Abstract
In astrocytes, the water-permeable channel aquaporin-4 (AQP4) is concentrated at the endfeet that abut the blood vessels of the brain. The asymmetric distribution of this channel is dependent on the function of dystroglycan (DG), a co-expressed laminin receptor, and its associated protein complex. We have demonstrated that the addition of laminin to astrocytes in culture causes the clustering of AQP4, DG, and lipid rafts. The last, in particular, have been associated with the initiation of cell signaling. As laminin binding to DG in muscle cells can induce the tyrosine phosphorylation of syntrophin and laminin requires tyrosine kinases for acetylcholine receptor clustering in myotubes, we asked if signal transduction might also be involved in AQP4 clustering in astrocytes. We analyzed the timecourse of AQP4, DG, and monosialotetrahexosylganglioside (GM1) clustering in primary cultures of rat astrocytes following the addition of laminin, and determined that the clustering of DG precedes that of AQP4 and GM1. We also showed that laminin induces the formation of phosphotyrosine-rich clusters and that the tyrosine kinase inhibitor, genistein, disrupts the laminin-induced clustering of both β-DG and AQP4. Using the Kinexus antibody microarray chip, we then identified protein-serine kinase C delta (PKCδ) as one of the main proteins exhibiting high levels of tyrosine phosphorylation upon laminin treatment. Selective inhibitors of PKC and siRNA against PKCδ disrupted β-DG and AQP4 clustering, and also caused water transport to increase in astrocytes treated with laminin. Our results demonstrate that the effects of laminin on AQP4 localization and function are relayed, at least in part, through PKC signaling.
Electronic supplementary material
The online version of this article (10.1007/s10571-020-00944-w) contains supplementary material, which is available to authorized users.
Keywords: Aquaporin-4, Laminin, Dystroglycan, Protein kinase C, Water permeability
Introduction
Dystroglycan (DG) was originally identified in muscle cells as being a component of the dystrophin glycoprotein complex (DGC). The DGC is composed of a heavily glycosylated α subunit of DG which binds with high affinity to the extracellular matrix (ECM) proteins laminin, agrin, perlecan and neurexin (Gee et al. 1993; Gee et al. 1994; Henry and Campbell 1998; Sugita et al. 2001), and a transmembrane β subunit that interacts with the other members of the DGC, dystrophin, dystrobrevin, and syntrophin, and through dystrophin, to filamentous actin. Therefore, DG serves as a bridge linking the basal lamina with the cytoskeleton (Ehmsen et al. 2002; Henry and Campbell 1996; Ibraghimov-Beskrovnaya et al. 1992; Jung et al. 1995; Way et al. 1992). It is thus believed to be essential for maintaining the integrity of the sarcolemma through repeated cycles of contraction and relaxation (Petrof et al. 1993). Indeed, mutations of various members of the DGC underlie the pathogenesis of many muscular dystrophies, a class of congenital disorders characterized primarily by progressive muscle weakness and degeneration and secondarily by defects in brain and ocular development, the etiologies of which are poorly understood (Hayashi et al. 2001; Kano et al. 2002; Longman et al. 2003).
The DGC is expressed in neurons and astrocytes of the central nervous system as well (Blake et al. 1999; Moukhles and Carbonetto 2001), and as a result these dystrophies often lead to defects in brain and retinal development (Beltran-Valero de Bernabe et al. 2002; Hayashi et al. 2001; Kano et al. 2002; Longman et al. 2003), although the causes for these are currently poorly understood. Of particular interest is the fact that the DGC is enriched at the astrocytic endfeet that separate the brain parenchyma from the permeant vasculature, where the water-carrying channel AQP4 is similarly highly expressed (Nielsen et al. 1997; Zaccaria et al. 2001), and mutations in the dystrophin gene or deletion of α-syntrophin result in a dramatic reduction of AQP4 amounts at these sites (Neely et al. 2001; Nicchia et al. 2004; Nico et al. 2003). This relationship appears to hold true in vitro as well, with the addition of laminin to primary cultures of rat astrocytes causing the formation of large clusters containing numerous members of the DGC and AQP4 (Guadagno and Moukhles 2004; Noël et al. 2009). Furthermore, loss of laminin binding to DG due to α-DG hypoglycosylation in the Largemyd mouse results in the mislocalization of AQP4 in the brain and retina (Michele et al. 2002; Rurak et al. 2007). From a functional point of view, it has been shown that the loss of either α-syntrophin or dystrophin recapitulate phenotypes seen in the AQP4 knockout mouse exhibiting a delay in the development of cytotoxic edema (Amiry-Moghaddam et al. 2004; Vajda et al. 2002). More recently, we have shown that laminin negatively regulates AQP4-mediated water transport in astrocytes (Noël et al. 2020). Based on these various lines of evidence, it was thus suggested that the DGC might serve as a scaffold, tethering AQP4 to the endfoot domain of astrocytes and regulating AQP4-mediated water transport (Neely et al. 2001).
DGC-ECM interactions play a crucial role in the signaling cascades that regulate the clustering of acetylcholine receptors (AChRs) at the neuromuscular junction (NMJ) (Campanelli et al. 1994; Grady et al. 2000; Jacobson et al. 2001; Peng et al. 1998). It is known, for instance, that laminin binding causes DG and syntrophin to be tyrosine-phosphorylated. The latter, notably, has the effect of increasing syntrophin’s binding to Grb2, which, through forming a complex with Sos1, activates Rac1 and PAK1-JNK, ultimately resulting in the remodeling of the actin cytoskeleton, activation of the PI3K/AKT pathway, and the aggregation of AChRs (Langenbach and Rando 2002; Marangi et al. 2002; Sadasivam et al. 2005; Sotgia et al. 2003; Spence et al. 2004; Weston et al. 2007; Zhou et al. 2007; Zhou et al. 2006).
We have previously shown that the laminin-induced clustering of AQP4 in astrocytes occurs via a lipid raft-dependent mechanism (Noël et al. 2009), and AChR clustering in muscle cells appears to be similar in this regard, with a number of studies demonstrating that the latter can be halted by treatments that perturb lipid raft integrity (Cartaud et al. 2011; Pato et al. 2008; Stetzkowski-Marden et al. 2006a; Stetzkowski-Marden et al. 2006b). This extends to the majority of molecules involved in signaling processes as well, such as PLCγ2, Syk, Src and Lyn (Gousset et al. 2004). In addition, β-DG can bind Grb2, and syntrophin can bind signaling molecules such as nNOS, Grb2, guanine nucleotide-binding protein alpha subunit (protein Go), stress-activated protein kinase-3 (ERK6), microtubule-associated serine/threonine kinase, syntrophin-associated serine/threonine kinase, ARMS and diacylglycerol kinase-zeta (Russo et al. 2000; Abramovici et al. 2003; Adams et al. 2001; Brenman et al. 1996; Hasegawa et al. 1999; Hogan et al. 2001; Lumeng et al. 1999; Luo et al. 2005; Oak et al. 2001; Okumura et al. 2008; Yakubchyk et al. 2005). Based on these lines of evidence, we therefore hypothesized that the co-clustering of AQP4, DG, and lipid rafts involves the participation of signal transduction events initiated by the binding of laminin to DG.
In the present study, we sought to determine whether signaling is involved in the clustering of AQP4. We determined via immunofluorescence, that following laminin binding, DG and laminin cluster first, followed by AQP4 and GM1. We then found by immunoblot that laminin caused protein tyrosine phosphorylation, and that this could be blocked using the tyrosine kinase inhibitor genistein, which results in the scattering of AQP4 and DG clusters, following which we showed that the clusters themselves contained a significant amount of these tyrosine-phosphorylated proteins. Using an antibody-based microarray, we identified the proline-rich/Ca2+-activated tyrosine kinase 2 (Pyk2) and the protein-serine kinase C delta (PKCδ) as being two targets that are highly phosphorylated downstream of laminin binding. Via the use of siRNA and selective inhibitors of PKC, we demonstrated that this serine/threonine kinase plays a role in the clustering process, regulating not only the accretion of AQP4 and DG but also the water-mediated transport of the former in laminin-treated astrocytes.
Methods
Antibody Characterization
Rabbit polyclonal anti-AQP4 targeting residues 249–323 of the rat sequence (RRID: AB_2039734; Catalog No. AQP-004, Alomone Laboratories) was used at a dilution of 1:100 in immunofluorescence-based experiments, and at 1:1000 for western blotting. The antibody stains a doublet of approximately 35 kDa by western blot, and this staining was eliminated by preincubation with the antigenic peptide (Noël et al. 2009).
Mouse monoclonal anti-β-DG recognizing the C-terminal tail of human DG (RRID: AB-2336241; Catalog No. VP-B205, Vector Laboratories), used at 1:1000 for western blotting and 1:50 for immunofluorescence, produces a band of significantly reduced intensity by western blot when cells are treated with siRNA targeting DG expression (Noël et al. 2009).
Mouse monoclonal anti-β-actin (RRID:AB_476744; Catalog No. A5441, Sigma-Aldrich), made against a KLH-conjugated peptide corresponding to the N-terminal cytoplasmic region of actin, produces a signal only in cytoplasmic and whole-cell fractions, but not in fractions containing cell-surface proteins only (Tham et al. 2016; data not shown).
Rabbit polyclonal anti-laminin-111, which recognizes all three subunits of laminin, was used at 1:100 in immunofluorescence experiments, and produces an immunofluorescence signal only in cells treated with laminin (Noël et al. 2009).
The rabbit polyclonal anti-PKCδ antibody was made against residues 662-673 of the unphosphorylated form of the human protein (RRID: AB_304315; Catalog No. ab4142, Abcam), and the monoclonal antibody targeting the Y313-phosphorylated form (RRID: AB_1524203; Catalog No. ab76181, Abcam) were both used at 1:1000 for western blotting, and the latter was used at 1:100 for immunofluorescence. Their specificities were knockdown-verified in this study.
Rabbit polyclonal antibodies against human Pyk2 in both its non-phosphorylated (RRID: AB_2174205; Catalog No. 06-559, Upstate) and Y579-phosphorylated forms (RRID: AB_568887; Catalog No. 07-893, Upstate) were both used at 1:1000 for western blotting, and their specificities were also knockdown-verified here.
Rabbit polyclonal antibody against FAK (RRID: AB_2300502; Catalog No. sc-558, Santa Cruz), raised against its target’s C-terminal sequence, was used at 1:1000 for western blotting, and produces a band of the expected molecular weight by western blotting.
Mouse monoclonal anti-phosphotyrosine, made using phosphotyramine-KLH as the antigen (RRID: AB_568858; Catalog No. 05-321X, Upstate), used at 1:1000 and 1:100 for western blotting and immunofluorescence, respectively, produces a much-decreased signal when used to probe extracts obtained from cells treated with genistein, which is known to reduce tyrosine phosphorylation levels.
Astrocyte Primary Culture
Cortices were dissected from embryonic day-18 Sprague–Dawley rats (RRID: RGD_734476; Charles River Laboratories International). After both the meninges and choroid plexus were removed, the remaining tissue was cut into small pieces and incubated for 25 min in trypsin (3.0 mg/ml; Gibco). The resultant suspension was then plated in culture flasks and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin and 1 mM l-glutamine (Gibco) for 18 h, following which the flasks were shaken so as to eliminate microglia and oligodendrocyte progenitors from the cultures. The astrocytes were then allowed to proliferate for 2–3 additional weeks, during which the culture medium was changed every 3 days. For immunofluorescence assays, astrocytes were trypsinized and plated on glass coverslips coated with poly-d-lysine (0.1 mg/ml; Sigma) in 24-well plates at a density of 200–250 × 103 cells/ml. Where required, the cells were treated with 15 nM Engelbreth-Holm-Swarm Sarcoma laminin-111 (Sigma-Aldrich) 2 days after plating for the lengths of time specified, added in serum-free medium.
RT-PCR
Total RNA extraction was performed on confluent mouse primary astrocyte cultures and on brains obtained from CO2-euthanized mice using a micro-homogenizing device (VWR, Mississauga, ON, Canada) and TRIZOL® reagent (Invitrogen) according to the supplied protocol. The quality and integrity of the RNA was assessed on formaldehyde agarose gel, and its concentration was determined using NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies, USA).
Total cDNA was prepared using Oligo dT(18) (Invitrogen) from 1 μg of total RNA from each sample and SuperScript™ III Reverse Transcriptase (RT; Invitrogen) following the included instructions. AQP4 M1 and M23 transcripts were amplified from 2 μl of synthesized total cDNA by PCR using the set of primers described in Noell et al. (2007), and Platinum®-Taq DNA polymerase (Invitrogen). GAPDH was amplified alongside using the forward primer 5′-ACC ACA GTC CAT GCC ATC AC-3′ and the reverse primer 5′-TCC ACC ACC CTG TTG CTG TA-3′ as an internal loading control. To verify that the signals did not originate from contaminating genomic DNA, RNA samples that had not been reverse transcribed (-RT) were also amplified.
siRNA Transfection
Astrocytes were transfected in suspension with 100 nM ON-TARGETplus SMARTpool siRNAs targeting either Pyk2 or PKCδ and non-targeting siRNAs as controls (both from Dharmacon) using Lipofectamine-2000 (Invitrogen), following the manufacturer’s protocol. Thirty-six hours after plating, the cells were treated with 15 nM of laminin-111 as described above for 4 h, and then processed for immunofluorescence.
Kinase Inhibition
For the tyrosine kinase inhibition experiments, astrocytes were incubated with 50 μM genistein, 0.5 μg/ml herbimycin or with 5 nM staurosporine (both Calbiochem) for 4 h at 37 °C in the presence of laminin. For the selective inhibition of Pyk2 or PKC kinase activity, the cells were treated with 50 μM dantrolene (which blocks the upstream calcium release steps necessary for the activation of Pyk2; Calbiochem), and 0.15–0.30 μg/ml Ro 31-8220 or 1.5–3 μg/ml GF-109203 (inhibitors of PKC; Sigma), respectively.
Kinexus Microarray
Primary cortical astrocytes, treated with 15 nM laminin or vehicle (PBS) for 3 h, were first washed twice in chilled (4 °C) PBS, and then sonicated in Kinex lysis buffer (20 mM MOPS, 1% Triton X-100, 2 mM EGTA, 5 mM EDTA, 30 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 5 μM pepstatin A, 1× complete protease inhibitor mixture (Roche Applied Science), 1 mM DTT). 100 μg of cleared lysate protein from each set of cells was shipped to Kinexus Bioinformatics Corporation for antibody array analysis. A replicate assay performed at a later date using a separate set of control and laminin-treated cells produced similar results.
Immunofluorescence
Where required, the lipid raft component monosialotetrahexosylganglioside (GM1) was labeled by first preincubating live astrocytes grown on glass coverslips in chilled (4 °C) PBS containing 10 μg/ml FITC-conjugated cholera toxin subunit B (CtxB; Sigma), which specifically labels this ganglioside, for 25 min prior to fixation. Otherwise, the cells were directly processed for immunofluorescence labeling by washing them with warm (37 °C) PBS, and then fixing them by immersing the coverslips in 4% (w/v) formaldehyde in 0.1 M PB for 20 min. Following this, they were washed thrice with room temperature (25 °C) PBS, for 15 min each time, and then blocked and permeabilized for 1 h at room temperature using a buffer consisting of 2% bovine serum albumin and 0.25% Triton X-100 (both Sigma) dissolved in PBS. Immunolabeling was performed by incubating the cells for 1 h in the presence of primary antibodies against β-DG (diluted 1:25 in blocking buffer), AQP4 (1:200), phosphotyrosine (1:400), laminin (1:1500), and phospho-PKCδ (1/100), added in various combinations. The cells were then rinsed with PBS, following which they were incubated for 1 h with Alexa Fluor 568 goat anti-mouse IgG, Alexa Fluor 647 goat anti-rabbit, Alexa Fluor 488 goat anti-mouse IgG, Alexa Fluor 568 goat anti-rabbit IgG or Alexa Fluor 568 goat anti-mouse IgG, and Alexa Fluor 488 goat anti-rabbit IgG (all diluted 1:200 in blocking buffer; Molecular Probes). Finally, after several more washes, the coverslips were mounted on glass slides using Prolong Gold Antifade Reagent with DAPI (Invitrogen). To confirm the specificity of the labeling, control cells were treated equivalently in the absence of primary antibodies. Fluorescent labeling of cultured cells was visualized using a confocal microscope (Fluoview 1000; Olympus) and an Uplan Apochromat 1.35 NA 60 × objective (Olympus).
Immunoblotting
Astrocyte cultures were first harvested and lysed on ice for 20 min in extraction buffer (25 mM Tris pH 7.4, 25 mM glycine and 150 mM NaCl) containing 1% Triton X-100, 1× complete protease inhibitor cocktail, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 30 mM sodium fluoride, 20 mM sodium pyrophosphate and 5 mM EDTA. After nuclei and cellular debris were removed from the suspension by centrifugation at 16,000×g for 10 min, extracted proteins were denatured by boiling for 9 min in reducing sample buffer, separated through a 10% sodium dodecyl sulfate–polyacrylamide electrophoresis gels, and then electrotransferred to nitrocellulose membranes (Bio-Rad). The resultant blots were then blocked in Tris-buffered saline Tween containing 5% low-fat milk (TBST-M) for 1 h at room temperature, and probed with primary antibodies against phosphotyrosine (diluted 1:1000 in TBST-M), PKCδ (1:500), phospho-PKCδ (1:500), β-DG (1:300), AQP4 (1:1000), Pyk2 (1:1000), phospho-Pyk2 (1:1000), FAK (1:1500) and β-actin (1:20000). Bound antibodies were detected using horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (1:2000; Jackson ImmunoResearch), and signals were visualized on BioFlex Econo Film (Interscience) using chemiluminescence (Amersham Biosciences).
Water Permeability Measurements
For the water permeability measurements, astrocytes grown on round coverglasses were incubated for 45 min with 6 µM calcein-AM (Molecular Probes) at 37 °C. After rinsing with PBS (pH 7.4), we placed the coverglasses in a perfusion chamber mounted to the stage of a Zeiss LSM510-Axioskop-2 two-photon laser-scanning microscope fitted with a 40X-W/0.80 numerical aperture objective lens directly coupled to a Mira Ti:sapphire laser (100-fs pulses, 76 MHz, pumped by a 5 W Verdi laser; Coherent), with which we monitored calcein fluorescence at 3.4-s intervals. The cells were initially perfused with isotonic CSF 330 mOsm (120 mM NaCl, 3.3 mM KCl, 26 mM NaHCO3, 1.3 mM MgSO4, 1.2 mM NaH2PO4, 1.8 mM CaCl2 and 11 mM d-Glucose, verified for the correct osmolarity using a freezing point-depression osmometer) introduced into the chamber at an approximate rate of 4 mL/min, and then subjected to an osmotic shock by switching the perfusate to a hypoosmotic, 250 mOsm, CSF, (same as above, but containing 80 mM, rather than 120 mM NaCl). The swelling of the cells results in a decrease in fluorescence levels, caused by the dilution of calcein by water entering the cell (Benfenati et al. 2007; Han et al. 1998; Hibino and Kurachi 2007; Huber et al. 2009; Huber et al. 2007). The individual fluorescence tracings for each cell were normalized against the initial calcein intensity, and averaged to produce the curves shown in Fig. 8. To account for variation in fluorescence intensity over time, cell swelling values quoted in the text were calculated from five consecutive data points associated with the minima of each averaged calcein intensity curve, and the SEMs provided were computed from the individual values used to calculate these averages.
Fig. 8.

PKC inhibition reverses the effects of laminin on water permeability. Untreated control astrocytes and astrocytes treated with 15 nM laminin alone, or laminin in combination with 0.3 μg/ml Ro 31-8220 were loaded with calcein and imaged. Averaged time-series recordings of calcein-loaded untreated and Ro 31-8220-treated astrocytes (− LAM and – LAM + Ro; (a) and laminin- and Ro 31-8220-treated astrocytes (+ LAM and + LAM + RO; (b). Cells were initially perfused with an isotonic fluid, and then subjected to a 120-s hypotonic challenge (marked by dashed lines), with isotonic conditions being re-established at the end of that period. Cell volume changes are reflected in the change in normalized calcein fluorescence intensity
Quantitative Analysis
The number and surface area of DG, AQP4, laminin and GM1 clusters were calculated from immunofluorescence images acquired under identical settings, using a common threshold value chosen to ensure that smaller clusters were included while the larger ones remained separate, via ImagePro Plus software (Media Cybernetics). Only clusters exceeding 1 μm2 in area were included in our analyses. A minimum of 30 images taken across three independent experiments were analyzed to produce the statistics presented in each figure. The significance of differences in the data was tested via ANOVA in combination with Tukey’s method. All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software).
Ethics Statement
All procedures involving animals were performed in accordance with Canadian Council on Animal Care guidelines, and our protocol was approved by the Animal Care Committee of the University of British Columbia (Approval Number A06-0319). Upon receipt, dams were euthanized using carbon dioxide. Embryos were then removed, decapitated with sharp surgical scissors, and processed for astrocyte culture as described above. Every effort was made to ensure that these animals were treated humanely.
Results
We previously described the co-clustering of DG, lipid rafts, and AQP4 in astrocyte cultures following laminin treatment (Noël et al. 2009). As it had been suggested by several studies that the ECM can play a role in initiating raft-associated signaling events, we therefore chose to investigate in the present study whether such a mechanism might be involved in this system as well, focusing primarily on the identification of the candidate molecules central to the clustering process.
The Laminin-Induced Clustering of DG and AQP4 is Regulated by Tyrosine Phosphorylation
In order to establish the approximate timeframe during which the signaling events that regulate AQP4 clustering might potentially occur, we began by first monitoring how GM1, DG, AQP4, and laminin distribution changes in primary astrocyte cultures following laminin treatment over the course of 8 h. Via the analysis of images collected over three independent experiments, we found that, while all four were initially diffusely distributed (Fig. 1a–d and u, Fig S1A-D), the number of clusters of DG and laminin was significantly increased between the 0- and 1-h timepoint (p < 0.001, n ≥ 30 fields in the case of the former and p < 0.001, n ≥ 30 for the latter), and the number of clusters peaking at 2 h post-treatment (Fig. 1e–h and u, Fig S1E-H). GM1 and AQP4 clustering, on the other hand, lagged slightly behind, increasing significantly between hours 2 and 3 (p < 0.001, n ≥ 30 for both), and plateauing an hour after that (Fig. 1i–l and u). The surface area occupied by the clusters appears to be governed by a logarithmic function, remaining well below 2000 μm2 on average until after the 4-h mark, at which point a coordinated exponential growth takes place (p ≤ 0.01 and n ≥ 31 in all cases), plateauing 2–3 h after that point (Fig. 1v). To control for the possibility that the increased clustering might be related to changes in AQP4 expression, we additionally investigated the effects of laminin treatment on the transcript levels of M1- and M23-AQP4, the two major isoforms of this channel in astrocytes. We found that neither was altered by the presence of laminin (Fig. 2) consistent with our earlier findings regarding AQP4 total protein levels (Tham et al. 2016).
Fig. 1.
Laminin-induced clustering of GM1, β-DG, and AQP4 increases over time. Rat cortical astrocyte cultures were treated with 15 nM laminin for various lengths of time, and then labeled for GM1, β-DG, and AQP4 (a–t). Scale bar, 30 μm. Curves depicting the mean number (u), and surface area (v) of these clusters (± SEMs), based on measurements obtained from a minimum of 30 randomly acquired fields per time point acquired over three independent experiments
Fig. 2.
Laminin causes certain cluster-associated components to be tyrosine-phosphorylated. Rat cortical astrocytes incubated in the absence (a–c) or the presence of 15 nM laminin (d–f) were labeled for phosphotyrosine (pY; a and d) and laminin (b and e). Arrowheads indicate clusters where both overlap. Scale bar, 30 μm. Histogram comparing the number (± SEMs) of phosphotyrosine-containing clusters in control and laminin-treated cultures (g), averaged across three independent experiments, for which 15 fields were analyzed per condition per experiment. * p< 0.05
Labeling astrocytes for tyrosine-phosphorylated protein species and for laminin (Fig. 2a–f), we found that, in comparison with the untreated controls, the laminin-treated cells not only exhibited increased global levels of tyrosine phosphorylation but also a greater number of phosphotyrosine clusters (compare Fig. 2a and d; quantified in g). Further, these clusters were frequently seen to overlap or coincide with clusters of laminin (Fig. 2f). The above is reminiscent of the manner in which laminin causes β-DG, GM1 and AQP4 to aggregate, and raises the possibility that certain molecules may be tyrosine-phosphorylated downstream of laminin binding, and become reorganized as a result.
Via immunoblot analysis, we confirmed the increase in the levels of tyrosine-phosphorylated proteins at 3 h, which coincides precisely with the period during which the clusters begin to expand in size (Fig. 1v). We found that this could be interrupted using the commonly used tyrosine phosphorylation inhibitor genistein, a phytoestrogen. However, the antibiotic herbimycin, also known to be an inhibitor of tyrosine kinases, did not have the same effect (Data not shown). Laminin appears to act on tyrosine phosphorylation through DG, as knocking down the latter by siRNA results in reduced phosphotyrosine levels in the presence of laminin (Fig. 3b). Consistent with the above, genistein added to astrocytes simultaneously with laminin was found to significantly reduce the number of β-DG and AQP4 clusters (compare Fig. 3c–e and f–h). Taken together, these results strongly suggest that the tyrosine phosphorylation of certain cluster-associated proteins is a critical step for the laminin-induced cluster formation and stabilization of β-DG and AQP4.
Fig. 3.
Genistein and dystroglycan silencing inhibit the laminin-induced increase in tyrosine phosphorylation and disrupts β-DG and AQP4 clustering. Astrocytes incubated in the presence of PBS (− LAM), 15 nM laminin (+ LAM), or laminin and 50 μM genistein (+ LAM + Gen) for three hours were analyzed for tyrosine phosphorylation (pY) by immunoblotting (a). Cells were transfected with either siRNA against dystroglycan (siDG) or with a scrambled non-targeting RNA (siCTL) as a control, and then treated with 15 nM laminin (+ LAM) or PBS (− LAM), following which phosphotyrosine levels were assessed by western blot (b). Immunofluorescence analysis of β-DG and AQP4 clustering in astrocytes treated with laminin alone (c–e), or laminin and genistein (f–h). Scale bar, 30 μm
We then performed a screen for molecules that exhibited the highest degrees of upregulation in their expression or phosphorylation levels in rat astrocytes following laminin treatment using the Kinexus antibody KAM-1.1 microarray. Out of a panel of thirty initial targets, the majority of which were kinases, proline-rich/Ca2+-activated tyrosine kinase 2 (Pyk2), a focal adhesion kinase (FAK) family member, and protein kinase C delta (PKCδ) exhibited the greatest degree of tyrosine phosphorylation in laminin-treated cells (highlighted in Fig. 4). We thus focused primarily on the role played by these kinases in mediating the phosphorylation events crucial to cluster formation for the remainder of this study.
Fig. 4.
Laminin binding affects the phosphorylation state of a variety of signaling molecules. Protein extracts prepared from control and 15 nM laminin-treated astrocytes were labeled with Cy5 and Cy3, respectively, and analyzed using the Kinexus KAM-1.1 microarray system to identify signaling molecules exhibiting the greatest degree of tyrosine phosphorylation (shown in red) or dephosphorylation (green) in response to laminin binding. Proline-rich/Ca2+-activated tyrosine kinase 2 (Pyk2) and the protein-serine kinase C delta (PKCδ) represent two of the most significant hits
PKCδ, But Not Pyk2, is Involved in the Co-clustering of DG and AQP4
By western blot, we determined that while laminin treatment does not affect the total levels of Pyk2, it does cause Pyk2 phosphorylation on tyrosine 579 to increase 1.39-fold (Fig S3A). To address the role of Pyk2 in the clustering response, we began by first assessing if its phosphorylation might be modulated by the general tyrosine phosphorylation inhibitors staurosporine, herbimycin, and genistein (Fig S3B). Of these, only staurosporine and genistein prevented Pyk2 phosphorylation following laminin treatment. To more directly assess the involvement of Pyk2, we proceeded to silence its expression by siRNA, but found that, despite the fact that the treatment was decidedly effective in reducing the expression of this kinase (Fig S3C), AQP4 clustering was not significantly perturbed in laminin-treated astrocytes (Fig S3D). Similar results were obtained with dantrolene, an inhibitor of Pyk2 (data not shown). Previous studies have suggested that focal adhesion kinase (FAK) can be upregulated to compensate for the loss of Pyk2. In addition, FAK was found to complex with DG in extracts prepared from brain synaptosomes (Cavaldesi et al. 1999). We therefore decided to determine if FAK levels were increased in Pyk2-silenced astrocytes. Contrary to our expectations, however, we found decreased amounts of FAK in these cells compared to cells transfected with a control non-silencing RNA (Fig S3C).
The phosphorylation of tyrosine 313 of PKCδ by comparison, increases in astrocytes treated with laminin (Fig. 5a), and is inhibited by the addition of genistein (Fig. 5b). Our immunofluorescence data also indicate that laminin causes PKCδ to assemble into aggregates that partially overlap with β-DG clusters (Fig. 5c–h). Further, we also determined that two inhibitors of PKC, GF-109203 and Ro 31-8220, significantly reduced the size of the AQP4 and β-DG aggregates induced by laminin treatment (Fig. 6a–i, j and k). Interestingly, our quantitative analysis revealed that, at certain doses, GF-109203 and Ro 31-8220 can also produce an increase in the number of β-DG clusters (Fig. 6k), perhaps suggesting that these compounds act by causing pre-existing clusters to fragment. Prompted by this finding, we proceeded to evaluate the effect that knocking down PKCδ (which undergoes significant tyrosine phosphorylation in response to laminin; Fig. 5a) would have on the clustering (Fig. 7b and c). We saw that even a modest loss of PKCδ significantly reduced the number of AQP4 clusters, on a level comparable to that which was seen with the lower dose of Ro 31-8220 (Fig. 7d). There was, however, no discernable effect on DG clusters (data not shown).
Fig. 5.
Phosphorylated PKCδ associates with laminin-induced β-DG clusters. PKCδ phosphorylation levels in control and 15 nM laminin-treated astrocyte cultures were compared by immunoblotting (a). The effect of genistein on the laminin-induced increase in PKCδ phosphorylation following laminin treatment was also investigated (b). The relative localizations of tyrosine-phosphorylated PKCδ and β-DG was visualized by immunofluorescence (c–h). Examples of β-DG-pPKC co-clustering in + LAM cells are marked with arrowheads. Scale bar, 30 μm
Fig. 6.
PKC inhibitors Ro 31-8220 and GF-109203 prevent the laminin-induced clustering of β-DG, and AQP4. Rat cortical astrocytes incubated in the presence of 15 nM laminin alone (+ LAM) or with laminin and 0.30 μg/ml Ro 31-8220 (+ LAM + Ro) or laminin and 3 μg/ml GF-109203 (+ LAM + GF) for three hours were double immunolabeled for β-DG (a, d and g) and AQP4 (b, e and h). Scale bar, 30 μm. Histograms comparing the number and surface area of laminin-induced AQP4 (j) and β-DG clusters (k) in the presence of the indicated concentrations of Ro 31-8220 and GF-109203. Numbers shown for each condition represent the means (± SEMs) calculated from 45 randomly acquired fields taken across 3 independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001
Fig. 7.
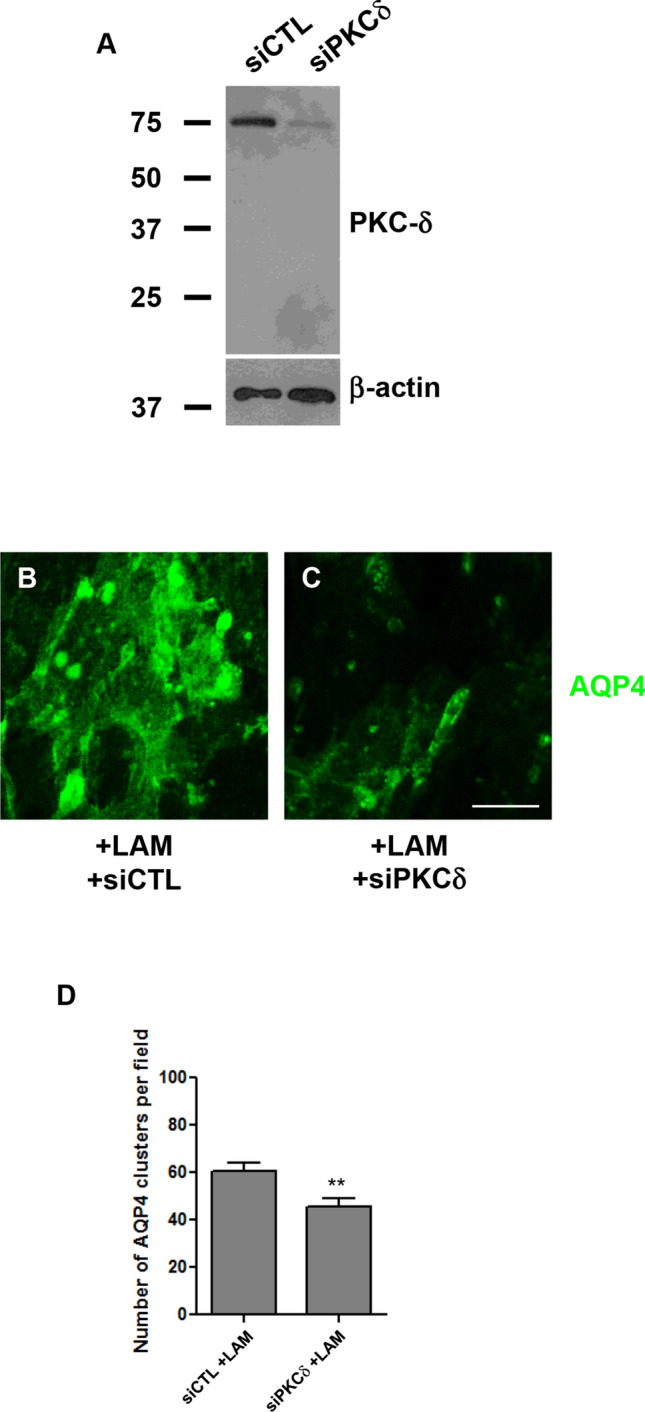
PKCδ knockdown reduces AQP4 clustering. PKCδ levels in rat cortical astrocytes treated with control siRNA (siCTL) and siRNA targeting PKCδ (siPKCδ) for 48 h, with β-actin as a loading control. Cells treated with siCTL (b) or siRNA against PKCδ (c) were incubated with 15 nM laminin (+ LAM) for 3 h, and then labeled for AQP4. Scale bar, 7.5 μm. Histogram comparing the number of laminin-induced AQP4 clusters in siCTL- and siPKCδ-treated cells (d). Numbers shown for each condition represent the means (± SEMs) calculated from > 30 randomly acquired fields per experiment, taken across 3 independent experiments. **p < 0.01
PKC Modulates AQP4 Function
Given our earlier observation that laminin treatment results in the phosphorylation of PKCδ resulting in the coalescence of AQP4 into clusters, and previous evidence demonstrating that the water permeability of AQP4 can be regulated by PKC-mediated phosphorylation (Fenton et al. 2010; Gunnarson et al. 2005; Han et al. 1998; McCoy et al. 2010; Moeller et al. 2009; Okuno et al. 2008; Yukutake and Yasui 2010; Zelenina et al. 2002), we next asked if laminin might also affect the functional properties of AQP4.
Via two-photon microscopy, we tested whether laminin treatment and PKC inhibition might affect the degree to which astrocytes swelled in response to a hypoosmotic challenge, which is used here as a surrogate measure of membrane water permeability. For this assay, astrocytes were first loaded with calcein, and then bathed in 330 mOsm artificial CSF. After a period of equilibration, the osmolarity of the CSF was changed abruptly to 250 mOsm for approximately 100 s, before being allowed to return to its previous value. Calcein fluorescence tracings taken during this assay revealed that laminin had the rather surprising effect of decreasing the magnitude of cell swelling by approximately 6.3 ± 2.0% (Fig. 8, compare a and b) on average compared to that for untreated control cells. Further, it was seen that while PKCδ inhibition using Ro 31-8220 did not have a major effect on cells that were not treated with laminin (Fig. 8a), it had the effect of increasing cell swelling in laminin-treated astrocytes by 5.6 ± 1.5% (Fig. 8b), thereby demonstrating that PKC may impinge on water transport processes as well.
Discussion
In the present study, we investigate the effects of exogenous laminin-111 on the clustering of GM1, β-DG and AQP4. We showed here that the clustering occurs in a time-dependent fashion, with aggregation beginning shortly after the addition of laminin, and the total number of clusters plateauing 2 h after for β-DG, or 3 h for GM1 and AQP4. These data indicate that laminin co-assembles with β-DG first, prior to the accretion of GM1-containing lipid rafts and AQP4. It is, however, noteworthy that the combined surface area of these clusters continues to increase past that point, and peaks simultaneously at 5 h, suggesting that despite the fact that the initial clustering of β-DG and laminin precedes that of GM1 and AQP4, the continued expansion of these clusters may require lipid raft involvement.
Lipid rafts are enriched for cholesterol and sphingolipids and are thought to form liquid-ordered islands in the plasma membrane, which is otherwise primarily composed of lipids in the liquid-disordered state (Rietveld and Simons 1998). Proteins that are doubly acylated, as well as certain subunits of heterotrimeric G-proteins and Src-family kinases (Resh 1999), are concentrated in lipid rafts, an arrangement that is believed to promote their interaction and thus facilitate coordinated signal transduction. This, combined with the observation that Src-dependent phosphorylation of β-DG on tyrosine 890 appears to regulate the SH2-SH3-mediated signaling events that play a role in membrane protrusion in certain systems (James et al. 2000; Sotgia et al. 2001; Thompson et al. 2008; Vogtlander et al. 2009), prompted us to ask if the laminin- and DG-mediated clustering of lipid rafts in astrocytes might involve specific signaling events.
Our initial finding that laminin increased the levels of tyrosine phosphorylation, and induced the formation of phosphotyrosine-rich aggregates, some of which co-localized with laminin clusters, seemed to support this hypothesis. As we had previously shown that laminin induces the clustering of α- and β-DG, but not that of β1-integrin (Noël et al. 2005; Noël et al. 2009), it is more than likely that the above was effected solely by the binding of laminin to α-DG. We further demonstrated that the tyrosine kinase inhibitor genistein significantly decreases the laminin-induced clustering of DG and AQP4.
We then determined that PKCδ was significantly tyrosine-phosphorylated following laminin treatment, and additionally showed that selective inhibitors of PKC caused the dissipation of AQP4 clusters, and the fragmentation of β-DG aggregates. siRNA inhibition of PKCδ expression similarly results in a reduction in the number of AQP4 clusters. These findings suggest that the laminin-induced reorganization of these clusters, and the lipid rafts with which they are associated, could be mediated via PKC phosphorylation.
Indeed, this would be consistent with studies showing that PKC is translocated to lipid rafts in T-cells as part of its activation, and that its regulation of transcription and apoptosis requires the presence of intact lipid rafts (El Fakhry et al. 2010; Jin et al. 2008; Liu et al. 2006; Shin et al. 2008; zum Buschenfelde et al. 2010), and with the observation that PKC activation can lead to changes in the composition of detergent-resistant membrane domains (Botto et al. 2007).
Several studies have shown that the export, internalization, and permeability of AQP4 may be regulated through its phosphorylation, mediated by kinases such as protein kinase A (Carmosino et al. 2007), Ca2+/calmodulin-dependent kinase II (Gunnarson et al. 2005), casein kinase II (Kadohira et al. 2008; Madrid et al. 2001), and PKC (Han et al. 1998; McCoy et al. 2010; Okuno et al. 2008; Yamamoto et al. 2007; Zelenina et al. 2002). In particular, it has been shown that PKC activation can downregulate AQP4 function via a number of routes, decreasing its expression at the mRNA level (Nakahama et al. 1999), and accelerating its internalization from the plasma membrane (Fazzina et al. 2010; Moeller et al. 2009; Yamamoto et al. 2007; Zhu et al. 2009) and negatively modulating channel permeability via the phosphorylation of serine180 (McCoy et al. 2010; Moeller et al. 2009; Zelenina et al. 2002). The above is largely in agreement with our finding in this study that the laminin-induced phosphorylation of PKCδ was associated with a decrease in water transport, but appears somewhat inconsistent with our previous observation that laminin also causes a 3.5-fold increase in the expression of AQP4 at the cell surface of astrocytes (Tham et al. 2016). It is worth noting, however, that laminin selectively enriches the M23 isoform of AQP4 (Tham et al. 2016), which is both less permeable to water, and whose permeability is much more severely reduced by PKC phosphorylation compared to M1-AQP4 (Fenton et al. 2010). This could account for the overall decrease in the water permeability of laminin-treated cells seen here.
Based on our data, we now hypothesize that the aggregation of AQP4 in response to laminin treatment occurs through the following sequence of events: laminin first binds to DG, and, through its tendency to self-assemble, causes DG to reorganize into aggregates, which expand as additional units of laminin-bound DG are introduced. These aggregates provide foci for the recruitment of additional components of the DGC, including syntrophin, and subsequently, AQP4. As a result of this, the lipid rafts with which AQP4 are associated, as well as the molecules contained within them, including PKCδ (Liu et al. 2006), are brought into close apposition, initiating a signaling cascade that results not only in the expansion and stabilization of these clusters but also in the modulation of water permeability. In keeping with the limitations of the cultures of astrocytes, it remains to be determined whether laminin plays a role in PKC-mediated mechanism that impinges on AQP4 organization and possibly its function in polarized astrocytes in vivo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (PDF 2254 kb) Figure S1. The clustering of GM1, β-DG, and laminin increases over time. Astrocytes incubated with 15 nM laminin for various lengths of time were immunolabeled for GM1, β-DG, and laminin. Scale bar, 30 μm. Figure S2. Laminin does not affect the expression of the M1 and M23 transcripts of AQP4. RT-PCR analysis of AQP4 M1 and M23 transcripts in brain tissue, and in control and laminin-treated astrocyte cultures. The reverse transcriptase step was omitted (-RT) for a duplicate set for brain tissue and –LAM and +LAM astrocytes as a control against genomic DNA contamination. Figure S3. Laminin-induced Pyk2 tyrosine phosphorylation does not play a significant role in AQP4 clustering. Western blot analysis of the levels of phospho-Pyk2 and overall Pyk2 levels in untreated control astrocytes (-LAM), and astrocytes treated with 15 nM laminin for 3 hours (+LAM), with β-actin as a loading control (A). Phospho-Pyk2, β-DG, and β-actin levels in cells treated with laminin only (+LAM), or with laminin in combination with 5 nM staurosporine (+Stau), 0.5 μg/ml herbimycin (+Herb), or 50 μM genistein (+Gen; B). Levels of phospho-Pyk2, FAK, Pyk2, and β-actin in cells treated with scrambled control RNA (siCTL), or with siRNA targeting Pyk2 (siPyk2; C). Number of AQP4 clusters per field (top) and cluster size (bottom) in astrocytes transfected with siCTL or siPyk2, prior to being treated with 15nM laminin (D). Numbers shown for each condition represent the means (± SEMs) calculated from 45 randomly-acquired fields taken from 3 independent experiments.
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), Muscular Dystrophy Canada (MDC), Amyotrophic Lateral Sclerosis (ALS) Society of Canada and the Natural Sciences and Engineering Research Council of Canada (NSERC) to H.M, and a University Graduate Fellowship and Edward Squires Memorial Scholarship to G. N.
Abbreviations
- AChR
Acetylcholine receptor
- AKT
Protein kinase B
- AQP4
Aquaporin-4
- BBB
Blood–brain barrier
- Cav1
Caveolin 1
- CSF
Cerebrospinal fluid
- DG
Dystroglycan
- DGC
Dystrophin glycoprotein complex
- ECM
Extracellular matrix
- ERK6
Extracellular signal-regulated kinase 6
- FAK
Focal adhesion kinase
- GM1
Monosialotetrahexosylganglioside
- Grb2
Growth factor receptor-bound protein 2
- JNK
c-Jun N-terminal kinase
- Lyn
Lck/yes novel tyrosine kinase
- NMJ
Neuromuscular junction
- nNOS
Neuronal nitric oxide synthases
- PAK1
p21 (RAC1) activated kinase 1
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PKC
Protein-serine kinase C delta
- PLCg2
Phospholipase C gamma 2
- Pyk2
Protein tyrosine kinase 2
- Rac1
Ras-related C3 botulinum toxin substrate 1
- SH2/SH3
Src homology 2/3
- Sos1
SOS Ras/Rac guanine nucleotide exchange factor 1
- Src
SRC proto-oncogene, non-receptor tyrosine kinase
- Syk
Spleen tyrosine kinase
Data Accessibility Statement
All data included in this report will be made available by the authors upon reasonable request.
Compliance with Ethical Standards
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abramovici H, Hogan AB, Obagi C, Topham MK, Gee SH (2003) Diacylglycerol kinase-zeta localization in skeletal muscle is regulated by phosphorylation and interaction with syntrophins. Mol Biol Cell 14:4499–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ME, Mueller HA, Froehner SC (2001) In vivo requirement of the alpha-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J Cell Biol 155:113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Xue R, Haug FM, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP (2004) Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J 18:542–544 [DOI] [PubMed] [Google Scholar]
- Beltran-Valero de Bernabe D, Currier S, Steinbrecher A et al (2002) Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet 71:1033–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati V, Nicchia GP, Svelto M, Rapisarda C, Frigeri A, Ferroni S (2007) Functional down-regulation of volume-regulated anion channels in AQP4 knockdown cultured rat cortical astrocytes. J Neurochem 100:87–104 [DOI] [PubMed] [Google Scholar]
- Blake DJ, Hawkes R, Benson MA, Beesley PW (1999) Different dystrophin-like complexes are expressed in neurons and glia. J Cell Biol 147:645–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto L, Masserini M, Palestini P (2007) Changes in the composition of detergent-resistant membrane domains of cultured neurons following protein kinase C activation. J Neurosci Res 85:443–450 [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH et al (1996) Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84:757–767 [DOI] [PubMed] [Google Scholar]
- Campanelli JT, Roberds SL, Campbell KP, Scheller RH (1994) A role for dystrophin-associated glycoproteins and utrophin in agrin-induced AChR clustering. Cell 77:663–674 [DOI] [PubMed] [Google Scholar]
- Carmosino M, Procino G, Tamma G, Mannucci R, Svelto M, Valenti G (2007) Trafficking and phosphorylation dynamics of AQP4 in histamine-treated human gastric cells. Biol Cell 99:25–36 [DOI] [PubMed] [Google Scholar]
- Cartaud A, Stetzkowski-Marden F, Maoui A, Cartaud J (2011) Agrin triggers the clustering of raft-associated acetylcholine receptors through actin cytoskeleton reorganization. Biol Cell 103:287–301 [DOI] [PubMed] [Google Scholar]
- Cavaldesi M, Macchia G, Barca S, Defilippi P, Tarone G, Petrucci TC (1999) Association of the dystroglycan complex isolated from bovine brain synaptosomes with proteins involved in signal transduction. J Neurochem 72:1648–1655 [DOI] [PubMed] [Google Scholar]
- Ehmsen J, Poon E, Davies K (2002) The dystrophin-associated protein complex. J Cell Sci 115:2801–2803 [DOI] [PubMed] [Google Scholar]
- El Fakhry Y, Alturaihi H, Diallo D, Merhi Y, Mourad W (2010) Critical role of lipid rafts in CD154-mediated T cell signaling. Eur J Immunol 40:770–779 [DOI] [PubMed] [Google Scholar]
- Fazzina G, Amorini AM, Marmarou CR, Fukui S, Okuno K, Dunbar JG, Glisson R, Marmarou A, Kleindienst A (2010) The protein kinase C activator phorbol myristate acetate decreases brain edema by aquaporin 4 downregulation after middle cerebral artery occlusion in the rat. J Neurotrauma 27:453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton RA, Moeller HB, Zelenina M, Snaebjornsson MT, Holen T, MacAulay N (2010) Differential water permeability and regulation of three aquaporin 4 isoforms. Cell Mol Life Sci 67:829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee SH, Blacher RW, Douville PJ, Provost PR, Yurchenco PD, Carbonetto S (1993) Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J Biol Chem 268:14972–14980 [PubMed] [Google Scholar]
- Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S (1994) Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell 77:675–686 [DOI] [PubMed] [Google Scholar]
- Gousset K, Tsvetkova NM, Crowe JH, Tablin F (2004) Important role of raft aggregation in the signaling events of cold-induced platelet activation. Biochim Biophys Acta 1660(1–2):7–15 [DOI] [PubMed] [Google Scholar]
- Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR (2000) Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin–glycoprotein complex. Neuron 25:279–293 [DOI] [PubMed] [Google Scholar]
- Guadagno E, Moukhles H (2004) Laminin-induced aggregation of the inwardly rectifying potassium channel, Kir4.1, and the water-permeable channel, AQP4, via a dystroglycan-containing complex in astrocytes. Glia 47:138–149 [DOI] [PubMed] [Google Scholar]
- Gunnarson E, Axehult G, Baturina G, Zelenin S, Zelenina M, Aperia A (2005) Lead induces increased water permeability in astrocytes expressing aquaporin 4. Neuroscience 136:105–114 [DOI] [PubMed] [Google Scholar]
- Han Z, Wax MB, Patil RV (1998) Regulation of aquaporin-4 water channels by phorbol ester-dependent protein phosphorylation. J Biol Chem 273:6001–6004 [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Cuenda A, Spillantini MG, Thomas GM, Buee-Scherrer V, Cohen P, Goedert M (1999) Stress-activated protein kinase-3 interacts with the PDZ domain of alpha1-syntrophin. A mechanism for specific substrate recognition. J Biol Chem 274:12626–12631 [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Ogawa M, Tagawa K, Noguchi S, Ishihara T, Nonaka I, Arahata K (2001) Selective deficiency of alpha-dystroglycan in Fukuyama-type congenital muscular dystrophy. Neurology 57:115–121 [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP (1996) Dystroglycan: an extracellular matrix receptor linked to the cytoskeleton. Curr Opin Cell Biol 8:625–631 [DOI] [PubMed] [Google Scholar]
- Henry MD, Campbell KP (1998) A role for dystroglycan in basement membrane assembly. Cell 95:859–870 [DOI] [PubMed] [Google Scholar]
- Hibino H, Kurachi Y (2007) Distinct detergent-resistant membrane microdomains (lipid rafts) respectively harvest K(+) and water transport systems in brain astroglia. Eur J Neurosci 26:2539–2555 [DOI] [PubMed] [Google Scholar]
- Hogan A, Shepherd L, Chabot J, Quenneville S, Prescott SM, Topham MK, Gee SH (2001) Interaction of gamma 1-syntrophin with diacylglycerol kinase-zeta. Regulation of nuclear localization by PDZ interactions. J Biol Chem 276:26526–26533 [DOI] [PubMed] [Google Scholar]
- Huber VJ, Tsujita M, Yamazaki M, Sakimura K, Nakada T (2007) Identification of arylsulfonamides as Aquaporin 4 inhibitors. Bioorg Med Chem Lett 17:1270–1273 [DOI] [PubMed] [Google Scholar]
- Huber VJ, Tsujita M, Nakada T (2009) Identification of aquaporin 4 inhibitors using in vitro and in silico methods. Bioorg Med Chem 17:411–417 [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP (1992) Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355:696–702 [DOI] [PubMed] [Google Scholar]
- Jacobson C, Cote PD, Rossi SG, Rotundo RL, Carbonetto S (2001) The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at neuromuscular junctions and formation of the synaptic basement membrane. J Cell Biol 152:435–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M, Nuttall A, Ilsley JL, Ottersbach K, Tinsley JM, Sudol M, Winder SJ (2000) Adhesion-dependent tyrosine phosphorylation of (beta)-dystroglycan regulates its interaction with utrophin. J Cell Sci 113:1717–1726 [DOI] [PubMed] [Google Scholar]
- Jin ZX, Huang CR, Dong L et al (2008) Impaired TCR signaling through dysfunction of lipid rafts in sphingomyelin synthase 1 (SMS1)-knockdown T cells. Int Immunol 20:1427–1437 [DOI] [PubMed] [Google Scholar]
- Jung D, Yang B, Meyer J, Chamberlain JS, Campbell KP (1995) Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J Biol Chem 270:27305–27310 [DOI] [PubMed] [Google Scholar]
- Kadohira I, Abe Y, Nuriya M, Sano K, Tsuji S, Arimitsu T, Yoshimura Y, Yasui M (2008) Phosphorylation in the C-terminal domain of Aquaporin-4 is required for Golgi transition in primary cultured astrocytes. Biochem Biophys Res Commun 377:463–468 [DOI] [PubMed] [Google Scholar]
- Kano H, Kobayashi K, Herrmann R et al (2002) Deficiency of alpha-dystroglycan in muscle-eye-brain disease. Biochem Biophys Res Commun 291:1283–1286 [DOI] [PubMed] [Google Scholar]
- Langenbach KJ, Rando TA (2002) Inhibition of dystroglycan binding to laminin disrupts the PI3K/AKT pathway and survival signaling in muscle cells. Muscle Nerve 26:644–653 [DOI] [PubMed] [Google Scholar]
- Liu Y, Belkina NV, Graham C, Shaw S (2006) Independence of protein kinase C-delta activity from activation loop phosphorylation: structural basis and altered functions in cells. J Biol Chem 281:12102–12111 [DOI] [PubMed] [Google Scholar]
- Longman C, Brockington M, Torelli S et al (2003) Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet 12:2853–2861 [DOI] [PubMed] [Google Scholar]
- Lumeng C, Phelps S, Crawford GE, Walden PD, Barald K, Chamberlain JS (1999) Interactions between beta 2-syntrophin and a family of microtubule-associated serine/threonine kinases. Nat Neurosci 2:611–617 [DOI] [PubMed] [Google Scholar]
- Luo S, Chen Y, Lai KO, Arevalo JC, Froehner SC, Adams ME, Chao MV, Ip NY (2005) {alpha}-Syntrophin regulates ARMS localization at the neuromuscular junction and enhances EphA4 signaling in an ARMS-dependent manner. J Cell Biol 169:813–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R, Le Maout S, Barrault MB, Janvier K, Benichou S, Merot J (2001) Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. EMBO J 20:7008–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangi PA, Wieland ST, Fuhrer C (2002) Laminin-1 redistributes postsynaptic proteins and requires rapsyn, tyrosine phosphorylation, and Src and Fyn to stably cluster acetylcholine receptors. J Cell Biol 157:883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy ES, Haas BR, Sontheimer H (2010) Water permeability through aquaporin-4 is regulated by protein kinase C and becomes rate-limiting for glioma invasion. Neuroscience 168:971–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP (2002) Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418:417–422 [DOI] [PubMed] [Google Scholar]
- Moeller HB, Fenton RA, Zeuthen T, Macaulay N (2009) Vasopressin-dependent short-term regulation of aquaporin 4 expressed in Xenopus oocytes. Neuroscience 164:1674–1684 [DOI] [PubMed] [Google Scholar]
- Moukhles H, Carbonetto S (2001) Dystroglycan contributes to the formation of multiple dystrophin-like complexes in brain. J Neurochem 78:824–834 [DOI] [PubMed] [Google Scholar]
- Nakahama K, Nagano M, Fujioka A, Shinoda K, Sasaki H (1999) Effect of TPA on aquaporin 4 mRNA expression in cultured rat astrocytes. Glia 25(3):240–246 [PubMed] [Google Scholar]
- Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME (2001) Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci USA 98:14108–14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchia GP, Nico B, Camassa LM, Mola MG, Loh N, Dermietzel R, Spray DC, Svelto M, Frigeri A (2004) The role of aquaporin-4 in the blood-brain barrier development and integrity: studies in animal and cell culture models. Neuroscience 129:935–945 [DOI] [PubMed] [Google Scholar]
- Nico B, Frigeri A, Nicchia GP et al (2003) Severe alterations of endothelial and glial cells in the blood-brain barrier of dystrophic mdx mice. Glia 42:235–251 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP (1997) Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël G, Belda M, Guadagno E, Micoud J, Klocker N, Moukhles H (2005) Dystroglycan and Kir4.1 coclustering in retinal Muller glia is regulated by laminin-1 and requires the PDZ-ligand domain of Kir4.1. J Neurochem 94:691–702 [DOI] [PubMed] [Google Scholar]
- Noël G, Tham DK, Moukhles H (2009) Interdependence of laminin-mediated clustering of lipid rafts and the dystrophin complex in astrocytes. J Biol Chem 284:19694–19704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël G, Tham DKL, MacVicar BA, Moukhles H (2020) Agrin plays a major role in the coalescence of the aquaporin-4 clusters induced by gamma-1 containing laminin. J Comp Neurol 528:407–418 [DOI] [PubMed] [Google Scholar]
- Noell S, Fallier-Becker P, Beyer C, Kröger S, Mack AF, Wolburg H (2007) Effects of agrin on the expression and distribution of the water channel protein aquaporin-4 and volume regulation in cultured astrocytes. Eur J Neurosci 6(8):2109–2118 [DOI] [PubMed] [Google Scholar]
- Oak SA, Russo K, Petrucci TC, Jarrett HW (2001) Mouse alpha1-syntrophin binding to Grb2: further evidence of a role for syntrophin in cell signaling. Biochemistry 40:11270–11278 [DOI] [PubMed] [Google Scholar]
- Okumura A, Nagai K, Okumura N (2008) Interaction of alpha1-syntrophin with multiple isoforms of heterotrimeric G protein alpha subunits. FEBS J 275:22–33 [DOI] [PubMed] [Google Scholar]
- Okuno K, Taya K, Marmarou CR, Ozisik P, Fazzina G, Kleindienst A, Gulsen S, Marmarou A (2008) The modulation of aquaporin-4 by using PKC-activator (phorbol myristate acetate) and V1a receptor antagonist (SR49059) following middle cerebral artery occlusion/reperfusion in the rat. Acta Neurochirurgica Suppl 102:431–436 [DOI] [PubMed] [Google Scholar]
- Pato C, Stetzkowski-Marden F, Gaus K, Recouvreur M, Cartaud A, Cartaud J (2008) Role of lipid rafts in agrin-elicited acetylcholine receptor clustering. Chem Biol Interact 175:64–67 [DOI] [PubMed] [Google Scholar]
- Peng HB, Ali AA, Daggett DF, Rauvala H, Hassell JR, Smalheiser NR (1998) The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes Commun 5:475–489 [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90:3710–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD (1999) Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta 1451:1–16 [DOI] [PubMed] [Google Scholar]
- Rietveld A, Simons K (1998) The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim Biophys Acta 137:467–479 [DOI] [PubMed] [Google Scholar]
- Rurak J, Noel G, Lui L, Joshi B, Moukhles H (2007) Distribution of potassium ion and water permeable channels at perivascular glia in brain and retina of the Large(myd) mouse. J Neurochem 103:1940–1953 [DOI] [PubMed] [Google Scholar]
- Russo K, Di Stasio E, Macchia G, Rosa G, Brancaccio A, Petrucci TC (2000) Characterization of the beta-dystroglycan-growth factor receptor 2 (Grb2) interaction. Biochem Biophys Res Commun 274:93–98 [DOI] [PubMed] [Google Scholar]
- Sadasivam G, Willmann R, Lin S, Erb-Vogtli S, Kong XC, Ruegg MA, Fuhrer C (2005) Src-family kinases stabilize the neuromuscular synapse in vivo via protein interactions, phosphorylation, and cytoskeletal linkage of acetylcholine receptors. J Neurosci 25:10479–10493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Yang CS, Lee JY, Lee SJ, Choi HH, Lee HM, Yuk JM, Harding CV, Jo EK (2008) Mycobacterium tuberculosis lipoprotein-induced association of TLR2 with protein kinase C zeta in lipid rafts contributes to reactive oxygen species-dependent inflammatory signalling in macrophages. Cell Microbiol 10:1893–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgia F, Lee H, Bedford MT, Petrucci T, Sudol M, Lisanti MP (2001) Tyrosine phosphorylation of beta-dystroglycan at its WW domain binding motif, PPxY, recruits SH2 domain containing proteins. Biochemistry 40:14585–14592 [DOI] [PubMed] [Google Scholar]
- Sotgia F, Bonuccelli G, Bedford M et al (2003) Localization of phospho-beta-dystroglycan (pY892) to an intracellular vesicular compartment in cultured cells and skeletal muscle fibers in vivo. Biochemistry 42:7110–7123 [DOI] [PubMed] [Google Scholar]
- Spence HJ, Dhillon AS, James M, Winder SJ (2004) Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep 5:484–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetzkowski-Marden F, Gaus K, Recouvreur M, Cartaud A, Cartaud J (2006a) Agrin elicits membrane lipid condensation at sites of acetylcholine receptor clusters in C2C12 myotubes. J Lipid Res 47:2121–2133 [DOI] [PubMed] [Google Scholar]
- Stetzkowski-Marden F, Recouvreur M, Camus G, Cartaud A, Marchand S, Cartaud J (2006b) Rafts are required for acetylcholine receptor clustering. J Mol Neurosci 30:37–38 [DOI] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC (2001) A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol 154:435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham DK, Joshi B, Moukhles H (2016) Aquaporin-4 cell-surface expression and turnover are regulated by dystroglycan, dynamin, and the extracellular matrix in astrocytes. PLoS ONE 11(10):e0165439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson O, Kleino I, Crimaldi L, Gimona M, Saksela K, Winder SJ (2008) Dystroglycan, Tks5 and Src mediated assembly of podosomes in myoblasts. PLoS ONE 3:e3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajda Z, Pedersen M, Füchtbauer EM, Wertz K, Stødkilde-Jørgensen H, Sulyok E, Dóczi T, Neely JD, Agre P, Frøkiaer J, Nielsen S (2002) Delayed onset of brain edema and mislocalization of aquaporin 4 in dystrophin-null transgenic mice. Proc Natl Acad Sci USA 99:13131–13136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogtlander NP, Visch HJ, Bakker MA, Berden JH, van der Vlag J (2009) Ligation of alpha-dystroglycan on podocytes induces intracellular signaling: a new mechanism for podocyte effacement? PLoS ONE 4:e5979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M, Pope B, Cross RA, Kendrick-Jones J, Weeds AG (1992) Expression of the N-terminal domain of dystrophin in E. coli and demonstration of binding to F-actin. FEBS Lett 301:243–245 [DOI] [PubMed] [Google Scholar]
- Weston CA, Teressa G, Weeks BS, Prives J (2007) Agrin and laminin induce acetylcholine receptor clustering by convergent, Rho GTPase-dependent signaling pathways. J Cell Sci 120:868–875 [DOI] [PubMed] [Google Scholar]
- Yakubchyk Y, Abramovici H, Maillet JC, Daher E, Obagi C, Parks RJ, Topham MK, Gee SH (2005) Regulation of neurite outgrowth in N1E − 115 cells through PDZ-mediated recruitment of diacylglycerol kinase zeta. Mol Cell Biol 25:7289–7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Kuramoto H, Kadowaki M (2007) Downregulation in aquaporin 4 and aquaporin 8 expression of the colon associated with the induction of allergic diarrhea in a mouse model of food allergy. Life Sci 81:115–120 [DOI] [PubMed] [Google Scholar]
- Yukutake Y, Yasui M (2010) Regulation of water permeability through aquaporin-4. Neuroscience 168:885–891 [DOI] [PubMed] [Google Scholar]
- Zaccaria ML, Di Tommaso F, Brancaccio A, Paggi P, Petrucci TC (2001) Dystroglycan distribution in adult mouse brain: a light and electron microscopy study. Neuroscience 104:311–324 [DOI] [PubMed] [Google Scholar]
- Zelenina M, Zelenin S, Bondar AA, Brismar H, Aperia A (2002) Water permeability of aquaporin-4 is decreased by protein kinase C and dopamine. Am J Physiol Renal Physiol 283:F309–F318 [DOI] [PubMed] [Google Scholar]
- Zhou YW, Thomason DB, Gullberg D, Jarrett HW (2006) Binding of laminin alpha1-chain LG4-5 domain to alpha-dystroglycan causes tyrosine phosphorylation of syntrophin to initiate Rac1 signaling. Biochemistry 45:2042–2052 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang D, Thomason DB, Jarrett HW (2007) Laminin-induced activation of Rac1 and JNKp46 is initiated by Src family kinases and mimics the effects of skeletal muscle contraction. Biochemistry 46:14907–14916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu SM, Xiong XX, Zheng YY, Pan CF (2009) Propofol inhibits aquaporin 4 expression through a protein kinase C-dependent pathway in an astrocyte model of cerebral ischemia/reoxygenation. Anesth Analg 109(5):1493–1499. 10.1213/ANE.0b013e3181b893f3 [DOI] [PubMed] [Google Scholar]
- zum Buschenfelde CM, Wagner M, Lutzny G, Oelsner M, Feuerstacke Y, Decker T, Bogner C, Peschel C, Ringshausen I (2010) Recruitment of PKC-betaII to lipid rafts mediates apoptosis-resistance in chronic lymphocytic leukemia expressing ZAP-70. Leukemia 24:141–152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (PDF 2254 kb) Figure S1. The clustering of GM1, β-DG, and laminin increases over time. Astrocytes incubated with 15 nM laminin for various lengths of time were immunolabeled for GM1, β-DG, and laminin. Scale bar, 30 μm. Figure S2. Laminin does not affect the expression of the M1 and M23 transcripts of AQP4. RT-PCR analysis of AQP4 M1 and M23 transcripts in brain tissue, and in control and laminin-treated astrocyte cultures. The reverse transcriptase step was omitted (-RT) for a duplicate set for brain tissue and –LAM and +LAM astrocytes as a control against genomic DNA contamination. Figure S3. Laminin-induced Pyk2 tyrosine phosphorylation does not play a significant role in AQP4 clustering. Western blot analysis of the levels of phospho-Pyk2 and overall Pyk2 levels in untreated control astrocytes (-LAM), and astrocytes treated with 15 nM laminin for 3 hours (+LAM), with β-actin as a loading control (A). Phospho-Pyk2, β-DG, and β-actin levels in cells treated with laminin only (+LAM), or with laminin in combination with 5 nM staurosporine (+Stau), 0.5 μg/ml herbimycin (+Herb), or 50 μM genistein (+Gen; B). Levels of phospho-Pyk2, FAK, Pyk2, and β-actin in cells treated with scrambled control RNA (siCTL), or with siRNA targeting Pyk2 (siPyk2; C). Number of AQP4 clusters per field (top) and cluster size (bottom) in astrocytes transfected with siCTL or siPyk2, prior to being treated with 15nM laminin (D). Numbers shown for each condition represent the means (± SEMs) calculated from 45 randomly-acquired fields taken from 3 independent experiments.
Data Availability Statement
All data included in this report will be made available by the authors upon reasonable request.








