Abstract
Parkinson’s disease (PD) seriously threatens human’s health. Researches have shown a close correlation between long non-coding RNAs (lncRNAs) and PD. However, the biological function of lncRNA homeobox transcript antisense RNA (HOTAIR) in PD remains largely unknown. In this study, we established PD models in vivo and in vitro by using 1-methyl-4-phenyl-2, 3, 6-tetrahydropyridine (MPTP) and 1-methyl-4-phenylpyridinium (MPP+) to assess the role of HOTAIR in pyroptotic cell death and neuronal damage. RNA immunoprecipitation (RIP) and dual luciferase reporter assay were used to verify the interaction between miR-326 and HOTAIR or ELAV like RNA binding protein 1 (ELAVL1). LncRNA HOTAIR was upregulated in PD mice and MPP+ induced SH-SY5Y cells. Additionally, knockdown of HOTAIR notably attenuated the symptom of PD in vivo. Downregulation of HOTAIR could obviously promoted cell viability and suppressed NLR family pyrin domain containing 3 (NLRP3) mediated pyroptotic cell death of SH-SY5Y cells in the presence of MPP+. Further, lncRNA HOTAIR positively regulated ELAVL1 expression by targeting miR-326, and downregulation of HOTAIR or ELAVL1 notably suppressed promotive effects of miR-326 inhibitor on MPP+ induced pyroptosis via activation of NLRP3 inflammasome. Collectively, HOTAIR silencing significantly inhibits neuronal damage through repressing NLRP3 mediated pyroptosis activation via regulation of miR-326/ELAVL1 axis in PD, which may contribute to a better understanding of PD pathogenesis and provide new treatment strategies for this disease.
Keywords: Parkinson’s disease, NLRP3, Pyroptosis, LncRNA HOTAIR, miR-326, ELAVL1
Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder all over the world, covering more than 1.5% of the population over the age of 60 (Massaquoi et al. 2020). The pathological marker of PD is a profound loss of nigrostriatal dopaminergic neurons that is preceded by the accumulation and spread of characteristic lewy body and neurite inclusions, consisting primarily of misfolded fibrillary α-synuclein (Saewanee et al. 2019). Despite the occurrence of symptomatic treatments, no effective therapies can slow or halt the development of PD (Fasciani et al. 2020). Nowadays, the treatment of PD is mainly to relieve pain (Hijazi et al. 2020), which is certainly not enough. Thus, it is urgent to make research efforts on the interpretation of pathogenic molecular mechanisms and find new strategies for treatment of PD.
Long non-coding RNAs (lncRNAs) are a group of non-coding RNA transcripts with the length of more than 200 nucleotides, which help regulate gene expression at transcriptional or post-transcriptional level (Huang et al. 2020). LncRNAs have been documented to be the key mediators notably involved in the progression of multiple cancers, including gastric adenocarcinoma and cervical carcinoma (Fu et al. 2020; Ling et al. 2020). Recently, researches have shown a close correlation between lncRNAs and PD (Gwinn et al. 2017). For instance, upregulation of lncRNA brain-derived neurotrophic factor antisense (BDNF-AS) could promote the symptom of PD via inhibition of miR-125b-5p expression (Fan et al. 2020). Long intergenic non-protein-coding RNA p53-induced transcript (LINC-PINT) was one of the major transcripts of neurons and deficiency of LINC-PINT aggravated the death of neuron cells under the condition of oxidative stress, highlighting its neuroprotective effects on neurodegenerative diseases (Simchovitz et al. 2020). Meanwhile, lncRNA homeobox transcript antisense RNA (HOTAIR) has been confirmed to be upregulated in the midbrain of PD mice and 1-methyl-4-phenylpyridinium (MPP+) treated neural cells, HOTAIR knockdown showed protective effects on tyrosine hydroxylase (TH) expression and cell viability (Liu et al. 2016). However, the mechanism by which HOTAIR regulates the occurrence of PD remains to be further explored.
As competitive endogenous RNAs (ceRNAs), lncRNAs could combine with miRNAs and play roles of miRNA sponge. Similarly, miRNAs have been confirmed to play key roles in many processes of diseases, including PD (Shu and Zhang 2017; Xue et al. 2019; Yu et al. 2020). Meanwhile, miR-326 has been considered as a key suppressor in the progression of various malignant tumors (Ghodousi et al. 2020; Tang et al. 2020). Besides, the biological function of miR-326 in PD has been authenticated to suppress inducible nitric oxide synthase (iNOS) expression and promote autophagy of dopaminergic neurons (Zhao et al. 2019a, b). On the other hand, it has been reported that activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome and pyroptosis could contribute to the occurrence of PD (Zeng et al. 2019). Moreover, ELAV like RNA binding protein 1 (ELAVL1) is a protein which can function as a modulator, mediating various proteins expressions through regulation of diverse mechanisms (Wang et al. 2020). In addition, ELAVL1 could promote the activation of NLRP3-mediated pyroptosis in multiple diseases (Jeyabal et al. 2016; Li et al. 2017). Since the effect of ELAVL1 on PD remains unclear, we sought to investigate the relation of miR-326 and ELAVL1 in PD progression in this research.
In the present study, we aimed to investigate the effects of HOTAIR on PD and explore its underlying mechanism. We found that lncRNA HOTAIR promotes the neuronal damage by facilitating NLRP3 mediated-pyroptosis activation in PD via regulation of miR-326/ELAVL1 axis. This finding provided us an evidence that HOTAIR/miR-326/ELAVL1 regulatory network may serve as a predictive biomarker in PD treatment.
Material and Methods
In Vivo Study
Male C57BL/6 mice (8 weeks old, 20–22 g) were purchased from Chinese Academy of Sciences (Shanghai, China). Mice were kept in a specific pathogen free (SPF) condition with drinking and food. This study was approved by the Medical Ethics Committee of Guizhou Provincial People’s Hospital (2015013). The animal experiments were strictly complied with the principle to minimize the pain and suffering to experimental animals. For PD model, mice were injected with 1-methyl-4-phenyl-2, 3, 6-tetrahydropyridine (MPTP, 20 mg/kg) in saline for 3 consecutive weeks as previously described (Liu and Lu 2018). 0.9% saline was injected into mice as the sham. For treatment of HOTAIR in PD mice, mice were randomly divided into 5 groups (6 mice/per group): control group (injected with nothing), sham group (injected with 0.9% sterile saline), MPTP group, MPTP + Adenovirus shRNA targeting HOTAIR (Ad-shHOTAIR) group and the negative control (Ad-shNC) group. Ad-shHOTAIR and Ad-shNC were obtained from Genepharma (Shanghai, China). Two days after vector injection, administration of MPTP or sterile saline was performed as above. Lastly, the ventral midbrain containing substantia nigra (SN) were collected and stored at − 80 °C for the following experiments.
Animal Behavior Test
For Rotation test, mice were placed in a spinning rod and the duration time of mice on the rod was recorded. The diameter of the roller is 6 cm. After adaption training with the speed of 20 r/min for 5 times, the speed was changed to 30 r/min according to the previous reference (Zhao et al. 2019a, b). The degree of bradykinesia was detected by Pole test. After MPTP pretreatment, mouse were placed at the top of the pole (8 mm in diameter, 50 cm in height), and the time from the mouse begin to move to the mouse put its head down (T-turn time) was noted. The locomotion activity time was that the mouse begin to move until the mouse moved to the bottom of the pole.
Hematoxylin–Eosin (H&E), Nissl and Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick-End Labeling (TUNEL) Staining
After treatment, the mice were euthanized and the brain tissues were collected and fixed with paraformaldehyde. Tissues were hydrated with 70%, 80% and 95% gradient alcohol for 12 h each, and n-butyl alcohol for 6 h. For H&E staining, paraffin sections containing SN were routinely washed with distilled water, stained with Mayer hematoxylin at room temperature for 5 min, flushed under tap water for l min. Next, the sections were re-stained with 1% eosin solution for l min. Histopathological changes were assessed by an optical microscope. For Nissl staining, samples were stained in 0.5% coke violet solution (pH 3.9) for 30 min, and rinsed with distilled water for 3 min according to a previous reference (Cai et al. 2019; Shiffman et al. 2018), the positive rate was calculated under a microscope. For TUNEL staining, paraffin sections were washed, permeabilized, and then incubated with 50 μL TUNEL reaction mixtures in a wet box for 60 min at 37 °C in the dark. For signal conversion, slides were incubated with 50 μL of peroxidase for 30 min at 37 °C, rinsed with phosphate buffer saline (PBS), and then incubated with 50 μL diaminobenzidine substrate solution for 10 min at 25 °C. Finally, the staining was observed under an optical microscope.
Immunohistochemical (IHC) Assay
For IHC staining, SN slices were incubated with the primary antibody to TH (Abcam; ab112, 1:1000) overnight and washed for several times. Then, the secondary antibody horseradish peroxidase-labeled IgG (Abcam; ab205718, 1:2000) was supplemented for 2 h. Lastly, samples were developed with diaminobenzidine, followed by hematoxylin counterstaining, dehydration, clearance, neutral balsam sealing. TH positive cells were observed and counted under a light microscope.
Detection of Dopamine (DA)
The mouse tissues were separated and preserved on an ice box. Next, the tissues were added with cold 0.1 mol/L perchloric acid and 100 ng/mL 2, 6-dihydroxy-benzoic acid. The supernatant was centrifuged (15 min, 4 °C, 13,000 r/min). The content of DA was measured using high performance liquid chromatography (HPLC) detection.
Cell Culture
SH-SY5Y cell line was obtained from Cell bank of Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fischer Scientific, Waltham, MA, USA) with 10% fetal bovine serum (FBS; Thermo Fischer Scientific), 1% penicillin (Thermo Fischer Scientific) and streptomycin (Thermo Fischer Scientific) at 37 °C, 5% CO2. To establish in vitro model of PD, SH-SY5Y cells were treated with MPP+ for 24 h.
Cell Transfection
shRNA against HOTAIR (shHOTAIR), shRNA against ELAVL1 (shELAVL1), shRNA control (shNC), miR-326 mimics and control (mimics NC), miR-326 inhibitor and negative control (inhibitor NC) were purchased from GenePharma (Shanghai, China). SH-SY5Y cells transfection was conducted by using lipofectamine 3000 transfection reagent (Thermo Fisher Scientific) following the manufacture’s instruction.
Luciferase Reporter Assay
The wild-type (WT) and mutant (MUT) HOTAIR or ELAVL1 were synthesized and inserted into the pEZX-MT06 vector (Genecopoeia, Guangzhou, China), respectively. Cells were co-transfected with miR-326 mimics or the corresponding control and the reporter vector containing WT or MUT HOTAIR/ELAVL1. Cells were collected 48 h later and the luciferase activity was quantified using the dual luciferase reporter assay system (Promega, Fitchburg, WI, USA).
Northern Blot Detection
RNA extracted from tissues was subjected to northern blot analysis. A biotin-16-dUTP (Roche, Mannheim, Germany)-labeled HOTAIR complementary DNA probe was prepared using PCR. Northern blot assay was performed using the NorthernMax Kit (Ambion, Grand Island, NY, USA) according to the manufacturer’s protocols. Then, washed membranes were detected and β-actin served as loading controls.
Cell Counting Kit-8 (CCK-8) Assay
To test the effects of MPP+ on the growth of SH-SY5Y cells, CCK-8 assay was allowed to detect the cell viability. Briefly, SH-SY5Y cells were seeded into 96-well plate overnight (3.0 × 103 cells/well). After treatments, CCK-8 reagent was added into each well. After incubation for 4 h, 150 μL dimethyl sulfoxide (DMSO; Thermo Fischer Scientific) was added into each well for 10 min. The absorbance at 450 nm was tested by Thermo Fischer Multiskan FC (Thermo Fischer Scientific).
Quantitative Real-Time PCR (qPCR)
The total RNA from cells or tissues of mice was extracted by using the RNA extraction kit (TaKaRa, Tokyo, Japan) and cDNA was synthesized using the RNA PCR Kit (TaKaRa, Ver.3.0). Real-time PCR was performed as follows: pre-denaturation at 94 °C for 2 min, followed by 40 cycles: denaturation at 94 °C for 30 s, annealing at 55 °C for 45 s and extension at 72 °C for 45 s, and a final extension at 72 °C for 10 min. PCR were carried out using SYBR premix Ex Taq II kit (TaKaRa, Tokyo, Japan) on an ABI 7500 Real-Time PCR system (ABI, NY, USA). The primers were obtained from GeneCreate Biological Engineering Co., Ltd (Wuhan, China). Relative quantification of gene expression was performed by using the method. U6 or β-actin was used as an internal control. The sequences of primers were listed in Table 1.
Table 1.
Primer sequence for qPCR
| Gene | Primer sequence (Tm: annealing temperature) |
|---|---|
| HOTAIR (mouse) | Forward: 5′-GGGCTGCAGAATTCACTCTC-3′ (Tm: 58.62 °C) |
| Reverse: 5′-GACTTCCTTCCTTCCGCTCT-3′ (Tm: 59.10 °C) | |
| HOTAIR (human) | Forward: 5′-GCCTTTCCCTGCTACTTGTG-3′ (Tm: 58.83 °C) |
| Reverse: 5′-AGAGCTTCCAAAGGCTAGGG-3′ (Tm: 59.08 °C) | |
| IL-6 (mouse) | Forward: 5′-TGCAAGAGACTTCCATCCAG-3′(Tm: 57.22 °C) |
| Reverse: 5′-TCCACGATTTCCCAGAGAAC-3′ (Tm: 57.24 °C) | |
| IL-6 (human) | Forward: 5′-CCTTCCAAAGATGGCTGAAA-3′ (Tm: 55.91 °C) |
| Reverse: 5′-CAGGGGTGGTTATTGCATCT-3′ (Tm: 57.56 °C) | |
| IL-1β (mouse) | Forward: 5′-GCCACCTTTTGACAGTGATGAG-3′ (Tm: 59.77 °C) |
| Reverse: 5′-AAGGTCCACGGGAAAGACAC-3′ (Tm: 59.89 °C) | |
| IL-1β (human) | Forward: 5′-CGATGCACCTGTACGATCAC-3′ (Tm: 58.80 °C) |
| Reverse: 5′-TCTTTCAACACGCAGGACAG-3′ (Tm: 58.42 °C) | |
| NLRP3 (mouse) | Forward: 5′-CCATCAATGCTGCTTCGACA-3′ (Tm: 58.91 °C) |
| Reverse: 5′-GAGCTCAGAACCAATGCGAG-3′ (Tm: 58.99 °C) | |
| NLRP3 (human) | Forward: 5′-AAGGCCGACACCTTGATATG-3′ (Tm: 57.67 °C) |
| Reverse: 5′-CCGAATGTTACAGCCAGGAT-3′ (Tm: 57.67 °C) | |
| ASC (mouse) | Forward: 5′-CTCTGTACGGGAAGGTCCTG-3′ (Tm: 59.18 °C) |
| Reverse: 5′-TCCTCCACCAGGTAGGACTG-3′ (Tm: 59.96 °C) | |
| ASC (human) | Forward: 5′-AAGCCAGGCCTGCACTTTAT-3′ (Tm: 59.96 °C) |
| Reverse: 5′-CAGGCTGGTGTGAAACTGAA-3′ (Tm: 58.32 °C) | |
| Caspase1 (human) | Forward: 5′-CTCAGGCTCAGAAGGGAATG-3′ (Tm: 57.67 °C) |
| Reverse: 5′-CGCTGTACCCCAGATTTTGT-3′ (Tm: 58.18 °C) | |
| miR-326 (human) | F: 5′-CGCCTCTGGGCCCTTC-3′ (Tm: 59.35 °C) |
| RT: 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGAC CTGGAG-3′ | |
| U6 | Forward: 5′-CTCGCTTCGGCAGCACA-3′ (Tm: 60.42 °C) |
| Reverse: 5′-AACGCTTCACGAATTTGCGT-3′ (Tm: 59.69 °C) | |
| ELAVL1 (human) | Forward: 5′-CGCAGAGATTCAGGTTCTCC-3′ (Tm: 58.07 °C) |
| Reverse: 5′-CCAAACCCTTTGCACTTGTT-3′ (Tm: 57.31 °C) | |
| β-actin (mouse) | Forward: 5′-AGCCATGTACGTAGCCATCC-3′ (Tm: 59.61 °C) |
| Reverse: 5′-TCTCAGCTGTGGTGGTGAAG-3′ (Tm: 59.61 °C) | |
| β-actin (human) | Forward: 5′-CCCTGGAGAAGAGCTACGAG-3′ (Tm: 58.97 °C) |
| Reverse: 5′-CGTACAGGTCTTTGCGGATG-3′ (Tm: 59.00 °C) |
Western Blot Assay
Total proteins were isolated from cell lysates by using RIPA buffer, and their concentrations were quantified by the bicinchoninic acid (BCA) protein assay kit (Beyotime, Shanghai, China). Proteins were separated by using 10% sodium dodecyl sulfate polyacrylamide gel and transported to polyvinylidene fluoride (PVDF) membranes (Thermo Fisher Scientific, Waltham, MA, USA). Then, membranes were blocked with 5% skim milk in tris-buffered saline-tween 20 (TBST) at room temperature for 1 h followed by incubation with primary antibodies: anti-interleukin (IL)-6 (ab6672, 1:1000), anti-NLRP3 (ab263899, 1:1000), anti-apoptosis-associated speck-like protein containing a CARD (ASC) (ab151700, 1:1000) anti-cysteinyl aspartate specific proteinase 1 (Caspase1) (ab207802, 1:1000), anti-α-synuclein (ab138501, 1:5000), anti-leucine rich repeat kinase (LRRK2) (ab133474, 1:10,000), anti-TH (ab112, 1:1000), anti-IL-1β (ab2105, 1:1000), anti-ELAVL1 (ab200342, 1:1000) and anti-β-actin (ab8226, 1:1000) overnight at 4 °C. All the antibodies were obtained from Abcam (Cambridge, MA, USA). After that, the PVDF membranes were incubated with secondary anti-rabbit antibody (ab205718, 1:5000) for 1 h at room temperature. Finally, membranes were visualized with the enhanced chemiluminescent detection system (GE Healthcare, Piscataway, NJ, USA). The densities of blots were normalized to β-actin.
Immunofluorescence Staining Assay
SH-SY5Y cells were cultured on glass coverslips until 80% confluent and then fixed with 4% paraformaldehyde for 30 min. Next, cells were blocked with 10% FBS for 30 min at room temperature and then incubated with anti-Caspase1 antibody (1:50; 22915-1-AP, proteintech, Wuhan, China) at 4 °C overnight, followed by incubation with goat anti-rabbit IgG (Abcam; ab150077, 1:200) at 37 °C for 1 h. Then, the nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Beyotime, Shanghai, China) for 5 min. Finally, the cells were observed under a fluorescence microscope.
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of IL-β in supernatants of SH-SY5Y cells were detected by using ELISA kits according to the manufacturer’s instructions. IL-β ELISA kits were purchased from NeoBioscience (Guangzhou, China).
RNA Binding Protein Immunoprecipitation (RIP) Assay
The RIP assay was performed applying the Magna RIP RNA Binding Protein Immunoprecipitation Kit (Millipore, Bedford, MA, USA) following the manufacturer’s protocol. Briefly, cells at 80–90% confluency were collected and lysed using RIP lysis buffer. One hundred microliters of cell extract was then incubated with RIP buffer containing magnetic beads conjugated to human anti-Ago2 antibody or negative control normal mouse IgG. The samples were incubated with Proteinase K to digest the protein and then the immunoprecipitated RNA was isolated. Then the relative enrichment of HOTAIR was measured via qPCR analysis.
Determination of Levels of Lactate Dehydrogenase (LDH)
Commercial LDH determination kits were obtained from Sangon Biotech (Shanghai, China). Transfected cells were harvested for detecting the levels based on the manufacturer’s instructions.
Hoechst 33342/Propidium Iodide (PI) Staining
Cells (5.0 × 105 cells/well) were seeded into 6-well plates followed by transfection or treatment with MPP+. Then, Hoechst 33342 solution (10 μL) was used to stain cells for 10 min at 37 °C in the dark. After that, cells were stained with PI (5 μL) for 10 min in the dark at 37 °C. The stained cells were observed under a fluorescence microscope.
Statistical Analysis
All data were presented as the mean ± standard deviation (SD), using GraphPad Prism 7 (GraphPad Software, Inc., LA Jolla, CA, USA) for data analysis via one‐way analysis of variance (ANOVA). p < 0.05 was considered statistically significant. All experiments were repeated three times.
Results
LncRNA HOTAIR was Upregulated in PD Mice
First, to investigate whether lncRNA HOTAIR is involved in PD, in vivo model of PD was established. As indicated in Fig. 1a (**p < 0.01), b (**p < 0.01) and c (*p < 0.05), the mice exhibited slower action after MPTP treatment, compared with Control and Sham groups. In addition, the results of qPCR (***p < 0.001; Fig. 1d) and Northern blot (**p < 0.01; Fig. 1e) revealed that lncRNA HOTAIR was upregulated in PD mice. Similarly, MPTP significantly increased the expression levels of IL-6, IL-1β, NLRP3 and ASC, suggesting the occurrence of PD may be related to the activation of NLRP3 inflammasome (**p < 0.01, ***p < 0.001; Fig. 1f). Taken together, HOTAIR might play an important role in the pathologic process of PD.
Fig. 1.
LncRNA HOTAIR was upregulated in PD mice. In vivo model of PD was established. Then, a T-tum time, b time for locomotion activity and c retention time of mice were measured. d, e The relative expression of HOTAIR in tissues of mice was measured by qPCR and Northern blot. The relative expression of HOTAIR was quantified normalizing to β-actin. f The relative expressions of IL-6, IL-1β, ASC and NLRP3 in brain tissues of mice were detected by qPCR. Data are analyzed using one-way ANOVA and presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
Silencing of HOTAIR Greatly Relieved the Neuronal Damage in PD Mice
In order to detect the effects of HOTAIR on neuronal injury, recombinant adenovirus vectors with shHOTAIR or shNC was constructed. As indicated in Fig. 2a, MPTP-induced increase of HOTAIR expression was notably reversed by knockdown of HOTAIR (**p < 0.01, ***p < 0.001). Additionally, the results of H&E and Nissl staining revealed that silencing of HOTAIR significantly attenuated the decrease of nigral cells, Nissl positive neuron number and neuron volume induced by MPTP (*p < 0.05, **p < 0.01; Fig. 2b). Consistently, HOTAIR silencing partially rescued the inhibitory effects of MPTP on DA content (**p < 0.01; Fig. 2c), α-synuclein (Fig. 2d), LRRK2 (Fig. 2d) and TH expression (**p < 0.01, ***p < 0.001; Fig. 2e). Besides, in TUNEL staining, HOTAIR downregulation obviously suppressed MPTP-induced cell apoptosis (***p < 0.001; Fig. 2f). Moreover, the effects of MPTP on IL-6, IL-1β, NLRP3 and ASC expression were partially reversed by shHOTAIR (*p < 0.05, **p < 0.01, ***p < 0.001; Fig. 2g). Altogether, suppression of HOTAIR greatly relieved the neuronal damage in PD mice model.
Fig. 2.
Silencing of HOTAIR greatly relieved the neuronal damage in PD mice. a The expression of HOTAIR in tissues was measured by qPCR. b The brain tissues containing SN were collected from mice. Then, H&E and Nissl staining were performed. c The content of DA was measured by HPLC assay. d The protein expressions of α-synuclein and LRRK2 were detected by western blot. The relative protein expressions were quantified by normalizing to β-actin. e The expression of TH in tissues of mice was measured by IHC. f TUNEL staining was allowed to detect the apoptotic cells. g The relative expressions of NLRP3, IL-6, IL-1β and ASC in tissues of mice were detected by qPCR. Data are analyzed using one-way ANOVA and presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
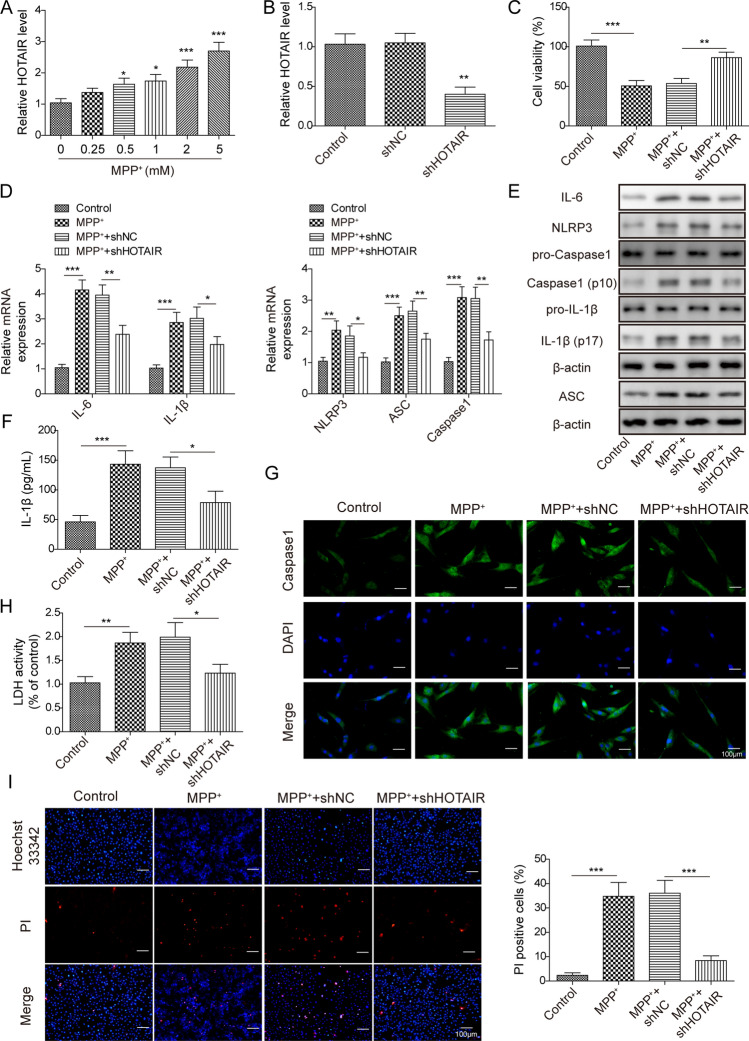
Knockdown of HOTAIR Suppressed the Pyroptosis of SH-SY5Y Cells
To mimic in vitro model of PD, SH-SY5Y cells were treated with MPP+. As showed in Fig. 3a, MPP+ increased the expression of HOTAIR in a dose-dependent manner (*p < 0.05, ***p < 0.001). Moreover, shHOTAIR was stably transfected into SH-SY5Y cells (**p < 0.01; Fig. 3b). In addition, the cell viability was significantly suppressed by MPP+, which was notably rescued in the presence of HOTAIR silencing (**p < 0.01, ***p < 0.001; Fig. 3c). Meanwhile, the expressions of pyroptosis related molecules (IL-6, IL-1β, Caspase1, NLRP3 and ASC) were upregulated by MPP+, while the effects of MPP+ on these agents were obviously attenuated by knockdown of HOTAIR (*p < 0.05, **p < 0.01, ***p < 0.001; Fig. 3d, e). In ELISA assay, silencing of HOTAIR partially inhibited the levels of IL-1β in supernatants of MPP+-treated SH-SY5Y cells (*p < 0.05, ***p < 0.001; Fig. 3f). Besides, the data of Caspase1 staining (Fig. 3g), LDH (*p < 0.05, **p < 0.01; Fig. 3h) and Hoechst 33,342/PI staining (***p < 0.001; Fig. 3i) further verified that silencing of HOTAIR could remarkably reversed the effect of MPP+ on pyroptosis of SH-SY5Y cells. Collectively, knockdown of HOTAIR suppressed NLRP3-mediated pyroptosis of MPP+-induced SH-SY5Y cells.
Fig. 3.
Knockdown of HOTAIR suppressed the pyroptosis of SH-SY5Y cells. SH-SY5Y cells were treated by different concentrations of MPP+. Then, a the expression of HOTAIR and b the transfection efficiency in SH-SY5Y cells were verified by qPCR. c The cell viability was measured by CCK-8 assay. d The expression of NLRP3, IL-6, IL-1β, Caspase1 and ASC in SH-SY5Y cells was detected by qPCR. e The protein expression of IL-1β, IL-6, ASC, NLRP3, pro-Caspase1, Caspase1 and pro-IL-1β in SH-SY5Y cells was detected by western blot. The relative expression was quantified normalizing to β-actin. f ELISA assay was performed to assess the level of IL-1β in supernatants of SH-SY5Y cells. g The expression of Caspase1 was tested by immunofluorescence staining. h, i Cell pyroptosis was measured by LDH detection and Hoechst 33,342/PI staining. Data are analyzed using one-way ANOVA and presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
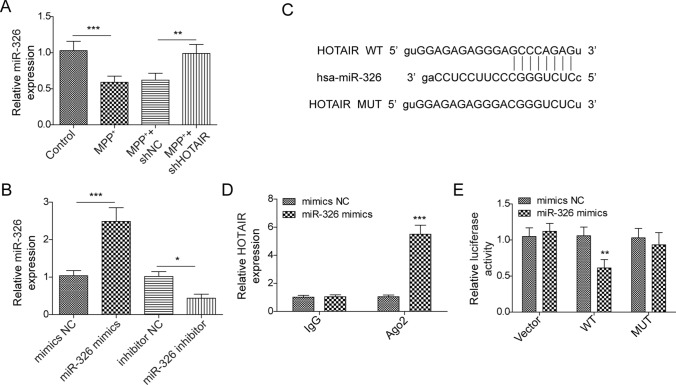
LncRNA HOTAIR Could Regulate the Expression of miR-326
The results indicated that the expression of miR-326 was significantly inhibited by MPP+, which was partly rescued by downregulation of HOTAIR (**p < 0.01, ***p < 0.001; Fig. 4a). Furthermore, miR-326 was upregulated by miR-326 mimics but downregulated by miR-326 silencing (*p < 0.05, ***p < 0.001; Fig. 4b). Meanwhile, the result of starBase revealed a potential binding site between HOTAIR and miR-326 (Fig. 4c), and the interaction of HOTAIR with miR-326 was further validated by RIP assay (***p < 0.001; Fig. 4d). Additionally, the result of dual luciferase reporter assay showed the luciferase activity of HOTAIR‐WT reporter was inhibited by miR‐326 mimics in SH-SY5Y cells, which further confirmed this finding (**p < 0.01; Fig. 4e). In summary, lncRNA HOTAIR could directly interact with miR-326 and negatively regulated its expression.
Fig. 4.
LncRNA HOTAIR could regulate the expression of miR-326. a The expression of miR-326 and b miRNA transfection in SH-SY5Y cells were measured by qPCR. c starBase was used to predict the target of HOTAIR. d RIP assay was performed to confirmed the correction with HOTAIR and miR-326. e The luciferase activity was measured after co-transfecting with WT/MUT HOTAIR plasmid and miR-326 mimics or NC in cells using the dual luciferase reporter assay. Data are analyzed using one-way ANOVA and presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
LncRNA HOTAIR Acted as a ceRNA by Sponging miR-326 and Regulated ELAVL1 Expression
As illustrated in Fig. 5a, the expression of ELAVL1 in SH-SY5Y cells was significantly upregulated in the presence of MPP+, while the effect was significantly inhibited by overexpression of miR-326 (*p < 0.05, **p < 0.01). In addition, this data was further verified by western blot detection (*p < 0.05, **p < 0.01; Fig. 5b). Furthermore, ELAVL1 was found to be the direct target of miR-326 (Fig. 5c). Moreover, the result of dual luciferase reporter assay confirmed the correlation between miR-326 and ELAVL1 (*p < 0.05; Fig. 5d). Besides, we found downregulation of miR-326 significantly suppressed the inhibitory effect of HOTAIR knockdown on ELAVL1 expression (*p < 0.05, **p < 0.01, ***p < 0.001; Fig. 5e, f). Taken together, lncRNA HOTAIR may regulate the progression of PD via miR-326/ELAVL1 axis.
Fig. 5.
LncRNA HOTAIR mediated the progression of PD via regulation of miR-326/ELAVL1 axis. a, b The expression of ELAVL1 in SH-SY5Y cells was tested by qPCR and western blot. The relative expression of ELAVL1 was quantified by normalizing to β-actin. c starBase database was used to predict the target of miR-326. d The luciferase activity was measured after co-transfecting with WT/MUT ELAVL1 3′-UTR plasmid and miR-326 mimics or NC in cells using the dual luciferase reporter assay. e, f The expression of ELAVL1 in shHOTAIR and miR-326 inhibitor transfected cells was detected by qPCR and western blot. The relative expression of ELAVL1 was quantified by normalizing to β-actin. Data are analyzed using one-way ANOVA and presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
LncRNA HOTAIR Induced the Activation of NLRP3-Mediated Pyroptosis via Regulation of miR-326/ELAVL1 Axis
In Fig. 6a, b, the expressions of IL-6, IL-1β, NLRP3, ASC and Caspase1 were significantly increased by miR-326 inhibitor, which were partially reversed in the presence of HOTAIR or ELAVL1 knockdown (**p < 0.01, ***p < 0.001). Similarly, the effect of miR-326 inhibitor on IL-1β level was significantly rescued by knockdown of ELAVL1 or HOTAIR (**p < 0.01, ***p < 0.001; Fig. 6c). In addition, the result of Caspase1 staining strongly confirmed the data of western blot (Fig. 6d). The results of LDH and Hoechst 33,342/PI staining showed that miR-326 inhibitor notably increased LDH activity and PI-positive cells, which were partially reversed by downregulation of ELAVL1 or HOTAIR (*p < 0.05, **p < 0.01, ***p < 0.001; Fig. 6e, f). In summary, HOTAIR induced the cell pyroptosis mediated by NLRP3 via regulation of miR-326/ELAVL1 axis.
Fig. 6.
LncRNA HOTAIR induced the activation of NLRP3 via regulation of miR-326/ELAVL1 axis. a The expression of IL-1β, IL-6, Caspase1, ASC and NLRP3 in SH-SY5Y cells was detected by qPCR. b The protein expression of Caspase1, pro-Caspase1, ASC, pro- IL-1β, IL-1β, IL-6 and NLRP3 in SH-SY5Y cells was detected by western blot. The relative expression was quantified normalizing to β-actin. c The level of IL-1β in SH-SY5Y cells was measured by ELISA. d The expression of Caspase1 in SH-SY5Y cells was detected by immunofluorescence staining. e LDH activity and f Hoechst 33342/PI staining was examined to assess pyroptotic cell death. Data are analyzed using one-way ANOVA and presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
PD is considered to enhance the burden on patients’ families and societies (Fan et al. 2020), and it is necessary to develop new strategies for PD treatment. In this study, we found that HOTAIR was upregulated in PD, and its knockdown could inhibit the progression of PD by suppressing NLRP3 inflammasome and pyroptosis through miR-326/ELAVL1 signal axis. This finding was similar to the previous references (Lang et al. 2020; Lin et al. 2019), further confirming that HOTAIR could act as a biomarker in PD. However, according to Lin Q et al. HOTAIR could promote the progression of PD via targeting miR-126-5p and Rab3a interacting protein (RAB3IP) in a ceRNA-dependent manner (Lin et al. 2019). This difference may be related to various mechanisms of HOTAIR in PD, and HOTAIR could target several specific miRNAs and downstream signal transduction pathways.
The incidence of PD frequently increases due to complicated network of pathological changes, such as dysfunctional network dynamics, apoptosis and autophagy (Borchert et al. 2019; Ge et al. 2020), as well as oxidative stress and mitochondrial dysfunction (Liu et al. 2019). In addition, recent studies have suggested that NLRP3 inflammasome mediated pyroptosis could contribute to the progression of PD (Wang et al. 2019a, b; Zeng et al. 2019), indicating a close correlation between pyroptosis and the occurrence and development of PD. In this research, we found that HOTAIR could act as a activator of NLRP3 inflammasome, which was consistent with previous data (Qiao et al. 2020), confirming that HOTAIR mediated the progression of PD via mediation of NLRP3 and regulation of pyroptosis.
Furthermore, it has been reported that lncRNA could exert their function mainly based on binding their sponged miRNAs (Jiang et al. 2020; Lou et al. 2020). LncRNAs can regulate the progression of PD by modulation of miRNAs expression. For instance, HAGLR opposite strand lncRNA (HAGLROS) could induce occurrence of PD by inhibiting apoptosis and autophagy via sponging miR-100 (Peng et al. 2019). In the present study, we found HOTAIR could sponge miR-326 and inhibit its expression. MiR-326 has been considered to serve as a prognostic factor and mediate the proliferation, invasion, and migration of multiple malignancies (Hu et al. 2019; Pan et al. 2019; Tang et al. 2019). Our research further supplemented the role of miR-326, suggesting that miR-326 could be a key suppressor in development of PD evidenced by repression of pyroptotic cell death due to NLRP3 inflammasome activation. Ding et al. found that lncRNA-p21 could regulate neuronal injury by targeting miR-625 and inhibiting transient receptor potential melastatin-related channel 2 (TRPM2) (Ding et al. 2019). Similarly, we found that HOTAIR regulated the occurrence of PD via sponging miR-326.
Next, to explore the potential mechanism of miR-326 in the occurrence of PD, starBase database was applied to identify target genes of miR-326. Our findings showed that ELAVL1 was a direct target of miR-326, which could repress ELAVL1 expression. ELAVL1 is an oncogene expressed in various malignant tumors, which played a key role during the progression (Liu and Tao 2020; Xue et al. 2019) and could be modulated by miRNAs in the regulation of multiple cancers (Wang et al. 2019a, b). Meanwhile, Li J et al. revealed that ELAVL1 could be involved in regulation of nervous system diseases (Li et al. 2019). Besides, ELAVL1 could be a promoter during the pyroptotic cell death by activation of NLRP3 (Li et al. 2017). Here, we found that suppression of HOTAIR or ELAVL1 could inhibit the effects of miR-326 inhibition on pyroptosis in vitro, suggesting that HOTAIR/miR-326/ELAVL1 regulatory network was implicated in PD progression via mediation of pyroptosis. Nevertheless, we recognize the need for an in-depth study of the mechanisms involved in such regulation.
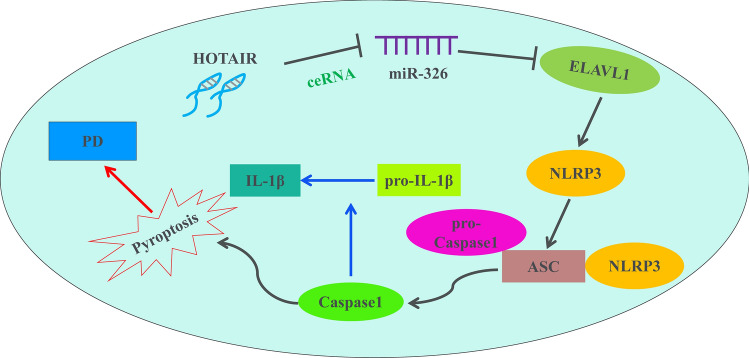
In conclusion, our study demonstrated that HOTAIR silencing significantly inhibits neuronal damage through suppressing NLRP3-mediated pyroptosis via regulation of miR-326/ELAVL1 axis in PD model (Fig. 7), which may provide a novel sight in PD pathogenesis and contribute to the exploration of new treatment strategies for this disease.
Fig. 7.
The mechanism of the regulatory network and function of lncRNA HOTAIR in PD. HOTAIR promoted the activation of NLRP3 inflammasome mediated pyroptosis at least in part by acting as a ceRNA for miR-326 and thus regulating ELAVL1 expression in PD. ┤: inhibition, → : promotion
Author Contributions
Guarantor of integrity of the entire study: J-YT, JH, C-CH, QZ, X-MH; study concepts: J-YT, QZ, JH, C-CH; study design: J-YT, J.-LZ, Y-KD; definition of intellectual content: J-YT, JH, Y-WT; literature research: J-YT, J.-LZ, C-CH, J-XL, DW; clinical studies: J-YT, QZ, JH, Y-KD; experimental studies: J-YT, J.-LZ, Y-KD; data acquisition: J-YT, JH, J-XL, DW; data analysis: J-YT, J.-LZ, J-XL, DW; statistical analysis: J-YT, QZ, JH, Y-KD; manuscript preparation: J-YT, JH, QZ, DW; manuscript editing: J-YT, JH, J-XL, DW; manuscript review: J-YT, J.-LZ, QZ, DW.
Funding
This work was supported by Natural Science Foundation of China (No. 81560201) and Doctor Foundation of Guizhou Provincial People's Hospital (No. GZSYBS [2015]03).
Data Availability
All data generated or analyzed during this study are included in this published article.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics Approval
This study was approved by the Medical Ethics Committee of Guizhou Provincial People’s Hospital. The animal experiments were strictly complied with the principle to minimize the pain and suffering to experimental animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Borchert RJ, Rittman T, Rae CL, Passamonti L, Jones SP, Vatansever D, Vazquez Rodriguez P, Ye Z, Nombela C, Hughes LE, Robbins TW, Rowe JB (2019) Atomoxetine and citalopram alter brain network organization in Parkinson's disease. Brain Commun. 10.1093/braincomms/fcz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Tu L, Li T, Yang X, Ren Y, Gu R, Zhang Q, Yao H, Qu X, Wang Q, Tian J (2019) Downregulation of lncRNA UCA1 ameliorates the damage of dopaminergic neurons, reduces oxidative stress and inflammation in Parkinson's disease through the inhibition of the PI3K/Akt signaling pathway. Int Immunopharmacol 75:105734 [DOI] [PubMed] [Google Scholar]
- Ding XM, Zhao LJ, Qiao HY, Wu SL, Wang XH (2019) Long non-coding RNA-p21 regulates MPP(+)-induced neuronal injury by targeting miR-625 and derepressing TRPM2 in SH-SY5Y cells. Chem Biol Interact 307:73–81 [DOI] [PubMed] [Google Scholar]
- Fan Y, Zhao X, Lu K, Cheng G (2020) LncRNA BDNF-AS promotes autophagy and apoptosis in MPTP-induced Parkinson's disease via ablating microRNA-125b-5p. Brain Res Bull 157:119–127 [DOI] [PubMed] [Google Scholar]
- Fasciani I, Petragnano F, Aloisi G, Marampon F, Rossi M, Francesca Coppolino M, Rossi R, Longoni B, Scarselli M, Maggio R (2020) A new threat to dopamine neurons: the downside of artificial light. Neuroscience 432:216–228 [DOI] [PubMed] [Google Scholar]
- Fu T, Ji X, Bu Z, Zhang J, Wu X, Zong X, Fan B, Jia Z, Ji J (2020) Identification of key long non-coding RNAs in gastric adenocarcinoma. Cancer Biomark 27:541–553 [DOI] [PubMed] [Google Scholar]
- Ge P, Dawson VL, Dawson TM (2020) PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson's disease. Mol Neurodegener 15:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghodousi ES, Aberuyi N, Rahgozar S (2020) Simultaneous changes in expression levels of BAALC and miR-326: a novel prognostic biomarker for childhood ALL. Jpn J Clin Oncol 50:671–678 [DOI] [PubMed] [Google Scholar]
- Gwinn K, David KK, Swanson-Fischer C, Albin R, Hillaire-Clarke CS, Sieber BA, Lungu C, Bowman FD, Alcalay RN, Babcock D, Dawson TM, Dewey RB Jr, Foroud T, German D, Huang X, Petyuk V, Potashkin JA, Saunders-Pullman R, Sutherland M, Walt DR, West AB, Zhang J, Chen-Plotkin A, Scherzer CR, Vaillancourt DE, Rosenthal LS (2017) Parkinson's disease biomarkers: perspective from the NINDS Parkinson's Disease Biomarkers Program. Biomark Med 11:451–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijazi MM, Buchmann SJ, Sedghi A, Illigens BM, Reichmann H, Schackert G, Siepmann T (2020) Assessment of cutaneous axon-reflex responses to evaluate functional integrity of autonomic small nerve fibers. Neurol Sci. 10.1007/s10072-020-04293-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZQ, Zhou SL, Li J, Zhou ZJ, Wang PC, Xin HY, Mao L, Luo CB, Yu SY, Huang XW, Cao Y, Jia F, Zhou J (2019) Circular RNA sequencing identifies CircASAP1 as a key regulator in hepatocellular carcinoma metastasis. Hepatology. 10.1002/hep.31068 [DOI] [PubMed] [Google Scholar]
- Huang T, Zhao HY, Zhang XB, Gao XL, Peng WP, Zhou Y, Zhao WH, Yang HF (2020) LncRNA ANRIL regulates cell proliferation and migration via sponging miR-339-5p and regulating FRS2 expression in atherosclerosis. Eur Rev Med Pharmacol Sci 24:1956–1969 [DOI] [PubMed] [Google Scholar]
- Jeyabal P, Thandavarayan RA, Joladarashi D, Suresh Babu S, Krishnamurthy S, Bhimaraj A, Youker KA, Kishore R, Krishnamurthy P (2016) MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun 471:423–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Peng A, Chen Y, Pang B, Zhang Z (2020) Long noncoding RNA EBLN3P promotes the recovery of the function of impaired spiral ganglion neurons by competitively binding to miR-204-5p and regulating TMPRSS3 expression. Int J Mol Med 45:1851–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang Y, Li Y, Yu H, Lin L, Chen X, Wang S, Zhang H (2020) HOTAIR drives autophagy in midbrain dopaminergic neurons in the substantia nigra compacta in a mouse model of Parkinson's disease by elevating NPTX2 via miR-221-3p binding. Aging (Albany, NY) 12:7660–7678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen F, Zhang Q, Meng X, Yao X, Risacher SL, Yan J, Saykin AJ, Liang H, Shen L, Alzheimer's Disease Neuroimaging Initiative (2019) Genome-wide Network-assisted association and enrichment study of amyloid imaging phenotype in Alzheimer's disease. Curr Alzheimer Res 16:1163–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zeng L, Cao C, Lu C, Lian W, Han J, Zhang X, Zhang J, Tang T, Li M (2017) Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res 350:327–335 [DOI] [PubMed] [Google Scholar]
- Lin Q, Hou S, Dai Y, Jiang N, Lin Y (2019) LncRNA HOTAIR targets miR-126-5p to promote the progression of Parkinson's disease through RAB3IP. Biol Chem 400:1217–1228 [DOI] [PubMed] [Google Scholar]
- Ling W, Yangchun X, Wei W, Qiang W (2020) Knockdown of long non-coding RNA GHET1 suppresses cervical carcinoma in vitro and in vivo. Cancer Biomark 28:21–32 [DOI] [PubMed] [Google Scholar]
- Liu Q, Sang Heon K, Yung-Wei S, Sok Cheon P, Wonwoong L, Jongki H, Jaehwan J, Kyoung Sang C, Songhee J, Byung-Soo K (2019) Neuroprotective effects of Suhexiang Wan on the in vitro and in vivo models of Parkinson's disease. J Tradit Chin Med 39:800–808 [PubMed] [Google Scholar]
- Liu S, Cui B, Dai ZX, Shi PK, Wang ZH, Guo YY (2016) Long non-coding RNA HOTAIR promotes Parkinson's disease induced by MPTP through up-regulating the expression of LRRK2. Curr Neurovasc Res 13:115–120 [DOI] [PubMed] [Google Scholar]
- Liu Y, Lu Z (2018) Long non-coding RNA NEAT1 mediates the toxic of Parkinson's disease induced by MPTP/MPP+ via regulation of gene expression. Clin Exp Pharmacol Physiol 45:841–848 [DOI] [PubMed] [Google Scholar]
- Liu Z, Tao H (2020) Small nucleolar RNA host gene 3 facilitates cell proliferation and migration in oral squamous cell carcinoma via targeting nuclear transcription factor Y subunit gamma. J Cell Biochem 121:2150–2158 [DOI] [PubMed] [Google Scholar]
- Lou W, Ding B, Fu P (2020) Pseudogene-derived lncRNAs and their miRNA sponging mechanism in human cancer. Front Cell Dev Biol 8:85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaquoi MS, Liguore WA, Churchill MJ, Moore C, Melrose HL, Meshul CK (2020) Gait deficits and loss of striatal tyrosine hydroxlase/trk-b are restored following 7,8-dihydroxyflavone treatment in a progressive mptp mouse model of parkinson's disease. Neuroscience 433:53–71 [DOI] [PubMed] [Google Scholar]
- Pan YJ, Wan J, Wang CB (2019) MiR-326: promising biomarker for cancer. Cancer Manag Res 11:10411–10418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng T, Liu X, Wang J, Liu Y, Fu Z, Ma X, Li J, Sun G, Ji Y, Lu J, Wan W, Lu H (2019) Long noncoding RNA HAGLROS regulates apoptosis and autophagy in Parkinson's disease via regulating miR-100/ATG10 axis and PI3K/Akt/mTOR pathway activation. Artif Cells Nanomed Biotechnol 47:2764–2774 [DOI] [PubMed] [Google Scholar]
- Qiao CM, Sun MF, Jia XB, Li Y, Zhang BP, Zhao LP, Shi Y, Zhou ZL, Zhu YL, Cui C, Shen YQ (2020) Sodium butyrate exacerbates Parkinson's disease by aggravating neuroinflammation and colonic inflammation in MPTP-induced mice model. Neurochem Res. 10.1007/s11064-020-03074-3 [DOI] [PubMed] [Google Scholar]
- Saewanee N, Praputpittaya T, Malaiwong N, Chalorak P, Meemon K (2019) Neuroprotective effect of metformin on dopaminergic neurodegeneration and alpha-synuclein aggregation in C. elegans model of Parkinson's disease. Neurosci Res S0168–0102:30440–30447 [DOI] [PubMed] [Google Scholar]
- Shiffman S, Basak S, Kozlowski C, Fuji RN (2018) An automated mapping method for Nissl-stained mouse brain histologic sections. J Neurosci Methods 308:219–227 [DOI] [PubMed] [Google Scholar]
- Shu L, Zhang X (2017) Shrimp miR-12 suppresses white spot syndrome virus infection by synchronously triggering antiviral phagocytosis and apoptosis pathways. Front Immunol 8:855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchovitz A, Hanan M, Yayon N, Lee S, Bennett ER, Greenberg DS, Kadener S, Soreq H (2020) A lncRNA survey finds increases in neuroprotective LINC-PINT in Parkinson's disease substantia nigra. Aging Cell 19:e13115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Chen Z, Zhao L (2019) Circular RNA hsa_circ_0000515 acts as a miR-326 sponge to promote cervical cancer progression through up-regulation of ELK1. Aging (Albany NY) 11:9982–9999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Chen Z, Zhao L (2020) Correction for: Circular RNA hsa_circ_0000515 acts as a miR-326 sponge to promote cervical cancer progression through up-regulation of ELK1. Aging (Albany, NY) 12:4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IK, Palanisamy K, Sun KT, Yu SH, Yu TM, Li CH, Lin FY, Chou AK, Wang GJ, Chen KB, Li CY (2020) The functional interplay of lncRNA EGOT and HuR regulates hypoxia-induced autophagy in renal tubular cells. J Cell Biochem. 10.1002/jcb.29669 [DOI] [PubMed] [Google Scholar]
- Wang S, Yuan YH, Chen NH, Wang HB (2019a) The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson's disease. Int Immunopharmacol 67:458–464 [DOI] [PubMed] [Google Scholar]
- Wang X, Yin H, Zhang L, Zheng D, Yang Y, Zhang J, Jiang H, Ling X, Xin Y, Liang H, Fang C, Ma J, Zhu J (2019b) The construction and analysis of the aberrant lncRNA-miRNA-mRNA network in non-small cell lung cancer. J Thorac Dis 11:1772–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Li QR, Xu YH, Zhou HB (2019) MicroRNA-139-3p inhibits the growth and metastasis of ovarian cancer by inhibiting ELAVL1. Onco Targets Ther 12:8935–8945 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yu H, Sun T, An J, Wen L, Liu F, Bu Z, Cui Y, Feng J (2020) Potential roles of exosomes in Parkinson's disease: from pathogenesis, diagnosis, and treatment to prognosis. Front Cell Dev Biol 8:86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R, Luo DX, Li HP, Zhang QS, Lei SS, Chen JH (2019) MicroRNA-135b alleviates MPP(+)-mediated Parkinson's disease in in vitro model through suppressing FoxO1-induced NLRP3 inflammasome and pyroptosis. J Clin Neurosci 65:125–133 [DOI] [PubMed] [Google Scholar]
- Zhao MW, Yang P, Zhao LL (2019a) Chlorpyrifos activates cell pyroptosis and increases susceptibility on oxidative stress-induced toxicity by miR-181/SIRT1/PGC-1alpha/Nrf2 signaling pathway in human neuroblastoma SH-SY5Y cells: implication for association between chlorpyrifos and Parkinson's disease. Environ Toxicol 34:699–707 [DOI] [PubMed] [Google Scholar]
- Zhao XH, Wang YB, Yang J, Liu HQ, Wang LL (2019b) MicroRNA-326 suppresses iNOS expression and promotes autophagy of dopaminergic neurons through the JNK signaling by targeting XBP1 in a mouse model of Parkinson's disease. J Cell Biochem 120:14995–15006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.