Abstract
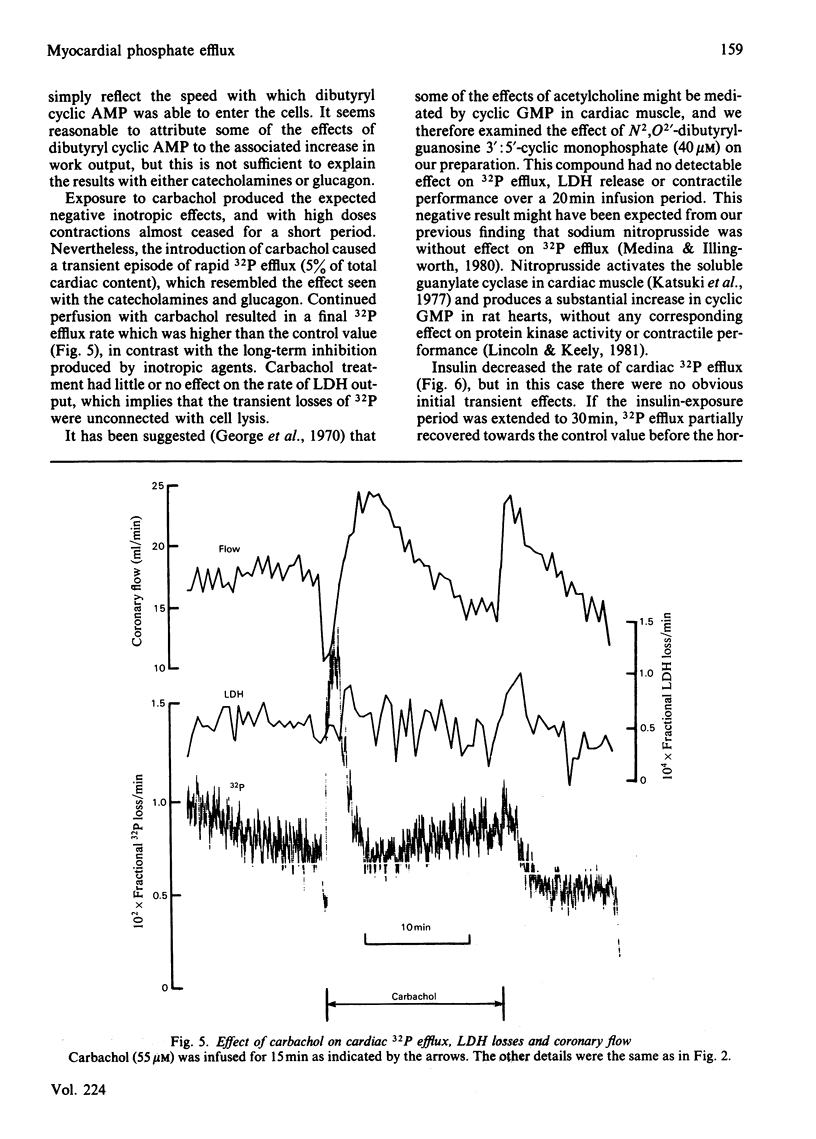
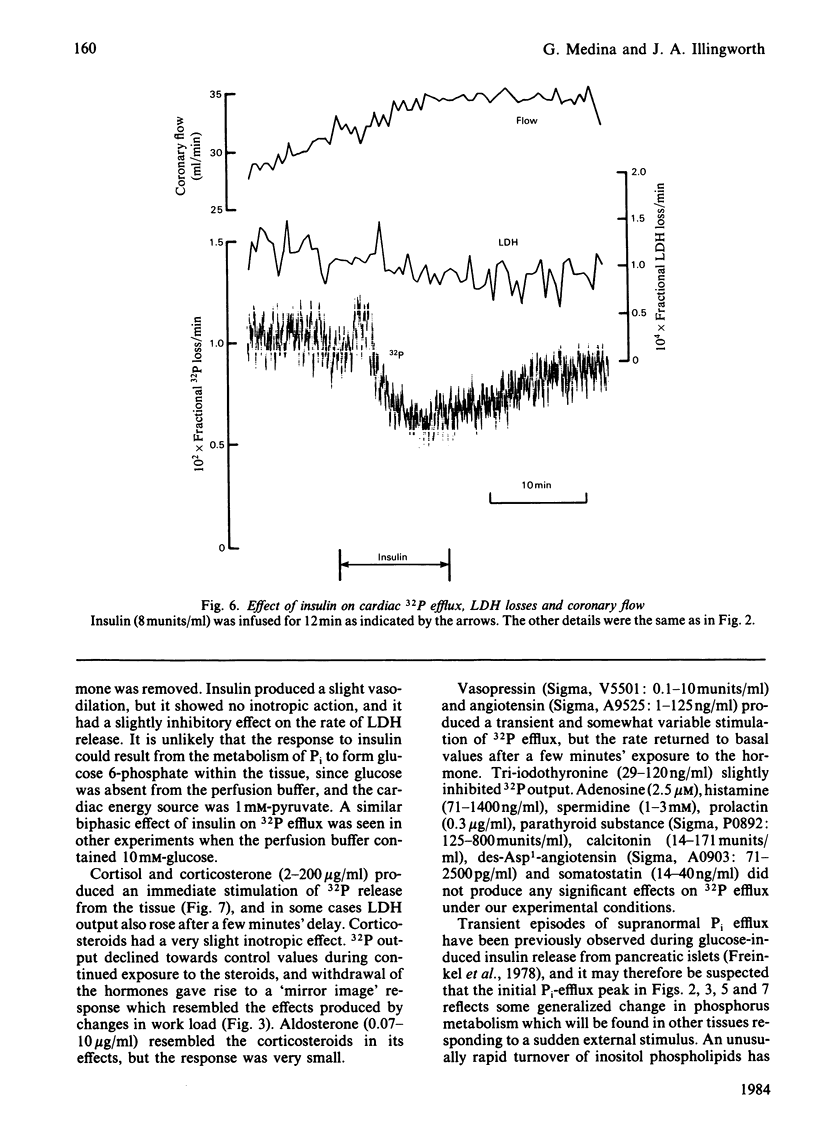
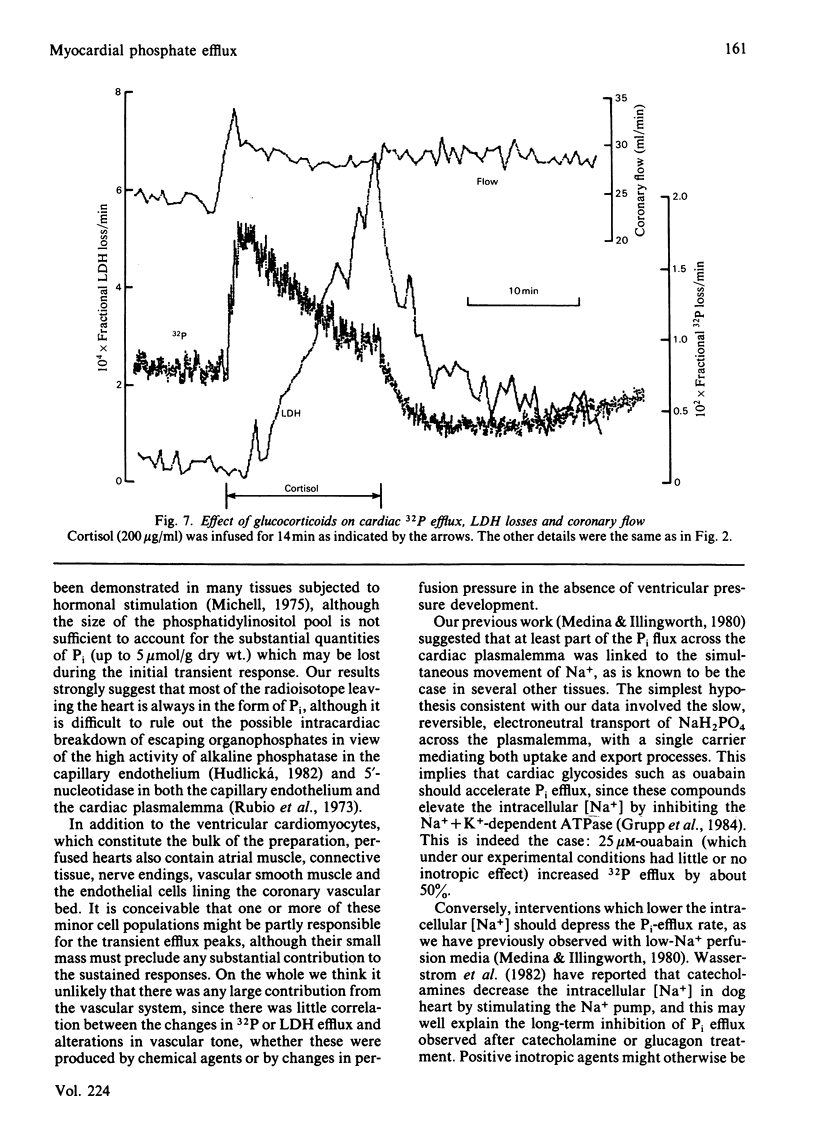
The effects of 23 agonists on the rates of cellular 32P efflux and lactate dehydrogenase (LDH) release were tested in a perfused rat heart preparation which had been prelabelled in vitro with [32P]Pi. Some 13 compounds produced detectable changes at high doses within 10 min, and in most cases a polyphasic response was observed. Six classes of compound gave rise to substantial effects, as follows. Catecholamines and glucagon produced a transient initial stimulation of Pi efflux, followed by a long-term inhibition of Pi transport and an increased rate of LDH release. These effects were clearly different from the response seen after treatment with dibutyryl cyclic AMP, which had a slower, stimulatory, effect on Pi output in doses which gave rise to a pronounced inotropic effect, and produced a marked increase in both coronary flow and LDH release. Carbachol also gave rise to a large transient stimulation of Pi efflux, which was followed by smaller sustained increase in Pi output without any obvious effect on LDH release. Dibutyryl cyclic GMP had no effect on Pi efflux or LDH release. Insulin decreased the rate of Pi efflux, although the loss rate partially recovered towards the control value after prolonged exposure to the hormone. Insulin had no obvious inotropic effects and produced no change in the rate of LDH release. Corticosteroids increased the rate of Pi efflux, although the loss rate partially declined towards the control value with prolonged exposure to the hormones. Corticosteroids produced a very slight inotropic response, and large doses sometimes increased the rate of LDH release from the tissue. Aldosterone slightly stimulated Pi output. A small, transient and somewhat variable stimulation of Pi efflux was observed with vasopressin and angiotensin, whereas tri-iodothyronine was slightly inhibitory, but adenosine, histamine, spermidine, des-Asp1-angiotensin, prolactin, parathyroid substances, calcitonin and somatostatin had no significant effects under our experimental conditions. Ouabain stimulated Pi efflux in doses that had no detectable inotropic effect. It is suggested that Pi efflux involves the electroneutral transport of NaH2PO4 across the cardiac plasmalemma and that many of the hormonal effects might be explained by changes in the intracellular [Na+] and pH in addition to changes in the intracellular [Pi].
Full text
PDF

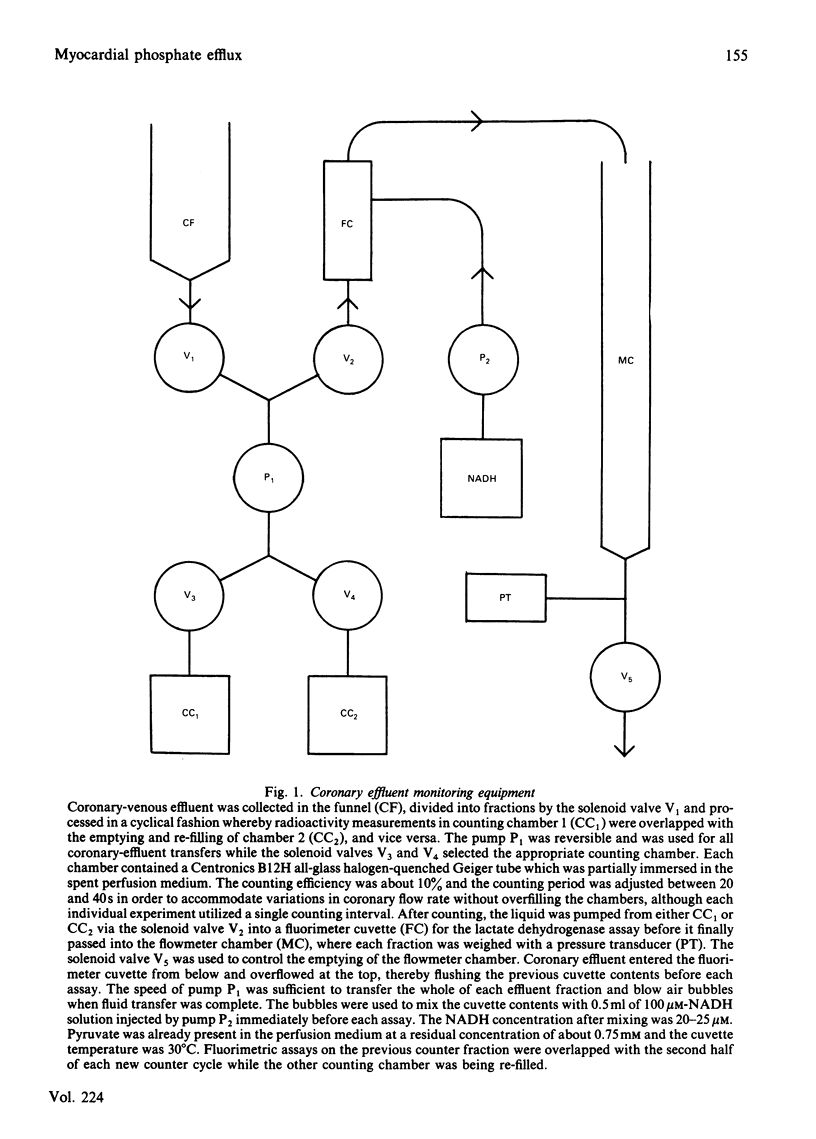
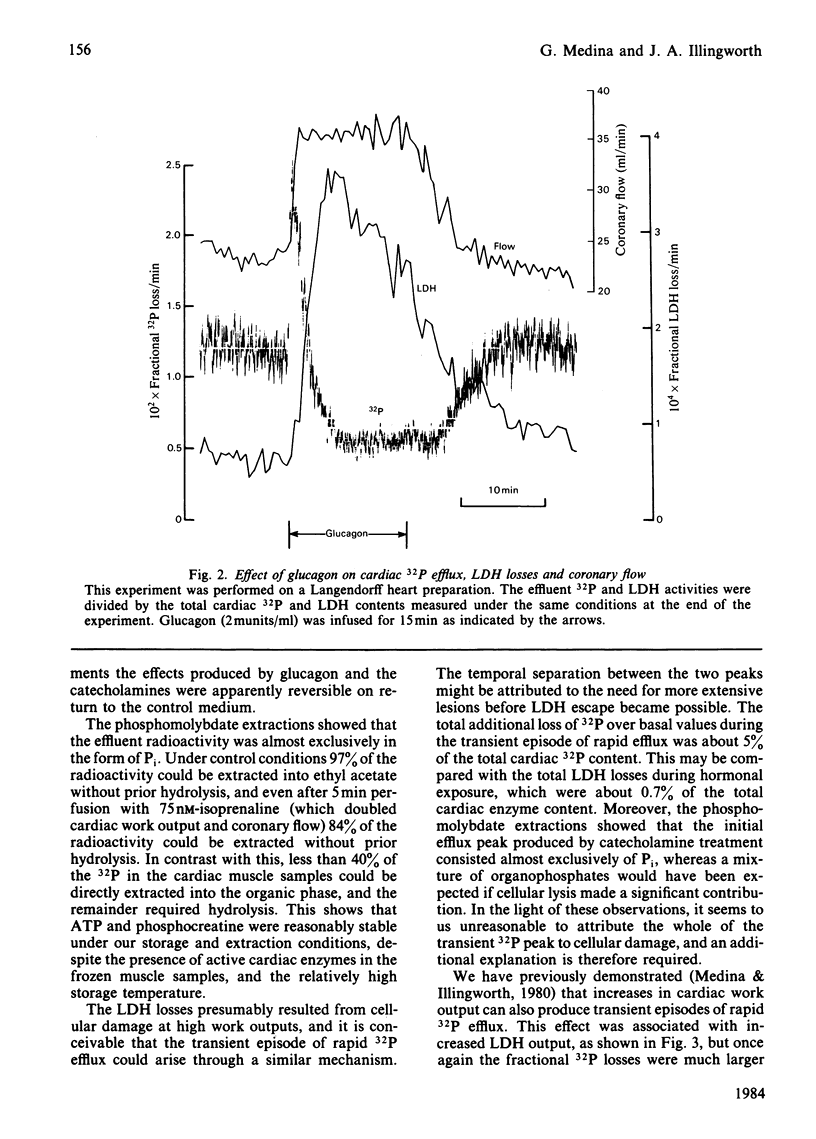
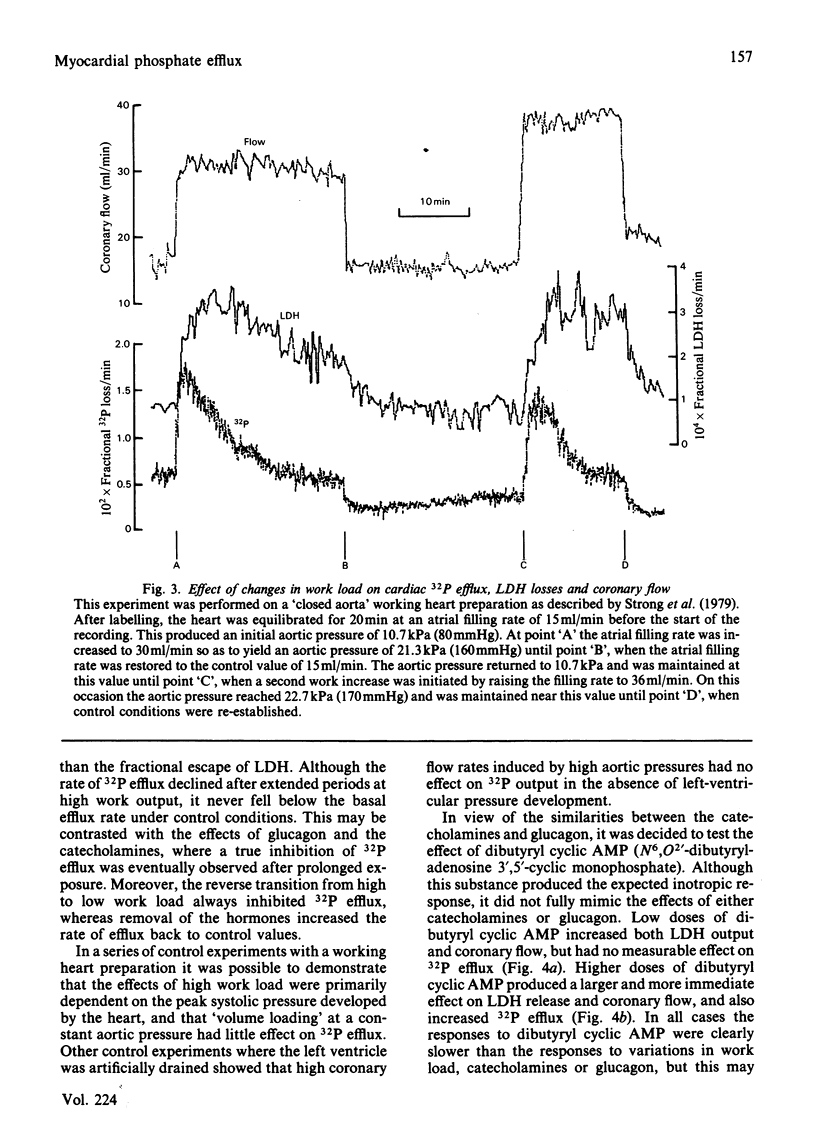
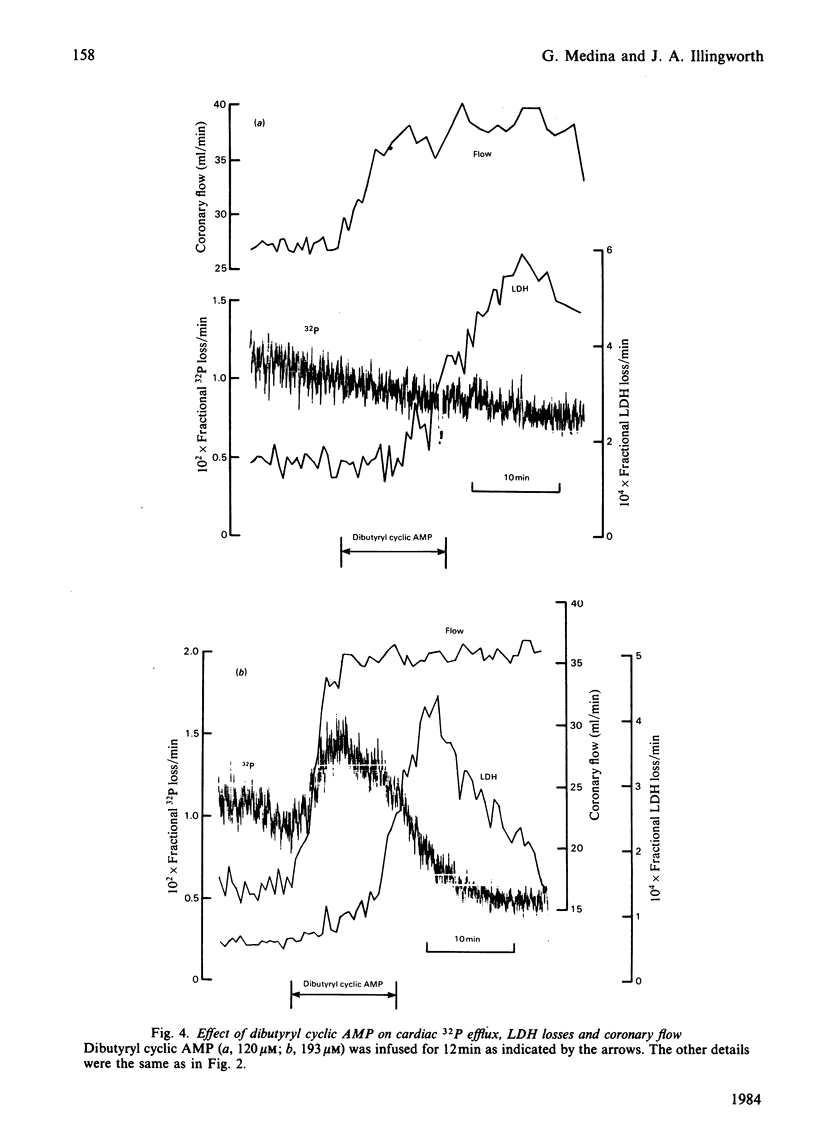

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fidelman M. L., Seeholzer S. H., Walsh K. B., Moore R. D. Intracellular pH mediates action of insulin on glycolysis in frog skeletal muscle. Am J Physiol. 1982 Jan;242(1):C87–C93. doi: 10.1152/ajpcell.1982.242.1.C87. [DOI] [PubMed] [Google Scholar]
- Freinkel N., Pedley K. C., Wooding P., Dawson R. M. Localization of inorganic phosphate in the pancreatic B cell and its loss on glucose stimulation. Science. 1978 Sep 22;201(4361):1124–1126. doi: 10.1126/science.356269. [DOI] [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp G., DePover A., Grupp I. L., Schwartz A. Analysis of the inotropic action of ouabain in rat ventricles: two apparent ouabain inotropic responses. Proc Soc Exp Biol Med. 1984 Jan;175(1):39–43. doi: 10.3181/00379727-175-41763. [DOI] [PubMed] [Google Scholar]
- Hudlická O. Growth of capillaries in skeletal and cardiac muscle. Circ Res. 1982 Apr;50(4):451–461. doi: 10.1161/01.res.50.4.451. [DOI] [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Knutson V. P., Ronnett G. V., Lane M. D. Rapid, reversible internalization of cell surface insulin receptors. Correlation with insulin-induced down-regulation. J Biol Chem. 1983 Oct 25;258(20):12139–12142. [PubMed] [Google Scholar]
- Langer G. A. Sodium-calcium exchange in the heart. Annu Rev Physiol. 1982;44:435–449. doi: 10.1146/annurev.ph.44.030182.002251. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Keely S. L. Regulation of cardiac cyclic GMP-dependent protein kinase. Biochim Biophys Acta. 1981 Aug 17;676(2):230–244. doi: 10.1016/0304-4165(81)90192-6. [DOI] [PubMed] [Google Scholar]
- Medina G., Illingworth J. Some factors affecting phosphate transport in a perfused rat heart preparation. Biochem J. 1980 May 15;188(2):297–211. doi: 10.1042/bj1880297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Moore R. D. Effects of insulin upon ion transport. Biochim Biophys Acta. 1983 Mar 21;737(1):1–49. doi: 10.1016/0304-4157(83)90013-8. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Munford J. W., Pillsworth T. J., Jr Effects of streptozotocin diabetes and fasting on intracellular sodium and adenosine triphosphate in rat soleus muscle. J Physiol. 1983 May;338:277–294. doi: 10.1113/jphysiol.1983.sp014673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H., Thandroyen F. T., Muller C., Bricknell O. L. Adrenaline-induced "oxygen-wastage" and enzyme release from working rat heart. Effects of calcium antagonism, beta-blockade, nicotinic acid and coronary artery ligation. J Mol Cell Cardiol. 1979 Oct;11(10):1073–1094. doi: 10.1016/0022-2828(79)90395-x. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson P. A., Langer G. A. Effect of pH on ionic exchange and function in rat and rabbit myocardium. Am J Physiol. 1975 Sep;229(3):570–581. doi: 10.1152/ajplegacy.1975.229.3.570. [DOI] [PubMed] [Google Scholar]
- Rubio R., Berne R. M., Dobson J. G., Jr Sites of adenosine production in cardiac and skeletal muscle. Am J Physiol. 1973 Oct;225(4):938–953. doi: 10.1152/ajplegacy.1973.225.4.938. [DOI] [PubMed] [Google Scholar]
- Streeten D. H., Moses A. M. Action of cortisol on sodium transport in canine erythrocytes. J Gen Physiol. 1968 Aug;52(2):346–362. doi: 10.1085/jgp.52.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong P., Mullings R., Illingworth J. A. Aerobic lactate synthesis by cardiac muscle. Eur J Biochem. 1979 Dec 17;102(2):625–636. doi: 10.1111/j.1432-1033.1979.tb04280.x. [DOI] [PubMed] [Google Scholar]
- Wasserstrom J. A., Schwartz D. J., Fozzard H. A. Catecholamine effects on intracellular sodium activity and tension in dog heart. Am J Physiol. 1982 Nov;243(5):H670–H675. doi: 10.1152/ajpheart.1982.243.5.H670. [DOI] [PubMed] [Google Scholar]


