epithelium are required for the barrier function and cell adhesion, some inflammatory cytokines such as IL-1 and TNF can disrupt the function of this barrier. Kimura et al, in two separate studies, investigated the therapeutic effect of curcumin on these two cytokines. They found that curcumin blocked the effects of IL-1 and TNF- on both TER and the subcellular localization of ZO-1. Finally, the authors proposed, curcumin can be used as an anti-inflammatory agent for eye diseases.[34,35]
In summary, the results of the present study showed that curcumin could reduce the expression and activity of MMP-2 and MMP-9 after their induction by CEES. The observed effectiveness of curcumin in inhibiting MMPs compared to doxycycline confirms its positive role as a natural substance in treating CEES-induced ocular inflammation. Further investigation is needed to confirm the safety and anti-inflammatory activity of curcumin under in vivo conditions.
Financial Support and Sponsorship
This work was supported by the Baqiyatallah University of Medical Sciences (Grant number: 97000503).
Conflicts of Interest
None.
References
- Jain AK, Tewari-Singh N, Gu M, Inturi S, White CW, Agarwal R. Sulfur mustard analog, 2-chloroethyl ethyl sulfide-induced skin injury involves DNA damage and induction of inflammatory mediators, in part via oxidative stress, in SKH-1 hairless mouse skin. Toxicol Lett. 2011;205:293–301. doi: 10.1016/j.toxlet.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt P, Lyman M, Swartz A, Tuznik K, Kniffin D, Whitten K, et al. Architectural and biochemical expressions of mustard gas keratopathy: Preclinical indicators and pathogenic mechanisms. PLoS One. 2012;7:e42837. doi: 10.1371/journal.pone.0042837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panahi Y, Naderi M, Zare MA, Poursaleh Z. Ocular effects of sulfur mustard. J Curr Ophthalmol. 2013;25:90. [Google Scholar]
- Pfister RR. Chemical injuries of the eye. Ophthalmology. 1983;90:1246–1253. [PubMed] [Google Scholar]
- Rastegar-Pouyani N, Sonam Dongsar T, Ataei M, Hassani S, Gumpricht E, Kesharwani P, et al. [DOI] [PubMed] [Google Scholar]
- Horwitz V, Dachir S, Cohen M, Gutman H, Cohen L, Fishbine E, et al. The beneficial effects of doxycycline, an inhibitor of matrix metalloproteinases, on sulfur mustard-induced ocular pathologies depend on the injury stage. Curr Eye Res. 2014;39:803–812. doi: 10.3109/02713683.2013.874443. [DOI] [PubMed] [Google Scholar]
- Ghiasi M, Jadidi K, Hashemi M, Zare H, Salimi A, Aghamollaei H. Application of mesenchymal stem cells in corneal regeneration. Tissue Cell. 2021;73:101600. doi: 10.1016/j.tice.2021.101600. [DOI] [PubMed] [Google Scholar]
- Shakibaie M, Tabandeh F, Shariati P, Norouzy A. Synthesis of a thin-layer gelatin nanofiber mat for cultivating retinal cell. J Bioact Compat Polym. 2018;33:371–381. [Google Scholar]
- Moir LM, Ng HY, Poniris MH, Santa T, Burgess JK, Oliver BG, et al. Doxycycline inhibits matrix metalloproteinase-2 secretion from TSC2-null mouse embryonic fibroblasts and lymphangioleiomyomatosis cells. Br J Pharmacol. 2011;164:83–92. doi: 10.1111/j.1476-5381.2011.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M, Manseau E, Law M, Aiken D. Ulceration is correlated with degradation of fibrin and fibronectin at the corneal surface. Invest Ophthalmol Vis Sci. 1983;24:1358–1366. [PubMed] [Google Scholar]
- Chung JH, Kang YG, Kim HJ. Effect of 0. 1% dexamethasone on epithelial healing in experimental corneal alkali wounds: Morphological changes during the repair process Graefes Arch Clin Exp Ophthalmol. 1998;236:537–545. doi: 10.1007/s004170050118. [DOI] [PubMed] [Google Scholar]
- De Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li DQ, Stern ME, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Yodkeeree S, Chaiwangyen W, Garbisa S, Limtrakul P. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA. J Nutr Biochem. 2009;20:87–95. doi: 10.1016/j.jnutbio.2007.12.003. [DOI] [PubMed] [Google Scholar]
- De Paiva CS, Corrales RM, Villarreal AL, Farley W, Li DQ, Stern ME, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47:2847–2856. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- Li DQ, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001;73:449–459. doi: 10.1006/exer.2001.1054. [DOI] [PubMed] [Google Scholar]
- Ge HY, Xiao N, Yin XL, Fu SB, Ge JY, Shi Y, et al. Comparison of the antiangiogenic activity of modified RGDRGD-endostatin to endostatin delivered by gene transfer in vivo rabbit neovascularization model. Mol Vis. 2011;17:1918–1928. [PMC free article] [PubMed] [Google Scholar]
- Turgut B, Guler M, Akpolat N, Demır T, Celıker U. The impact of tacrolimus on vascular endothelial growth factor in experimental corneal neovascularization. Curr Eye Res. 2011;36:34–40. doi: 10.3109/02713683.2010.516620. [DOI] [PubMed] [Google Scholar]
- Hosseindokht M, Zare H, Salehi R, Pourgholi L, Ziaee S, Boroumand M, et al. Association between polymorphisms in microRNA seed region and warfarin stable dose. Postgrad Med J. 2020;96:737–741. doi: 10.1136/postgradmedj-2019-137197. [DOI] [PubMed] [Google Scholar]
- Tahmasebi T, Nosrati R, Zare H, Saderi H, Moradi R, Owlia P. Isolation of indigenous Glutathione producing Saccharomyces cerevisiae strains. Iran J Pathol. 2016;11:354–362. [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD. Real-time quantitative PCR. Methods. 2001;25:383–385. doi: 10.1006/meth.2001.1260. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Desantis A, Deshmukh M, Lacey CJ, Hahn RA, Beloni J, et al. Doxycycline hydrogels as a potential therapy for ocular vesicant injury. J Ocul Pharmacol Ther. 2010;26:407–419. doi: 10.1089/jop.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi MA, Yazdani S, Sajjadi H, Jadidi K, Karimian F, Einollahi B, et al. Chronic and delayed-onset mustard gas keratitis: Report of 48 patients and review of literature. Ophthalmology. 2005;112:617–625. doi: 10.1016/j.ophtha.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Kadar T, Dachir S, Cohen L, Sahar R, Fishbine E, Cohen M, et al. Ocular injuries following sulfur mustard exposure—Pathological mechanism and potential therapy. Toxicology. 2009;263:59–69. doi: 10.1016/j.tox.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Shohrati M, Peyman M, Peyman A, Davoudi M, Ghanei M. Cutaneous and ocular late complications of sulfur mustard in Iranian veterans. Cutan Ocul Toxicol. 2007;26:73–81. doi: 10.1080/15569520701212399. [DOI] [PubMed] [Google Scholar]
- Kehe K, Balszuweit F, Steinritz D, Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, et al. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicol Sci. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Inturi S, Ammar DA, Agarwal C, Tyagi P, et al. Silibinin, dexamethasone, and doxycycline as potential therapeutic agents for treating vesicant-inflicted ocular injuries. Toxicol Appl Pharmacol. 2012;264:23–31. doi: 10.1016/j.taap.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith VA, Cook SD. Doxycycline-a role in ocular surface repair. Br J Ophthalmol. 2004;88:619–625. doi: 10.1136/bjo.2003.025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara O, Takimoto K, Morikawa T, Harada F, Takai R, Adhikari BR, et al. Upregulated expression of MMP-9 in gingival epithelial cells induced by prolonged stimulation with arecoline. Oncol Lett. 2017;14:1186–1192. doi: 10.3892/ol.2017.6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji A, Chakrabarti J, Mitra A, Chatterjee A. Effect of curcumin on gelatinase A (MMP-2) activity in B16F10 melanoma cells. Cancer Lett. 2004;211:235–242. doi: 10.1016/j.canlet.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Shao ZM, Shen ZZ, Liu CH, Sartippour MR, Go VL, Heber D, et al. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int J Cancer. 2002;98:234–240. doi: 10.1002/ijc.10183. [DOI] [PubMed] [Google Scholar]
- Chao SC, Hu DN, Roberts J, Shen X, Lee CY, Nien CW, et al. Inhibition effect of curcumin on UVB-induced secretion of pro-inflammatory cytokines from corneal limbus epithelial cells. Int J Ophthalmol. 2017;10:827–833. doi: 10.18240/ijo.2017.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Hu DN, Pan Z, Lu CW, Xue CY, Aass I. Curcumin protects against hyperosmoticity-induced IL-1β elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res. 2010;90:437–443. doi: 10.1016/j.exer.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Kimura K, Teranishi S, Fukuda K, Kawamoto K, Nishida T. Delayed disruption of barrier function in cultured human corneal epithelial cells induced by tumor necrosis factor-α in a manner dependent on NF-kappaB. Invest Ophthalmol Vis Sci. 2008;49:565–571. doi: 10.1167/iovs.07-0419. [DOI] [PubMed] [Google Scholar]
- Kimura K, Teranishi S, Nishida T. Interleukin-1β-induced disruption of barrier function in cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:597–603. doi: 10.1167/iovs.08-2606. [DOI] [PubMed] [Google Scholar]