Abstract
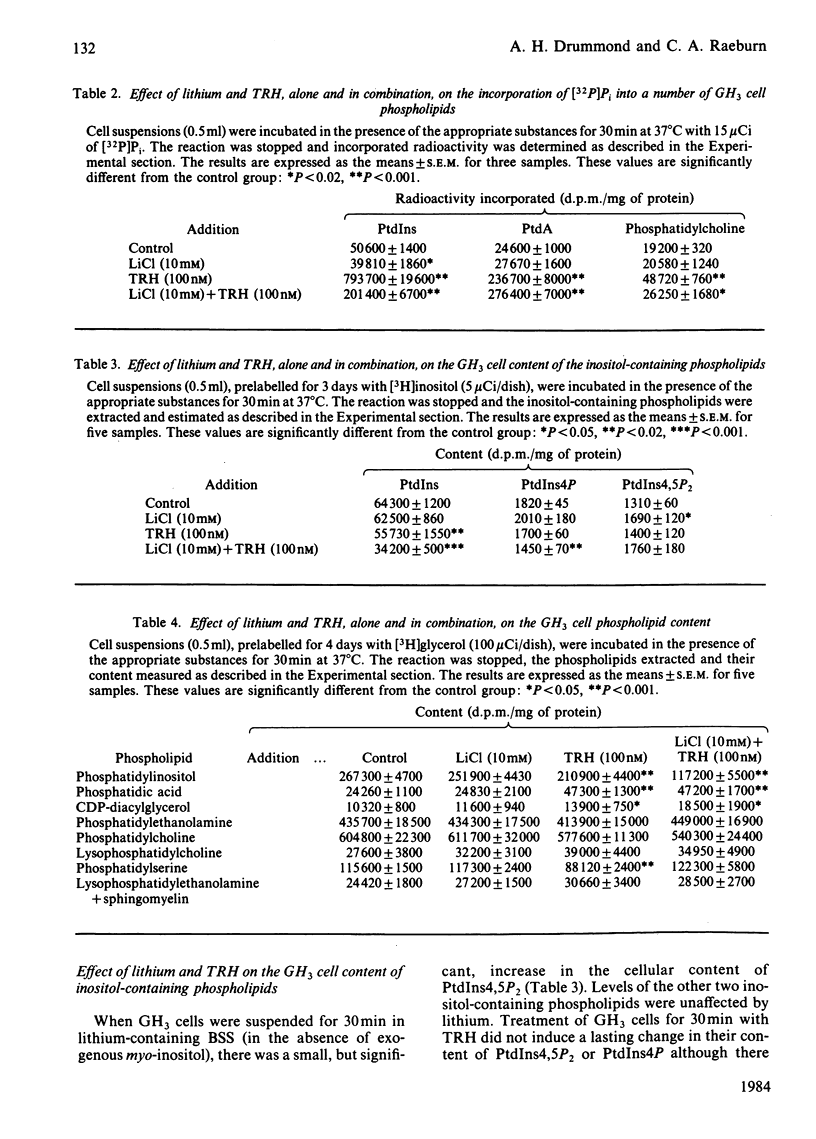
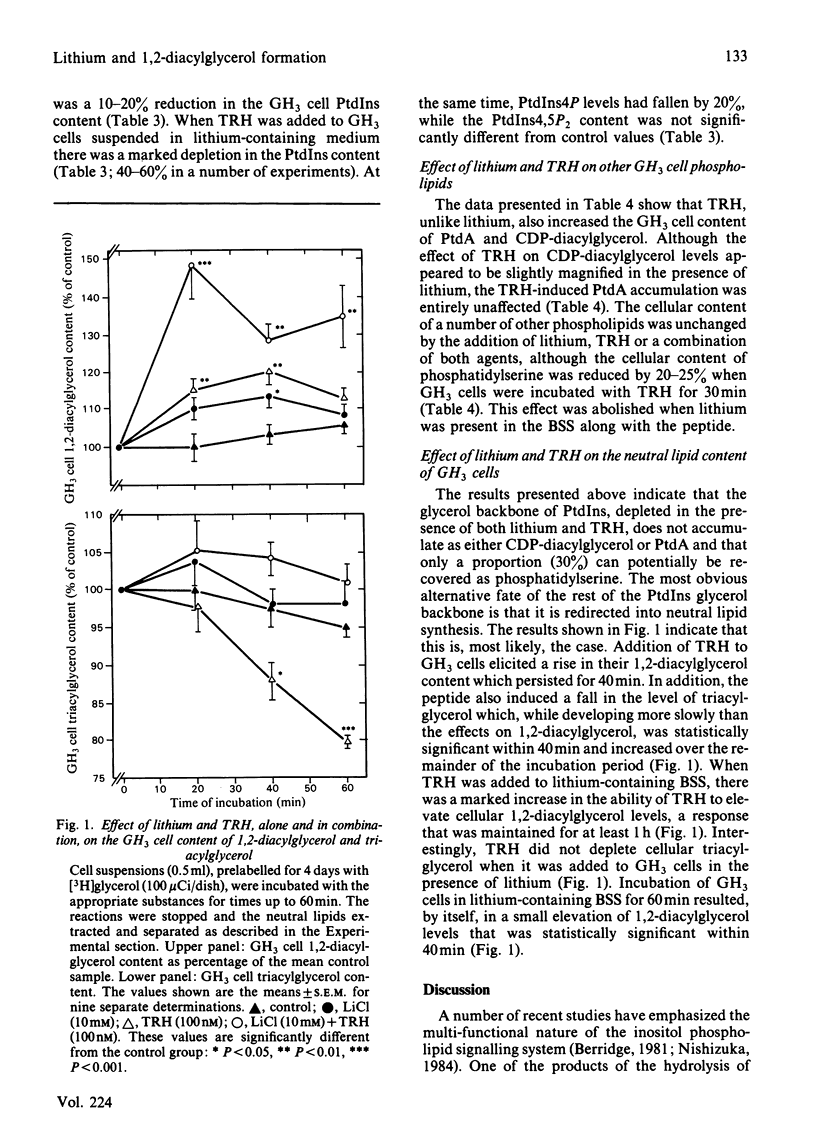
Treatment of GH3 cells with thyrotropin-releasing hormone (TRH) for periods up to 60 min resulted in a prolonged reduction in the cellular content of phosphatidylinositol (PtdIns) with no lasting change in the levels of the other inositol-containing phospholipids. Accompanying this was a maintained increase in the GH3 cell 1,2-diacylglycerol content and a slower decline in the level of cellular triacylglycerol. When the cells were suspended in lithium-containing balanced salt solution for 30 min (in the absence of exogenous myo-inositol), there was a 15% decrease in GH3 cell inositol levels. This was associated with a small, but significant, increase in the cellular content of phosphatidylinositol 4,5-bisphosphate (PtdIns4,5P2) and 1,2-diacylglycerol. Addition of TRH to cells suspended in lithium-containing medium depleted cellular inositol levels by around 65% within 30 min. By this time, there was also a 50% reduction in the cellular content of PtdIns and a 20% reduction in phosphatidylinositol 4-phosphate (PtdIns4P). Control levels of PtdIns4,5P2 were maintained in the combined presence of TRH and lithium. Under those conditions, TRH no longer depleted cellular triacylglycerol and there was a marked increase in the ability of TRH to elevate the GH3 cell content of 1,2-diacylglycerol. The effect of TRH on the cellular content of phosphatidic acid was not altered by the presence of lithium. The results show, firstly, that when PtdIns resynthesis is inhibited by lithium-induced inositol depletion, its glycerol backbone accumulates, at least in part, in 1,2-diacylglycerol and, secondly, that GH3 cells preserve their cellular levels of PtdIns4,5P2 in the face of a considerable reduction in the cellular content of PtdIns.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar R. A., Taft W. C., Abdel-Latif A. A. Effects of corticotropin-(1-24)-tetracosapeptide on polyphosphoinositide metabolism and protein phosphorylation in rabbit iris subcellular fractions. J Neurochem. 1983 Nov;41(5):1460–1468. doi: 10.1111/j.1471-4159.1983.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Allison J. H., Blisner M. E. Inhibition of the effect of lithium on brain inositol by atropine and scopolamine. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1332–1338. doi: 10.1016/0006-291x(76)90342-9. [DOI] [PubMed] [Google Scholar]
- Allison J. H., Stewart M. A. Reduced brain inositol in lithium-treated rats. Nat New Biol. 1971 Oct 27;233(43):267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- Aloyo V. J., Zwiers H., Gispen W. H. B-50 protein kinase and kinase C in rat brain. Prog Brain Res. 1982;56:303–315. doi: 10.1016/S0079-6123(08)63781-4. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Phosphatidylinositol hydrolysis: a multifunctional transducing mechanism. Mol Cell Endocrinol. 1981 Nov;24(2):115–140. doi: 10.1016/0303-7207(81)90055-1. [DOI] [PubMed] [Google Scholar]
- Calderon P., Furnelle J., Christophe J. In vitro lipid metabolism in the rat pancreas. I. Basal lipid metabolism. Biochim Biophys Acta. 1979 Sep 28;574(3):379–390. doi: 10.1016/0005-2760(79)90234-0. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes P., Michell R. H. Phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: lipids in search of a function. Cell Calcium. 1982 Oct;3(4-5):467–502. doi: 10.1016/0143-4160(82)90031-8. [DOI] [PubMed] [Google Scholar]
- Drummond A. H., Bushfield M., Macphee C. H. Thyrotropin-releasing hormone-stimulated [3H]inositol metabolism in GH3 pituitary tumor cells. Studies with lithium. Mol Pharmacol. 1984 Mar;25(2):201–208. [PubMed] [Google Scholar]
- Enyedi A., Faragó A., Sarkadi B., Szász I., Gárdos G. Cyclic AMP-dependent protein kinase stimulates the formation of polyphosphoinositides in the plasma membranes of different blood cells. FEBS Lett. 1983 Sep 5;161(1):158–162. doi: 10.1016/0014-5793(83)80751-0. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C., Thaw C. Calcium influx is not required for TRH to elevate free cytoplasmic calcium in GH3 cells. Endocrinology. 1983 Oct;113(4):1522–1524. doi: 10.1210/endo-113-4-1522. [DOI] [PubMed] [Google Scholar]
- Gillon K. R., Hawthorne J. N. Sorbitol, inositol and nerve conduction in diabetes. Life Sci. 1983 Apr 25;32(17):1943–1947. doi: 10.1016/0024-3205(83)90045-0. [DOI] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., Dekker A., Wirtz K. W., Gispen W. H. Corticotropin-(1--24)-tetracosapeptide affects protein phosphorylation and polyphosphoinositide metabolism in rat brain. Biochem J. 1981 Jan 15;194(1):283–291. doi: 10.1042/bj1940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles J., Zwiers H., van Dongen C. J., Schotman P., Wirtz K. W., Gispen W. H. Modulation of brain polyphosphoinositide metabolism by ACTH-sensitive protein phosphorylation. Nature. 1980 Aug 7;286(5773):623–625. doi: 10.1038/286623a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Litosch I., Lin S. H., Fain J. N. Rapid changes in hepatocyte phosphoinositides induced by vasopressin. J Biol Chem. 1983 Nov 25;258(22):13727–13732. [PubMed] [Google Scholar]
- Macphee C. H., Drummond A. H. Thyrotropin-releasing hormone stimulates rapid breakdown of phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 4-phosphate in GH3 pituitary tumor cells. Mol Pharmacol. 1984 Mar;25(2):193–200. [PubMed] [Google Scholar]
- Martin T. F. Thyrotropin-releasing hormone rapidly activates the phosphodiester hydrolysis of polyphosphoinositides in GH3 pituitary cells. Evidence for the role of a polyphosphoinositide-specific phospholipase C in hormone action. J Biol Chem. 1983 Dec 25;258(24):14816–14822. [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pollard A. D., Brindley D. N. Effects of vasopressin and corticosterone on fatty acid metabolism and on the activities of glycerol phosphate acyltransferase and phosphatidate phosphohydrolase in rat hepatocytes. Biochem J. 1984 Jan 15;217(2):461–469. doi: 10.1042/bj2170461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi M. J., Gershengorn M. C. Thyroliberin stimulates rapid hydrolysis of phosphatidylinositol 4,5-bisphosphate by a phosphodiesterase in rat mammotropic pituitary cells. Evidence for an early Ca2+-independent action. Biochem J. 1983 Nov 15;216(2):287–294. doi: 10.1042/bj2160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebecchi M. J., Kolesnick R. N., Gershengorn M. C. Thyrotropin-releasing hormone stimulates rapid loss of phosphatidylinositol and its conversion to 1,2-diacylglycerol and phosphatidic acid in rat mammotropic pituitary cells. Association with calcium mobilization and prolactin secretion. J Biol Chem. 1983 Jan 10;258(1):227–234. [PubMed] [Google Scholar]
- Rebecchi M. J., Monaco M. E., Gershengorn M. C. Thyrotropin releasing hormone rapidly enhances [32P]orthophosphate incorporation into phosphatidic acid in cloned GH3 cells. Biochem Biophys Res Commun. 1981 Jul 16;101(1):124–130. doi: 10.1016/s0006-291x(81)80019-8. [DOI] [PubMed] [Google Scholar]
- Schacht J. Extraction and purification of polyphosphoinositides. Methods Enzymol. 1981;72:626–631. doi: 10.1016/s0076-6879(81)72054-8. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Roduit C., Zahnd G. R. Polyphosphoinositide hydrolysis by phospholipase C is accelerated by thyrotropin releasing hormone (TRH) in clonal rat pituitary cells (GH3 cells). FEBS Lett. 1984 Mar 12;168(1):54–60. doi: 10.1016/0014-5793(84)80205-7. [DOI] [PubMed] [Google Scholar]
- Schlegel W., Roduit C., Zahnd G. Thyrotropin releasing hormone stimulates metabolism of phosphatidyl inositol in GH3 cells. A possible mechanism in stimulus-response coupling. FEBS Lett. 1981 Nov 2;134(1):47–49. doi: 10.1016/0014-5793(81)80547-9. [DOI] [PubMed] [Google Scholar]
- Sobel A., Tashjian A. H., Jr Distinct patterns of cytoplasmic protein phosphorylation related to regulation of synthesis and release of prolactin by GH cells. J Biol Chem. 1983 Sep 10;258(17):10312–10324. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Sutton C. A., Martin T. F. Thyrotropin-releasing hormone (TRH) selectively and rapidly stimulates phosphatidylinositol turnover in GH pituitary cells: a possible second step of TRH action. Endocrinology. 1982 Apr;110(4):1273–1280. doi: 10.1210/endo-110-4-1273. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Egawa K. CDP-diglyceride:inositol transferase from rat liver. Purification and properties. J Biol Chem. 1977 Aug 10;252(15):5419–5423. [PubMed] [Google Scholar]
- Yavin E., Zutra A. Separation and analysis of 32P-labeled phospholipids by a simple and rapid thin-layer chromatographic procedure and its application to cultured neuroblastoma cells. Anal Biochem. 1977 Jun;80(2):430–437. doi: 10.1016/0003-2697(77)90665-0. [DOI] [PubMed] [Google Scholar]
- Zawalich W., Brown C., Rasmussen H. Insulin secretion: combined effects of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Dec 16;117(2):448–455. doi: 10.1016/0006-291x(83)91221-4. [DOI] [PubMed] [Google Scholar]


