Graphical Abstract
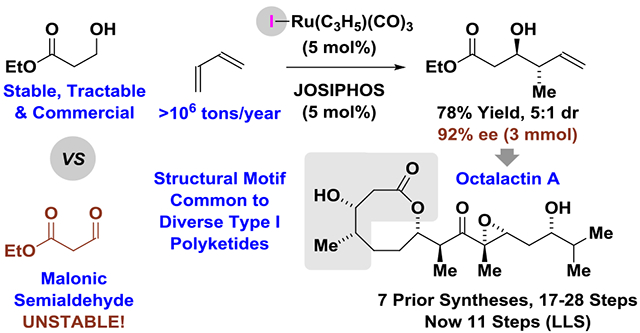
Tractable and commercially available esters (and amides) of β-hydroxy propionic acid serve as malonic semialdehyde proelectrophiles in enantioselective ruthenium-catalyzed hydrogen auto-transfer crotylations mediated by butadiene. Through iterative asymmetric butadiene-mediated crotylations of ethyl 3-hydroxypropanoate, total syntheses of the polyketide natural products octalactins A and B were achieved in fewer steps than previously possible.
As evident in the structure of erythromycin A, the first commercial polyketide antibiotic (1952), propionate motifs are pervasive among polyketides natural products, including macrolides (Figure 1). In connection with efforts to develop hydrogen auto-transfer reactions for the stereo- and site-selective conversion of lower alcohols to higher alcohols, our laboratory has advanced catalytic methods for the assembly of diverse polyketide substructures,1 including methods for diastereo- and enantioselective carbonyl crotylation via hydrogen auto-transfer from primary alcohol proelectrophiles using iridium2 or ruthenium3 catalysts and α-methyl allyl acetate or butadiene as crotylmetal pronucleophiles, respectively. Applicability of these methods to alcohols that dehydrogenate to form unstable aldehydes4 suggested the feasibility of utilizing 3-hydroxy propionates as malonic semialdehyde proelectrophiles in carbonyl crotylations via hydrogen auto-transfer. Here, using iodide-bound ruthenium-JOSIPHOS complexes recently developed in our laboratory,5 we report efficient diastereo- and enantioselective butadiene-mediated anti-crotylations3f of 3-hydroxy propionate esters and amides to form a structural motif found in diverse polyketide natural products. Using this method, total syntheses of octalactins A and B, were completed in fewer steps than previously possible.6,7
Figure 1.
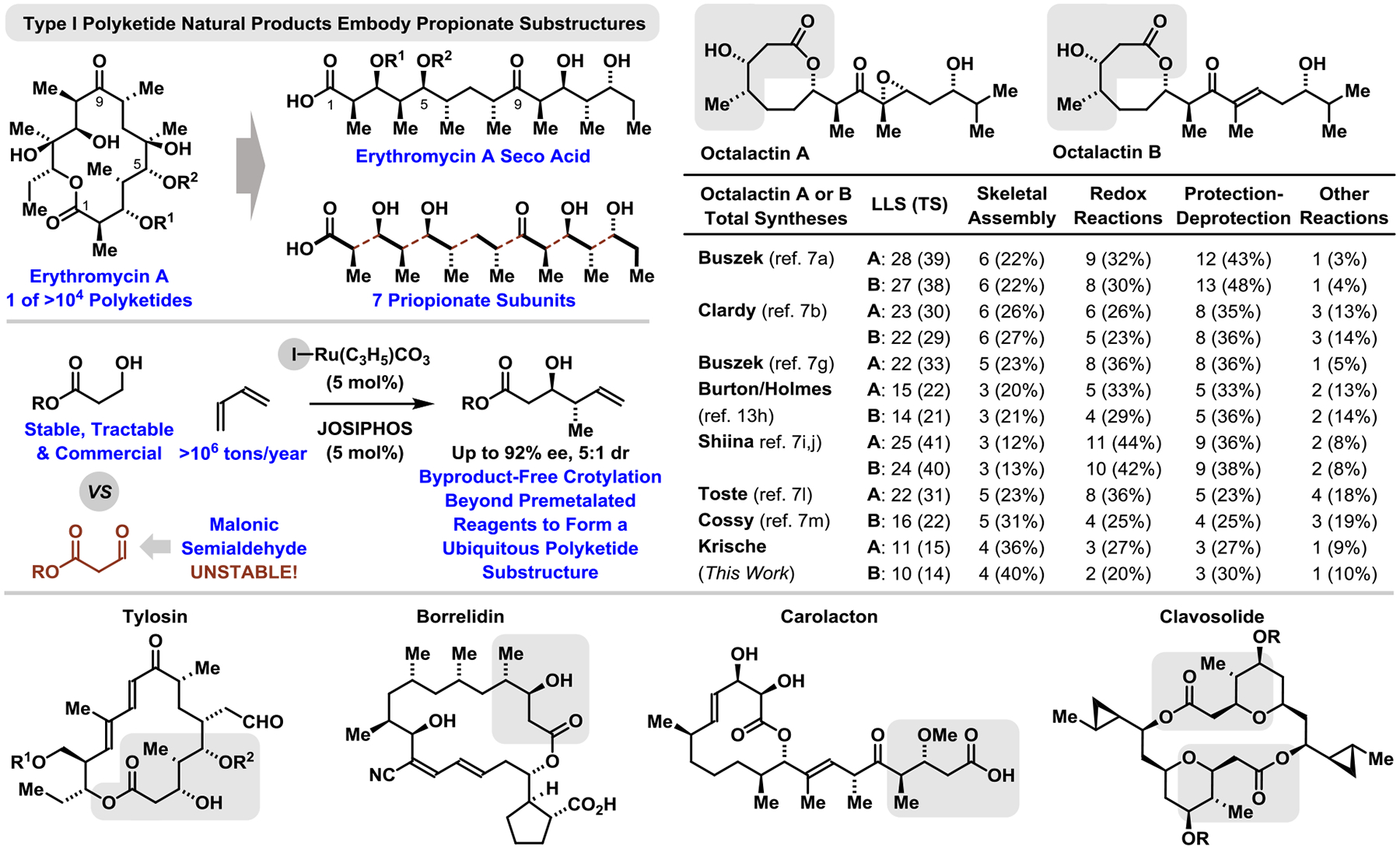
Crotylation of 3-hydroxy propionates streamlines the total syntheses of octalactins A and B (LLS = Longest Linear Sequence). See Supporting Information for graphical summaries of prior syntheses.
In an initial experiment, ethyl 3-hydroxy propionate 2a (100 mol%) was exposed to butadiene 1 (1 atm) in the presence of the iodide-bound ruthenium catalyst derived from Ru(I)(CO)3(η3-C3H5) (5 mol%) and the JOSIPHOS ligand SL-J502–1 (5 mol%) in isooctane solvent (0.5 M) with added trifluoroethanol (300 mol%) at 100 °C.3f To our dismay, only trace quantities of the targeted product of anti-crotylation 3a was formed. However, upon a survey of different solvents it was found that reactions run in methyl tert-butyl ether (MTBE) were highly efficient, delivering the product of anti-crotylation 3a in 78% yield as a 5:1 mixture of diastereomers favoring the anti-isomer with high levels of enantiomeric enrichment (92% ee) at 3 mmol scale (Scheme 1). These conditions for butadiene-mediated diastereo- and enantioselective anti-crotylation were applicable to diverse 3-hydroxy propionate esters and amides 2a-2h. Reactions of β-hydroxy ketones were inefficient, but conversion of Weinreb amide 2f to adduct 3f potentially provides access to ketone-containing derivatives.8
Scheme 1.

Enantioselective butadiene-mediated anti-crotylation of β-hydroxy esters and amides 2a-2h.a
aYields of material isolated by silica gel chromatography. Diastereoselectivities were determined by 1H NMR analysis of crude reaction mixtures. Enantioselectivities were determined by chiral stationary phase HPLC analysis. bCsI (10 mol%). c115 ⁰C. See Supporting Information for further details.
As described above, compound 3a comprises a structural motif that is ubiquitous among type I polyketide natural products (Figure 1). Hence, to highlight the utility of the present method for anti-crotylation, it was applied to the synthesis of octalactins A and B.6,7 Retrosynthetically, it was envisioned that octalactin A, the more complex congener, could be accessed via triorganozincate-mediated vinylation9 of the aldehyde, Fragment A (Scheme 2). While this disconnection has been used frequently in prior syntheses of the octalactins (under Nozaki-Hiyama-Kishi (NHK) conditions7a,g,h,l or using vinyllithium reagents7b,i,j,m), it has never been achieved with high levels of substrate-directed or catalyst-directed stereocontrol. However, our experience with triorganozincate-mediated vinylation4,9f suggest these reagents are uniquely effective in substrate-directed stereoselective aldehyde vinylations. Fragment A is potentially accessible via iterative butadiene-mediated anti-crotylation of ethyl hydroxy propionate 2a. Fragment B was envisioned to arise via enantioselective iridium-catalyzed allylation of isobutanol10 followed by cross-metathesis of the resulting homoallylic alcohol with vinylpinacolboronate followed by iodination of the carbon-boron bond.11
Scheme 2.

Retrosynthetic analysis of octalactin A.
The synthesis of Fragment A begins with butadiene-mediated anti-crotylations of ethyl hydroxy propionate 2a, which on 3 mmol scale gave the homoallylic alcohol 3a in 78% yield with 5:1 the anti-diastereoselectivity and 92% ee (Scheme 3). In Cossy’s synthesis of octalactin B, an analogous crotylation was performed.7m The malonic semi-aldehyde (generated via ozonlysis of methyl 3-butenoate) was reacted with a TADDOL-modified crotyltitanium reagent that requires a 5-step preparation.12 Hence, our butadiene-mediated process streamlines the formation of 3a by removing a total of 6 steps. Triisopropylsilylation of the secondary alcohol followed by hydroformylation-reduction using a rhodium-xantphos catalyst provides the homologous alcohol 4 with good linear vs branched regioselectivity (8:1).13 A second butadiene-mediated anti-crotylation provided adduct 5 in 91% yield with high stereocontrol. Silanolate-mediated ester hydrolysis14 followed by Shiina lactonization15 and oxidative cleavage of the terminal olefin16 provides Fragment A.
Scheme 3.

Synthesis of Fragment A.a
aYields of material isolated by silica gel chromatography. Diastereoselectivities and linear:branched regioselectivities were determined by 1H NMR analysis of crude reaction mixtures. See Supporting Information for further details.
The preparation of Fragment B begins with the π-allyliridium-C,O-benzoate-catalyzed allylation of isobutanol10 to provide homoallylic alcohol 6 in 88% yield with high levels of enantiocontrol (94% ee) (Scheme 4). Exposure of homoallylic alcohol 6 to (2-propenyl)pinacolboronate in the presence of the Hoveyda-Grubbs II catalyst (added via syringe pump addition), affects cross-metathesis with complete (Z)-stereoselectivity.11a Subsequent TBS protection and iodination of the C-B bond11 provides Fragment B in a total of 4 steps (LLS). O-Silylation prior to cross-metathesis led to an inseparable mixture of (Z:E)-diastereomers (5:1).
Scheme 4.

Synthesis of Fragment B.a
aYields of material isolated by silica gel chromatography. Enantioselectivities were determined by chiral stationary phase HPLC analysis. See Supporting Information for further details.
With Fragments A and B in hand, the triorganozincate-mediated fragment union was attempted. To our delight, the allylic alcohol 7 was isolated in 60% yield with complete diastereocontrol (>20:1) to form the desired C9-(R) epimer, as confirmed by conversion to octalactin A (vide infra) (Scheme 5). The observed stereochemistry is consistent with Evans’ models for merged 1,2- and 1,3-asymmetric induction.17 Notably, in all prior syntheses that enact fragment union under NHK conditions7a,g,h,l or using vinyllithium reagents,7b,i,j,m modest selectivities were observed (1:1 to 2:1 dr). Evans observed in aldol additions to β-OAc aldehydes that 1,3-anti- and 1,3-syn-products formed with low diastereocontrol (43:57 ratio) with calculations suggesting the minimally reinforcing nature of the β-acyloxy moiety.17 Hence, stereocontrol in additions to aldehydes akin to Fragment A are anticipated to depend more on the α-stereocenter vis-à-vis the Felkin-Anh model, which, in turn, depends on nucleophile size. Triorganozincates are larger than neutral vinylchromium (NHK) and vinyllithium reagents, augmenting interactions with the α-stereocenter for heightened Felkin-Anh control. Indeed, beyond octalactin, the enhanced diastereoselectivity of triorganozincate versus NHK or vinyllithium addition has been documented,9c,f and the triorganozincate-mediated fragment union requires only a slight excess of the vinyliodide, unlike corresponding NHK reactions.7a,g,h,l The allylic alcohol 7 was subjected to hydroxyl-directed epoxidation per Buszek’s report,7a Dess-Martin oxidation and HF•pyridine-mediated silyl deprotection to deliver octalactin A in 11 steps (LLS) from tractable, commercial ethyl 3-hydroxy propionate. In accordance with previous synthetic reports,7 an analogous oxidation-deprotection sequence delivered octalactin B in 10 steps (LLS).
Scheme 5.
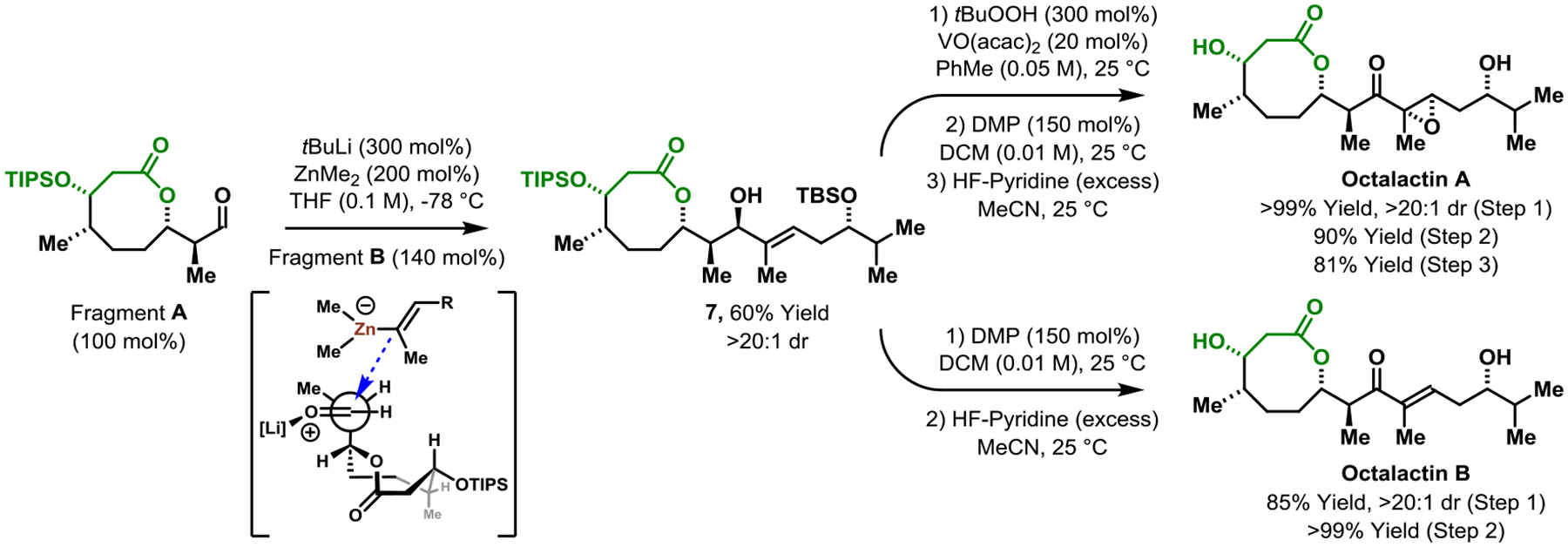
Union of Fragments A and B via triorganozincate addition and total syntheses of octalactins A and B a
aYields of material isolated by silica gel chromatography. See Supporting Information for further details.
In summary, we demonstrate that highly tractable β-hydroxy propionates serve as malonic semialdehyde proelectrophiles in enantioselective butadiene-mediated crotylations, thus enabling direct access to ubiquitous polyketide substructures. Using this method, octalactins A and B were prepared in fewer steps than previously possible. It is our hope the present method will aid other researchers engaged in the daunting enterprise of target-oriented synthesis.
Supplementary Material
Acknowledgments.
We thank the Welch Foundation (F-0038) and the NIH (R01 GM093905) for support of this research.
Footnotes
The authors declare no competing financial interest.
Supporting Information Available. Experimental procedures, spectroscopic data for all new compounds (1H NMR, 13C NMR, IR, HRMS). This material is available free of charge via the internet at http://pubs.acs.org.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
REFERENCES
- (1).For a recent review on polyketide construction via hydrogen auto-transfer, see:Ortiz E; Saludares C; Wu J; Cho Y; Santana CG; Krische MJ Carbonyl Allylation and Crotylation: Historical Perspective, Relevance to Polyketide Synthesis, and Evolution of Enantioselective Ruthenium-Catalyzed Hydrogen Auto-Transfer Processes. Synthesis 2023, 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) For iridium-catalyzed aldehyde crotylations of alcohol proelectrophiles mediated by α-methyl allyl acetate, see: Kim IS; Han SB; Krische MJ anti-Diastereo- and Enantioselective Carbonyl Crotylation from the Alcohol or Aldehyde Oxidation Level Employing a Cyclometallated Iridium Catalyst: α-Methyl Allyl Acetate as a Surrogate to Preformed Crotylmetal Reagents. J. Am. Chem. Soc 2009, 131, 2514–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gao X; Townsend IA; Krische MJ Enhanced anti-Diastereo- and Enantioselectivity in Alcohol-Mediated Carbonyl Crotylation Using an Isolable Single Component Iridium Catalyst. J. Org. Chem 2011, 76, 2350–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gao X; Han H; Krische MJ Direct Generation of Acyclic Polypropionate Stereopolyads via Double Diastereo- and Enantioselective Iridium-Catalyzed Crotylation of 1,3-Diols: Beyond Stepwise Carbonyl Addition in Polyketide Construction. J. Am. Chem. Soc 2011, 133, 12795–12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) For ruthenium-catalyzed aldehyde crotylations of alcohol proelectrophiles mediated by butadiene, see: Shibahara F; Bower JF; Krische MJ Ruthenium-Catalyzed C−C Bond Forming Transfer Hydrogenation: Carbonyl Allylation from the Alcohol or Aldehyde Oxidation Level Employing Acyclic 1,3-Dienes as Surrogates to Preformed Allyl Metal Reagents. J. Am. Chem. Soc 2008, 130, 6338–6339. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zbieg JR; Moran J; Krische MJ Diastereo- and Enantioselective Ruthenium-Catalyzed Hydrohydroxyalkylation of 2-Silyl-Butadienes: Carbonyl syn-Crotylation from the Alcohol Oxidation Level. J. Am. Chem. Soc 2011, 133, 10582–10586. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zbieg JR; Yamaguchi E; McInturff EL; Krische MJ Enantioselective CH Crotylation of Primary Alcohols via Hydrohydroxyalkylation of Butadiene. Science 2012, 336, 324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) McInturff EL; Yamaguchi E; Krische MJ Chiral-Anion-Dependent Inversion of Diastereo- and Enantioselectivity in Carbonyl Croylation via Ruthenium-Catalyzed Butadiene Hydrohydroxyalkylation. J. Am. Chem. Soc 2012, 134, 20628–20631. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Pantin M; Hubert JG; Söhnel T; Brimble MA; Furkert DP Stereochemical Characterization of Polyketide Stereotriads Synthesized via Hydrogen-Mediated Asymmetric syn-Crotylation. J. Org. Chem 2017, 82, 11225–11229. [DOI] [PubMed] [Google Scholar]; (f) Ortiz E, Spinello BJ, Cho Y, Wu J & Krische MJ Stereo- and Site-Selective Crotylation of Alcohol Pronucleophiles via Ruthenium-Catalyzed Hydrogen Auto-Transfer Mediated by Methylallene and Butadiene. Angew. Chem. Int. Ed 2022, 61, e202212814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For a recent example, see:Meyer CC; Verboom KL; Evarts MM; Jung W-O; Krische MJ Allyl Alcohol as an Acrolein Equivalent in Enantioselective C-C Coupling: Total Synthesis of Amphidinolides R, J, and S. J. Am. Chem. Soc 2023, 145, 8242–8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ortiz E; Shezaf JZ; Chang Y-H; GoncÇalves TP; Huang K-W; Krische MJ Understanding Halide Counterion Effects in Enantioselective Ruthenium-Catalyzed Carbonyl (α-aryl)allylation: Alkynes as Latent Allenes and Trifluoroethanol-Enhanced Turnover in the Conversion of Ethanol to Higher Alcohols via Hydrogen Auto-Transfer. J. Am. Chem. Soc 2021, 143, 16709–16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).For isolation and structure determination of octalactins A and B, see:Tapiolas DM; Roman M; Fenical W; Stout TJ; Clardy J Octalactins A and B: cytotoxic eight-membered-ring lactones from a marine bacterium, Streptomyces sp. J. Am. Chem. Soc 1991, 113, 4682–4683. [Google Scholar]
- (7).(a) For total and formal syntheses of octalactins A and B, see: Buszek KR; Sato N; Jeong Y Total Synthesis of Octalactin A and B. J. Am. Chem. Soc 1994, 116, 5511–5512. [Google Scholar]; (b) McWilliams JC; Clardy J Total Synthesis of (+)-Octalactins A and B: Unusual Metabolites from a Marine Microbe. J. Am. Chem. Soc 1994, 116, 8378–8379. [Google Scholar]; (c) Buszek KR; Jeong Y An Improved Synthesis of the Octalactins. Tetrahedron Lett. 1995, 36, 7189–7192. [Google Scholar]; (d) Andrus MB; Argade AB Synthesis of Octalactin Lactone and Side Chain. Tetrahedron Lett. 1996, 37, 5049–5052. [Google Scholar]; (e) Kodama M; Matsushita M; Terada Y; Takeuchi A; Yoshio S; Fukuyama Y Enantioselective Synthesis of Octalactin A. Chem. Lett 1997, 117–118. [Google Scholar]; (f) Inoue S; Iwabuchi Y; Irie H; Hatakeyama S A New Stereoselective Route to (−)-Octalactin A Based on Intramolecular SmI2 Promoted Reformatsky Reaction. Synlett 1998, 7, 735–736. [Google Scholar]; (g) Buszek KR; Sato N; Jeong Y Total Synthesis of Octalactin A via Ring-Closing Metathesis Reaction. Tetrahedron Lett. 2002, 43, 181–184. [Google Scholar]; (h) O’Sullivan PT; Buhr W; Fuhry MAM; Harrison JR; Davies JE; Feeder N; Marshall DR; Burton JW; Holmes AB A Concise Synthesis of the Octalactins. J. Am. Chem. Soc 2004, 126, 2194–2207. [DOI] [PubMed] [Google Scholar]; (i) Shiina I; Oshiumi H; Hashizume M; Yamai Y; Ibuka R Asymmetric Total Synthesis of Octalactin B Using a New and Rapid Lactonization. Tetrahedron Lett. 2004, 45, 543–547. [Google Scholar]; (j) Shiina I; Hashizume M; Yamai Y; Oshiumi H; Shimazaki T; Takasuna Y; Ibuka R Enantioselective Total Synthesis of Octalactin A Using Asymmetric Aldol Reactions and a Rapid Lactonization to Form a Medium-Sized Ring. Chem. Eur. J 2005, 11, 6601–6608. [DOI] [PubMed] [Google Scholar]; (k) Aird JI; Hulme AN; White JW An Evans−Tishchenko−Ring-Closing Metathesis Approach to Medium-Ring Lactones. Org. Lett 2007, 9, 631–634. [DOI] [PubMed] [Google Scholar]; (l) Radosevich AT; Chan VS; Shih H-W; Toste FD Synthesis of (−)-Octalactin A by a Strategic Vanadium-Catalyzed Oxidative Kinetic Resolution. Angew. Chem., Int. Ed 2008, 47, 3755–3758. [DOI] [PubMed] [Google Scholar]; (m) Dinh M-T; Bouzbouz S; Péglion J-L; Cossy J Synthetic Efforts toward the Synthesis of Octalactins. Tetrahedron 2008, 64, 5703–5710. [Google Scholar]
- (8).For a review, see:Nowak M Weinreb Amides. Synlett 2015, 561–562. [Google Scholar]
- (9).(a) For use of triorganozincates in diastereoselective aldehyde vinylation, see ref. 4 and the following reports: Williams DR & Kissel WS Total Synthesis of (+)-Amphidinolide J. J. Am. Chem. Soc 1998, 120, 11198–11199. [Google Scholar]; (b) Messenger BT; Davidson BS Synthetic Studies toward the Microtubule-stabilizing Agent Laulimalide: Synthesis of the C15–C28 Fragment. Tetrahedron Lett. 2001, 42, 801–803. [Google Scholar]; (c) Hayashi Y; Shoji M; Ishikawa H; Yamaguchi J; Tamura T; Imai H; Nishigaya Y; Takabe K; Kakeya H; Osada H The Asymmetric Total Synthesis of (+)-Cytotrienin A, an Ansamycin-Type Anticancer Drug. Angew. Chem. Int. Ed 2008, 47, 6657–6660. [DOI] [PubMed] [Google Scholar]; (d) Zenato C; Pignataro L; Ambrosi A; Hao Z; Gennari C A Highly Stereoselective Total Synthesis of (+)-9-epi-Dictyostatin. Eur. J. Org. Chem 2010, 5767–5771. [Google Scholar]; (e) Geist JG; Barth R; Roush WR Enantioselective Synthesis of the C(1)-C(11) Fragment of Tedanolide C. Org. Lett 2013, 15, 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Lee DS; Maejima S; Krische MJ Synthesis of the C28–C41 Side Chain of (Proposed) Neaumycin B: A Contiguous Stereohextet. Org. Lett 2023, 25, 6763–6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).For a recent perspective, see:Shezaf JZ; Santana CG; Ortiz E; Meyer CC; Liu P; Sakata K; Huang K-W; Krische MJ Leveraging the Stereochemical Complexity of Octahedral Diastereomeric-at-Metal Catalysts to Unlock Regio-, Diastereo-, and Enantioselectivity in Alcohol-Mediated C–C Couplings via Hydrogen Transfer. J. Am. Chem. Soc 2024, 146, 7905–7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11) (a).Morrill C; Grubbs RH Synthesis of Functionalized Vinyl Boronates via Ruthenium-Catalyzed Olefin Cross-Metathesis and Subsequent Conversion to Vinyl Halides. J. Org. Chem 2003, 68, 6031–6034. [DOI] [PubMed] [Google Scholar]; (b) Brown HC; Hamaoka T; Ravindran N Reaction of Alkenylboronic Acids with Iodine under the Influence of Base. A Simple Procedure for the Stereospecific Conversion of Terminal Alkynes into trans-1-Alkenyl Iodides via Hydroboration. J. Am. Chem. Soc 1973, 95, 5768–5788. [Google Scholar]
- (12).Hafner A; Duthaler RO; Marti R; Rihs G; Rothe-Streit P; Schwarzenbach FJ Enantioselective Allyltitanation of Aldehydes with Cyclopentadienyldialkoxyallyltitantium Complexes. J. Am. Chem. Soc 1992, 114, 2321–2336. [Google Scholar]
- (13).Kranenburg M; van der Burgt YEM; Kamer PCJ; van Leeuwen PWNM; Goubitz KG; Fraanje J New Diphosphine Ligands Based on Heterocyclic Aromatics Inducing Very High Regioselectivity in Rhodium-Catalyzed Hydroformylation: Effect of the Bite Agle. Organometallics 1995, 14, 3081–3089. [Google Scholar]
- (14).Laganis ED; Chenard BL Metal Silanoates: Organic Soluble Equivalents for O−2. Tetrahedron Lett. 1984, 25, 5831–5834. [Google Scholar]
- (15).Shiina I; Kubota M; Ibuka R A Novel and Efficient Macrolactonization of ω-Hydroxycarboxylic Acids Using 2-Methyl-6-Nitrobenzoic Anhydride (MNBA). Tetrahedron Lett. 2002, 43, 7535–7539. [Google Scholar]
- (16).Pappo R; Allen D; Lemieux R; Johnson W Osmium Tetroxide-Catalyzed Periodate Oxidation of Olefinic Bonds. J. Org. Chem 1956, 21, 478–479. [Google Scholar]
- (17).Evans DA; Duffy JL; Yang MG A Stereochemical Model for Merged 1,2- and 1,3-Asymmetric Induction in Diastereoselective Mukaiyama Aldol Addition Reactions and Related Processes. J. Am. Chem. Soc 1996, 118, 4322–4343. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.


