Abstract
Guillain-Barré syndrome (GBS) stands out as the most prevalent and severe acute immune-mediated paralytic neuropathy. Approximately 30% of patients experience respiratory failure necessitating admission to the intensive care unit (ICU) and invasive mechanical ventilation. The management of diseases concomitant with acute respiratory distress syndrome (ARDS) poses significant challenges. This case report illustrates the swift development of ARDS in a patient with GBS, explores the utility of the biomarker neurofilament light chain, and highlights the unexpected advantages of proactive ARDS intervention.
Keywords: Guillain-Barré syndrome, severe acute respiratory distress syndrome, case, ventilation
Introduction
Guillain-Barré syndrome (GBS) stands out as the most prevalent and severe acute immune-mediated paralytic neuropathy, characterized by polyneurogenic involvement, with an estimated annual incidence of 100,000 new cases globally.1,2 Patients typically present with the acute onset of symmetrical delayed paralysis, often preceded by a history of upper respiratory tract infection or recent vaccination. The disease duration usually spans approximately two to four weeks. Commonly associated pathogenic microorganisms include Campylobacter jejuni, Zika virus, and the Severe Acute Respiratory Syndrome (SARS) coronavirus.3 Guillain-Barré syndrome commonly manifests with progressive (episodic) limb weakness, though the clinical presentation can vary among patients. The disease typically reaches its peak within two weeks, and a characteristic diagnostic finding is cerebrospinal fluid demonstrating protein-cell separation. Simultaneously, refined electrophysiological examination confirms the presence of multiple demyelinating peripheral neuropathies. Approximately 30% of patients experience respiratory failure necessitating admission to the intensive care unit (ICU) and invasive mechanical ventilation. The underlying mechanism for respiratory failure is the progressive weakening of inspiratory and expiratory muscles.4 In cases where the respiratory system is implicated and progresses to acute respiratory distress syndrome (ARDS) requiring tracheal intubation, physicians face heightened challenges in assessing and managing the condition. Although mechanical ventilation supports a patient’s respiratory function, comprehensive airway management and the extubation process are critical components of the overall treatment strategy. Evaluating the effectiveness of specialized interventions and determining the optimal timing for extubation present significant challenges for clinicians.
In December 2023, a patient with Guillain-Barré syndrome complicated by severe acute respiratory distress syndrome was admitted to the People’s Hospital of Peking University. Initially, the patient’s neurological condition was not the primary focus; however, the patient’s condition rapidly deteriorated, progressing to acute respiratory distress syndrome (ARDS). In response to elevated immune markers and poor prognostic indicators, an aggressive pharmacological approach was employed to halt disease progression. This intervention ultimately led to successful extubation, facilitating the patient’s recovery and expediting discharge from the hospital.
Case Presentation
A 59-year-old female patient initially presented with cough, fever, and flu-like symptoms two weeks ago, receiving moxifloxacin anti-infective treatment at a local hospital. Subsequently, the patient experienced soreness, numbness, and dryness in both lower limbs, leading to progressive activity limitation. Limb weakness extended to the upper extremities, accompanied by hyperalgesia. The patient later manifested significant cold symptoms. One week before admission, the patient developed pronounced limb dyskinesia and urinary incontinence, prompting referral to Peking University First Hospital for consideration of “Acute Inflammatory Demyelinating Polyneuropathies (AIDP)”. Due to limited inpatient beds, the patient was admitted to Fuxing Hospital of Capital Medical University. Basic tests, including cerebrospinal fluid analysis (Table 1), were conducted upon admission. Of note, the patient’s symptoms suggested a potential immune abnormality induced by infection. Antinuclear antibody profiling revealed a granular pattern with 1:100 titers in the cytoplasm and nucleus. The patient tested positive for anti-Mitochondrial M2 type antibody (AMA-M2), anti-SSA (52kDe), and anti (anti-Ro-52). Additionally, there was a suspiciously positive result for SS-A60 and antibody (anti-SSA). In the course of treatment, 27.5 g of gamma globulin was administered on an anti-infective basis.
Table 1.
The Laboratory Findings (Fuxing Hospital of Capital Medical University)
| Variable | Value |
|---|---|
| Complete blood count | |
| Red blood cells (109/L) | 3.72 |
| White blood cells(109/L) | 5.52 |
| Platelets count (109/L) | 297 |
| Hemoglobin (g/l) | 104 |
| Venus blood gas analysis | |
| PH | 7.441 |
| PCO2 (mm Hg) | 38.10 |
| PO2 (mm Hg) | 62.40 |
| Electrolyte | |
| Sodium (mmol/L) | 131.46 |
| Potassium (mmol/L) | 3.45 |
| Total calcium (mg/dl) | |
| Ionized calcium (mg/dl) | 1.92 |
| Magnesium (mmol/L) | 0.83 |
| Phosphorus (mmol/L) | 0.65 |
| CSF analysis | |
| CSF appearance | Transparent, colourless |
| CSF protein (g/L) | 1.15 |
| CSF glucose (mmol/L) | 3.3 |
| CSF chloride (mmol/l) | 104.8 |
| CSF white blood cell count (106/l) | 1 |
| CSF red blood cell count (106/l) | 20 |
| Percentage of CSF polymorphonuclear cells(%) | 0 |
| Percentage of CSF monocytes(%) | 100 |
| Bacteria | |
| Pathological evidence | Negative |
Note: *CSF: cerebrospinal fluid.
Twenty-four hours ago, the patient experienced a loss of consciousness. Chest CT results revealed typical “Acute respiratory distress syndrome (ARDS)”, characterized by widespread patchy or solid shadows in both lungs, predominantly in the lower lungs, and without significant pleural effusion. Blood gas analysis indicated “type I respiratory failure.” Consequently, the patient underwent endotracheal intubation and was subsequently transferred to the intensive care unit at the People’s Hospital of Peking University.
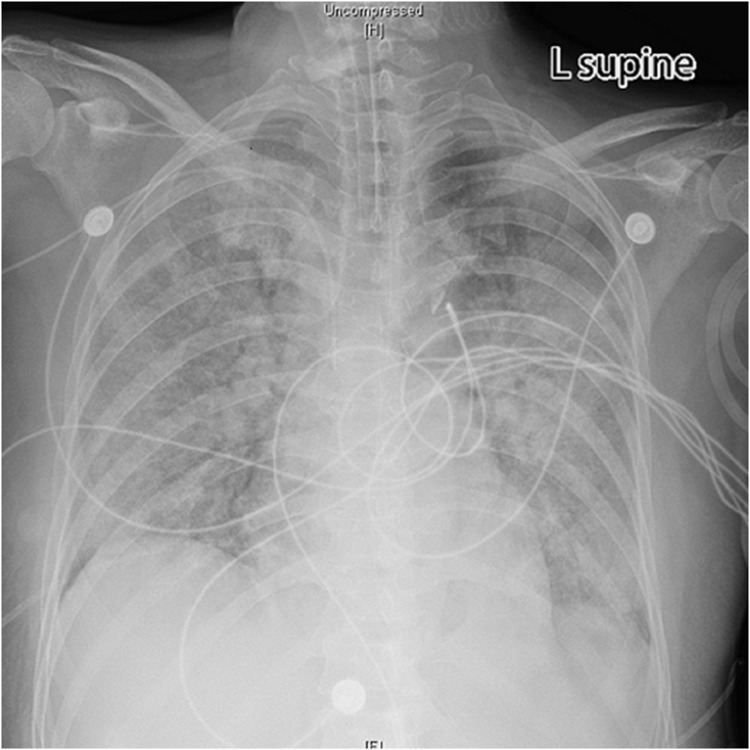
Upon admission to the Intensive Care Unit (ICU), the patient’s APACHE II score was 24, and the SOFA score was 11. The Glasgow Coma Scale (GCS) score was 5, with a breakdown of E1V1M3. Pupils in both eyes were isometric and equidistant, measuring about 5 mm, with the light reflex absent. Coarse breath sounds and wet rales were noted in both lungs. The patient exhibited weak muscle tone in all four limbs, and pathological signs were positive on the left side. Of particular concern was the patient’s respiratory status (Figures 1 and 2, Table 2). Ventilator monitoring indicated a peak airway pressure of 28 cmH2O, a tidal volume of 514 mL, and a respiratory rate of 24 breaths per minute.
Figure 1.
Bedside chest X-ray showed diffuse infiltrative shadows in both lungs and no significant cardiac shadow enlargement (the X-ray on the day of admission).
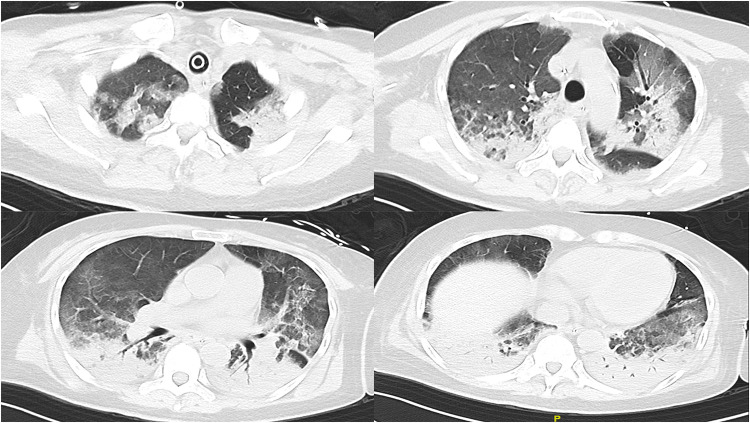
Figure 2.
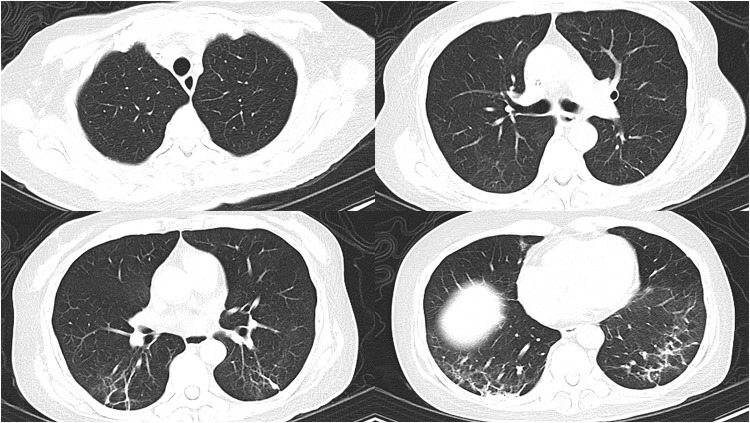
Chest CT showed diffuse infiltrative shadows in both lungs, combined with some solid lesions, visible inflatable air duct sign, and gravity-dependent lesion sites (Chest CT on 14 December).
Table 2.
Arterial Blood Gas Analysis
| Arterial Blood Gas Analysis | Value |
|---|---|
| PH | 7.39 |
| PCO2 (mmHg) | 32 |
| PO2 (mmHg) | 75 |
| A-aDO2 | 604 |
| Pa02/FiO2 | 75 |
In response to the challenging clinical scenario, we initiated a comprehensive therapeutic approach. The patient received a combination of oseltamivir, ceftriaxone, and levofloxacin to address potential infections. Additionally, immunomodulatory treatment was administered, comprising 20 mg of immunoglobulin and 500 mg of methylprednisolone daily. Given the severe pulmonary impairment, we employed intermittent lung re-expansion through mechanical ventilation, implementing lung-protective measures throughout. On the second day of admission, recognizing the possibility of “Neuromyelitis optica”, the patient was referred for evaluation and initiated on inelizumab. Notably, autoimmune peripheral antibodies were negative. Testing for four demyelinating antibodies, including anti-aquaporin-4 (AQP4), anti-myelin basic protein antibody (MBP), anti-myelin oligodendroglial fine allyl glycoprotein antibody (MOG), and anti-glial fibrillary acidic protein antibody (GPAP), all returned negative results. However, the patient exhibited a significant elevation in neurofilament light chain (Nfl), measuring 2441.57 pg/mL. This finding underscores the complexity of the neurological involvement in this case. Following the implementation of a daily 16-hour prone ventilation protocol, the patient’s oxygenation exhibited gradual improvement. A subsequent chest CT validated substantial amelioration in the pulmonary status (Figure 3). On the 5th day of admission, a judicious reduction in sedative and analgesic medications facilitated the patient’s increased consciousness. Encouragingly, successful extubation was achieved on the 6th day after a meticulous assessment via an extubation readiness test. This positive evolution in respiratory function reflects the effectiveness of our therapeutic interventions and underscores the importance of vigilant management in critical care settings.
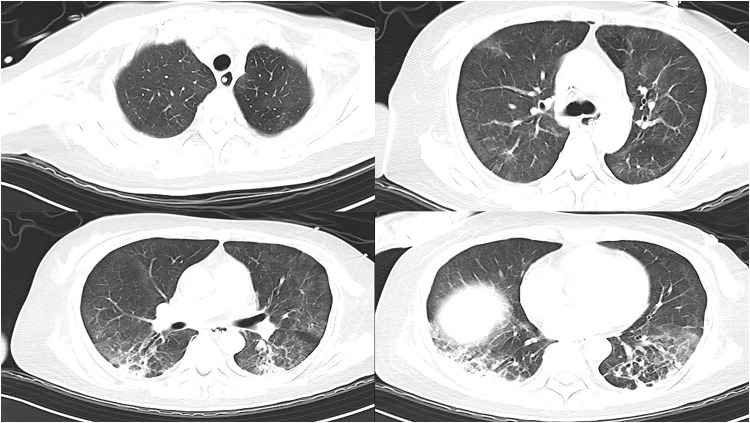
Figure 3.
The bilateral lung lesions were significantly less severe than before, with relatively incomplete absorption of inflammation on the dorsal side, and fibrous streak shadows were prominent on the dorsal side (Chest CT on 19 December).
Upon the patient’s successful extubation and restoration of consciousness, accompanied by the regained muscle strength, the patient was seamlessly transitioned back to the general ward within the Department of Neurology for ongoing therapeutic management. During this phase, alveolar lavage fluid analysis revealed the presence of Klebsiella pneumoniae, with the pathogen exhibiting sensitivity to tigecycline. Given the persistent elevation in white blood cell and neutrophil counts, the patient’s treatment regimen was accordingly adjusted to incorporate tigecycline therapy. This adaptive approach aligns with our commitment to optimizing treatment strategies based on real-time clinical indicators. Following the resumption of enteral feeding, the gastrointestinal tube was subsequently removed. The patient was discharged for ongoing rehabilitation and presented for a follow-up evaluation at our hospital one month later. Cranial MRI results revealed no significant abnormalities (Figure 4), while chest CT findings indicated the presence of residual fibrous streaks in the lower lungs bilaterally (Figure 5). The streaks observed in the lungs were residual fibrotic lesions resulting from the patient’s severe intrapulmonary infection, persisting despite early extubation. Despite these radiological improvements, the patient persisted with prolonged lethargy and mild anxiety. The short-term impact of the disease can be physically and mentally devastating. The confined environment where monitoring and multiple instruments are concentrated necessitates optimized patient comfort. As patients transition back to their daily routines, it is crucial that they are able to fully reintegrate both mentally and physically into their everyday lives.
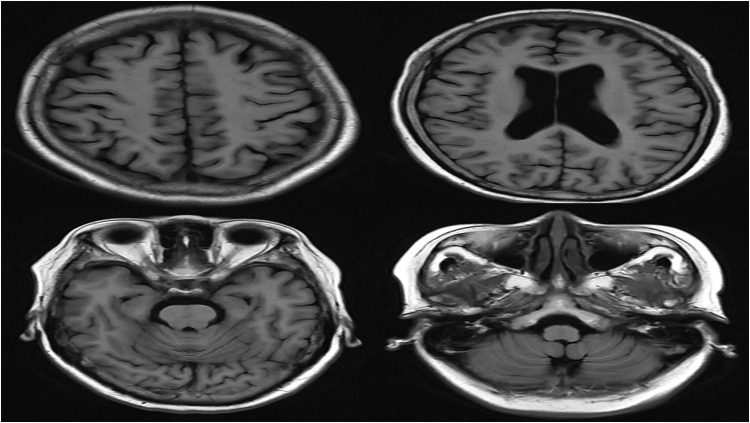
Figure 4.
Cranial MRI results revealed no significant abnormalities (MRI of T1 flair on 6 January).
Figure 5.
The chest CT findings indicated the presence of residual fibrous streaks in the lower lungs bilaterally (Chest CT on 8 January).
Discussion
Guillain-Barré syndrome (GBS) manifests with a rapid onset, frequently culminating in a high incidence of respiratory failure necessitating intensive care unit (ICU) admission. The cumulative incidence of respiratory complications, including acute respiratory distress syndrome (ARDS), poses a formidable challenge for intensivists. Despite the absence of AQP4 positivity and overt visual manifestations, the patient’s clinical presentation, characterized primarily by an acute spinal cord lesion, warranted consideration of AQP4-negative optic nerve myelitis spectrum disorders (neuromyelitis optica spectrum disorders, NMOSD).5
Most antecedents had an infectious trigger, and the relationship between SARS-CoV-2 and Guillain-Barré Syndrome (GBS) has been documented in cases during the COVID-19 pandemic.6,7 This case shares similarities with previous reports, where the urgent need to save the patient’s life was constrained by time and space, limiting the possibility of further electrophysiologic monitoring. In response to the onset of neurological symptoms, shock therapy with immunoglobulin and corticosteroids was administered, as is common in GBS cases.7 However, this approach remains an emergency measure, particularly in the context of critical ARDS, and there is still no definitive literature prior to Manu clarifying whether corticosteroids increase the risk of spreading infection. In the early stages of this case, there was no clear pathogenic evidence, including SARS-CoV-2, although the patient exhibited clear signs of infection. The specific infectious trigger leading to the autoimmune response could not be identified, though it is recognized that immune diseases are associated with certain lung abnormalities, such as MDA5-positive dermatomyositis.8 The autoimmune complications induced by GBS, especially when involving the respiratory system, pose significant challenges in diagnosis and treatment. Fortunately, the early adoption of lung-protective ventilation in this case allowed for effective management without further deterioration, and prone ventilation proved particularly beneficial.
In light of this diagnostic uncertainty, inebilizumab, a humanized monoclonal antibody targeting CD19 of the IgG subtype, was administered. This therapeutic approach aims to deplete B cells and plasma cells expressing CD19, thereby inhibiting antibody- and complement-dependent cytotoxicity. The implementation of inebilizumab has been associated with a significant reduction in disease flares and a deceleration in the progression of disability.9 The existing literature does not provide equivocal guidance on the appropriateness of administering inebilizumab as a treatment for patients diagnosed with Guillain-Barré Syndrome (GBS). Interestingly there are studies showing that inebilizumab treatment of optic neuromyelitis optica results in lower sNfL levels.10 After discussion with the specialist, second-line immunotherapy could be attempted with an adequate dose of gamma globulin already in use. Thanks to the fact that the patient was already intubated for respiratory assistance, the side effects of the immunotherapy storm were sufficiently reduced under appropriate conditions of sedation and analgesia. The entire medical team has been fulfilling the task of continuously improving the monitoring indicators, clarifying the diagnosis and adapting the treatment strategy according to the patient’s condition.
In the case presented, the patient experienced a gradual progression of muscle strength and sensory abnormalities, with the sudden onset of loss of consciousness, despite prior gamma globulin treatment. Although the patient’s condition had progressed for over a week, imaging revealed diffuse shadows in both lungs, a PaO2/FiO2 ratio of less than 300, and a relatively clear diagnosis of ARDS. Coupled with the new onset of a conscious coma, these findings aligned with the indications for urgent intubation. Ongoing close monitoring of vital signs is imperative, as the patient’s condition may not be effectively managed solely through pharmacological interventions. Imported immunoglobulins, acting as a protective barrier, might not cover antibodies generated by the persisting immune imbalance triggered by the infection. While guidelines may not specify interventions for the progression of respiratory deterioration in GBS patients, proactive measures, including admission to an ICU for vigilant vital sign monitoring, are recommended. Life support, particularly respiratory assistance, may become necessary at any point, emphasizing the unpredictable nature of the disease’s impact on respiratory function.
On the flip side, evaluating neurological symptoms in patients under intubation sedation and analgesia can be challenging. While adhering to a standard treatment regimen, it is crucial to regularly assess the patient’s mental and muscle strength recovery. The administration of sedative and analgesic drugs to intubated patients poses a significant obstacle to neurological function assessment and interferes with voluntary muscle movement exercises. In these cases, a precise and individualized adjustment of opioid or benzodiazepine dosages is essential to ensure that the patient remains under light sedation whenever possible. Recognizing the dynamic nature of this process, fine adjustments may be necessary, and only close supervision allows for such adjustments. In the early stages of acute respiratory distress syndrome (ARDS), patients often experience extremely high peak airway pressure, leading to a state of double-inspiratory respiratory distress. Following the infusion of complete gamma globulin, the patient’s symptoms did not significantly improve. Unprotected ventilation was initiated to maintain the patient’s plateau pressure below 30 cmH2O and to ensure adequate ventilation, while the monitoring room closely tracked the patient’s blood gases to assess oxygenation status. Achieving this requires high doses of medication to maintain deep sedation, interrupt spontaneous respiration, and facilitate prone position ventilation.
Furthermore, this case underscores the benefits of prone ventilation in ARDS. Oxygenation typically improves with changes in position, altering ventilation/perfusion distribution. Numerous studies have concluded that this simple, bedside measure leads to reduced mortality in ARDS patients with diminished tension in the non-dependent regional lung.11,12 The COVID-19 pandemic highlighted the importance of the prone ventilation strategy, offering additional clinical evidence for the practical value of this approach. Innovations in its implementation have further simplified the procedure.13,14 This patient underwent prone ventilation using the “envelope” method, ensuring adequate enteral nutrition before turning and securing each line. Care was taken to prevent pressure sores, especially in lax skin zones, and attention was given to conditions such as scrotal edema in male patients. Prone ventilation contributed to the rapid improvement of the patient’s ventilatory function, leading to a shorter duration of mechanical ventilation and reduced reliance on sedative and analgesic drugs. This approach also minimized the risk of nosocomial infections and tracheotomy while increasing the likelihood of successful extubation—a crucial step toward the patient’s transfer to the general ward and eventual discharge. Notably, the patient’s immune profile indicated positivity for anti-AMA-M2, anti-SSA, and anti-Ro-52, suggesting potential associations with conditions such as lupus erythematosus, Sjögren’s syndrome, or rheumatoid arthritis. This abnormal immunologic result likely reflects the production of multiple autoantibodies due to an infection-induced immune imbalance. However, this differs from MDA5-positive dermatomyositis, where a worsening pulmonary condition typically indicates a poor prognosis. In previous cases, treatment with multiple monoclonal antibodies has been employed, offering the possibility of controlling the immune imbalance more effectively. When gamma globulin fails to halt disease progression, introducing second-line monoclonal antibodies may provide an earlier intervention and potentially improve outcomes.15 In this case, inebilizumab was used, a drug that received Breakthrough Therapy designation from the FDA in 2019 for the treatment of the rare autoimmune disease neuromyelitis optica spectrum disorder (NMOSD). Additional indications for inebilizumab, including other potential inflammatory and autoimmune conditions as well as hematologic malignancies, are currently being explored in China.
Two other antibodies, while only suspected positives, suggest the patient is in a state of immune imbalance, especially when comprehensive evaluations cannot be conducted promptly. Although subsequent refinement of encephalitis-associated antibodies did not reveal significant abnormalities, a notable increase in neurofilament light chain (Nfl) levels was observed, potentially indicating neurological manifestations. Neurofilament light chain serves as a crucial subunit of the neuronal neurofilament proteins (NfFs), the primary cytoskeletal protein in neuronal axons. It is highly expressed in the axonal region of neurons, playing essential roles in maintaining morphological stability and ensuring the transmission of neuronal signals.16 Elevated levels of this biomarker indicate severe damage to the patient’s nervous system. It has been utilized as a diagnostic, prognostic, follow-up, and treatment indicator, serving as a novel biomarker in various chronic neurological conditions, including Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis, and Alzheimer’s disease.17–19 Concerning Guillain-Barré syndrome, an acute or subacute polyneuropathy, studies have demonstrated a correlation between neurofilament light chains in the cerebrospinal fluid and blood with both disease severity and mortality.20–23 While many studies have explored the prognostic value of neurofilament light chain (NfL) in neurological disorders, its clinical impact on the treatment principles of ARDS in cases complicated by Guillain-Barré Syndrome (GBS) remains limited. In this case, the focus shifted to the timely extubation process, with controlling the immune response being of greater importance. Given that the recovery period for GBS extends over several months, it is not yet possible to determine whether NfL more clearly reflects specific neurological impairments and residual symptoms.
Conclusion
This case underscores the importance of early recognition and aggressive intervention for acute respiratory distress syndrome (ARDS) in patients with Guillain-Barré Syndrome (GBS). It highlights the necessity of integrating clinical presentation, laboratory findings, and imaging results to develop an individualized treatment plan for GBS. Additionally, while monitoring serum neurofilament light chain (sNfL) levels can be useful in assessing disease severity and prognosis, more aggressive multidisciplinary treatment, early application of a comprehensive regimen, and standardized ARDS management are likely to offer significant benefits. Clinicians are also reminded to closely monitor disease progression and promptly adjust treatment strategies in accordance with the latest clinical guidelines and research advancements when managing such complex cases.
Acknowledgment
We thank Dr. Shuguang Yang for his invaluable guidance in the clinical management of this case.
Funding Statement
The research received the grant from the National Natural Science Foundation of China (NSFC) (NO. 82202366) and Beijing Key Clinical Specialty Outstanding Project.
Data Sharing Statement
All data of the correspondence are publicly available on databases such as PubMed and Google scholar.
Ethics Approval and Consent to Participate
Patient details were omitted, and a de-identification process was implemented to ensure confidentiality and privacy. Written informed consent was obtained from the patient in this case report, and publication of the case did not require ethics from the institution; therefore, no further ethical review was required.
Consent for Publication
All the authors have approved the publication. Study participants agreed to use journal republishing links and to upload revised documents.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the study was conducted without any commercial or financial relationships that could be interpreted as potential conflicts of interest.
References
- 1.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717–727. doi: 10.1016/s0140-6736(16)00339-1 [DOI] [PubMed] [Google Scholar]
- 2.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36:123–133. doi: 10.1159/000324710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahrizaila N, Lehmann HC, Kuwabara S. Guillain-Barré syndrome. Lancet. 2021;397:1214–1228. doi: 10.1016/s0140-6736(21)00517-1 [DOI] [PubMed] [Google Scholar]
- 4.Orlikowski D, Prigent H, Sharshar T, Lofaso F, Raphael JC. Respiratory dysfunction in Guillain-Barré syndrome. Neurocrit Care. 2004;1:415–422. doi: 10.1385/ncc:1:4:415 [DOI] [PubMed] [Google Scholar]
- 5.Jarius S, Paul F, Weinshenker BG, et al. Neuromyelitis optica. Nat Rev Dis Primers. 2020;6:85. doi: 10.1038/s41572-020-0214-9 [DOI] [PubMed] [Google Scholar]
- 6.Rane RP, Jain A, Hussain KM, Naik S, Shahab A. A rare case of Guillain-Barré syndrome associated with SARS-CoV-2 infection requiring mechanical ventilation. Cureus. 2022;14:e25810. doi: 10.7759/cureus.25810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean A, Said A, Marri K, Chelius D. Stridor due to cranial nerve X palsy progressing to polyneuropathy in a teenager with COVID-19. Pediatrics. 2021;148:e2021051534. doi: 10.1542/peds.2021-051534 [DOI] [PubMed] [Google Scholar]
- 8.He W, Cui B, Chu Z, et al. Radiomics based on HRCT can predict RP-ILD and mortality in anti-MDA5 + dermatomyositis patients: a multi-center retrospective study. Respir Res. 2024;25:252. doi: 10.1186/s12931-024-02843-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled Phase 2/3 trial. Lancet. 2019;394:1352–1363. doi: 10.1016/s0140-6736(19)31817-3 [DOI] [PubMed] [Google Scholar]
- 10.Aktas O, Hartung H-P, Smith MA, et al. Serum neurofilament light chain levels at attack predict post-attack disability worsening and are mitigated by inebilizumab: analysis of four potential biomarkers in neuromyelitis optica spectrum disorder. J Neurol Neurosurg. 2023;94:757–768. doi: 10.1136/jnnp-2022-330412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guérin C, Albert RK, Beitler J, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46:2385–2396. doi: 10.1007/s00134-020-06306-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med. 2013;188:1286–1293. doi: 10.1164/rccm.201308-1532CI [DOI] [PubMed] [Google Scholar]
- 13.Rampon GL, Simpson SQ, Agrawal R. Prone positioning for acute hypoxemic respiratory failure and ARDS: a review. Chest. 2023;163:332–340. doi: 10.1016/j.chest.2022.09.020 [DOI] [PubMed] [Google Scholar]
- 14.Papazian L, Munshi L, Guérin C. Prone position in mechanically ventilated patients. Intensive Care Med. 2022;48:1062–1065. doi: 10.1007/s00134-022-06731-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yen TH, Tseng CW, Wang KL, Fu PK. Combination therapy with rituximab, tofacitinib and pirfenidone in a patient with rapid progressive interstitial lung disease (RP-ILD) due to MDA5 antibody-associated dermatomyositis: a case report. Medicina. 2021;57:1358. doi: 10.3390/medicina57121358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Rumeileh S, Abdelhak A, Foschi M, et al. The multifaceted role of neurofilament light chain protein in non-primary neurological diseases. Brain. 2023;146:421–437. doi: 10.1093/brain/awac328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21:246–257. doi: 10.1016/s1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 18.Aamodt WW, Waligorska T, Shen J, et al. Neurofilament light chain as a biomarker for cognitive decline in Parkinson disease. Mov Disord. 2021;36:2945–2950. doi: 10.1002/mds.28779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arslan B, Zetterberg H. Neurofilament light chain as neuronal injury marker - what is needed to facilitate implementation in clinical laboratory practice? Clin Chem Lab Med. 2023;61:1140–1149. doi: 10.1515/cclm-2023-0036 [DOI] [PubMed] [Google Scholar]
- 20.Martín-Aguilar L, Camps-Renom P, Lleixà C, et al. Serum neurofilament light chain predicts long-term prognosis in Guillain-Barré syndrome patients. J Neurol Neurosurg. 2020. doi: 10.1136/jnnp-2020-323899 [DOI] [PubMed] [Google Scholar]
- 21.Altmann P, De Simoni D, Kaider A, et al. Increased serum neurofilament light chain concentration indicates poor outcome in Guillain-Barré syndrome. J Neuroinflammation. 2020;17(86). doi: 10.1186/s12974-020-01737-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Körtvelyessy P, Kuhle J, Düzel E, et al. Ratio and index of neurofilament light chain indicate its origin in Guillain-Barré syndrome. Ann Clin Transl Neurol. 2020;7:2213–2220. doi: 10.1002/acn3.51207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breville G, Sukockiene E, Vargas MI, Lascano AM. Emerging biomarkers to predict clinical outcomes in Guillain-Barré syndrome. Expert Rev Neurother. 2023;23:1201–1215. doi: 10.1080/14737175.2023.2273386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data of the correspondence are publicly available on databases such as PubMed and Google scholar.