Abstract
Background:
Bladder cancer is one of the most common cancers in the world and is associated with high treatment costs and mortality. The role of different enzymes and molecules in this cancer has been the subject of extensive research in recent years. Among these, the role of metabolic enzymes such as FASN and LDH has been studied less than others. Therefore, the present study was designed to investigate the role of FASN and LDH in bladder cancer patients.
Methods:
One hundred cases diagnosed with bladder cancer and 50 sex-age- matched healthy individuals as control were examined. FASN and LDH serum levels in both patients and controls were determined by human-specific sandwich ELISA kits.
Results:
Serum levels of FASN and LDH elevated in bladder cancer patients in comparison to healthy individuals (P= 0.03, P= 0.01, respectively). We also found that than higher stages of bladder cancer (III-IV) had higher serum levels of LDH and FASN compared to early stages (I-II) (P= 0.007 and P= 0.006, respectively). Moreover, there was a statistically significant association between smoking history and serum FASN levels in bladder cancer patients (P=0.015). However, there were no remarkable associations between the serum levels of LDH and FASN with other clinicopathological features including sex, age, tumor grade, and tumor size.
Conclusion:
The data indicate that LDH and FASN may be good and useful biomarkers in the diagnosis and clinical management of bladder cancer. However, further studies are needed.
Key Words: Bladder cancer, ELISA, FASN, LDH
Bladder cancer is one of the most common cancers worldwide and is associated with high treatment costs, morbidity, and mortality. In 2022, 81180 new cases and 17100 deaths from bladder cancer are estimated to occur in the United States (1). Bladder cancer affects men three times more than women (the ratio is roughly 3:1). The risk of this cancer increases with age as the median age at diagnosis is 65 years, and is more common in white populations than in black individuals. The most common type (80-90%) of bladder cancer mainly arises from the epithelium (urothelium) of the bladder and is known as urothelial carcinoma (transitional cell carcinoma). Other histomorphological types of bladder cancer, which account for 10–25% of cases, include squamous cell carcinoma, adenocarcinoma, and small-cell carcinoma (2). Roughly 75% of newly diagnosed patients have non-muscle-invasive bladder cancer (NMIBC) of low grade and 25% have muscle-invasive bladder cancer (MIBC) with metastatic behavior. These subtypes are highly heterogeneous tumors that are completely different according to survival rates, progression, recurrence, and biological behavior (3). Despite a great deal of study on bladder cancer, the underlying cellular and molecular mechanisms are still not fully understood.
Therefore, it is essential to identify new pathways and molecules that are critical in the initiation, proliferation, invasion, and metastasis of the bladder tumor. During malignant transformation, cancer cells exhibit unique metabolic reprogramming to support the energetic and biosynthetic demands of rapidly proliferating tumor cells. In contrast to normal cells, most malignant cells, such as bladder cancer cells, exhibit an increased rate of aerobic glycolysis (the Warburg effect). This phenomenon is a major hallmark of cancer which generates ATP quicker than oxidative phosphorylation and results in the production of key precursors for biomolecule synthesis (4). Lactate dehydrogenase (LDH) is a key cytoplasmic enzyme that plays a substantial role in the glycolysis process. Tumor-derived lactate is a potent immune suppressor that modifies the tumor microenvironment and blocks anti-tumor immune reactions. A previous study found that LDHA suppression by diclofenac can reduce the accumulation of CD4+FoxP3+ Tregs in a murine model of glioma (5). High LDH levels can promote the malignant alterations and survival of tumor cells. The tumor burden had a positive correlation with serum levels of LDH and surgical removal of primary cancers causes a decrease in serum levels of LDH (6).
In human malignancies, not only glucose metabolism but also fatty acid synthesis is accelerated. Fatty acids (FAs) are critical components of all biological membranes and are essential substrates for energy storage and mediating signaling (7). It has been reported that various lipogenic enzymes are up-regulated in tumors and correlated with cancer progression, therapeutic resistance, and relapse. Fatty acid synthase (FASN) is involved in the biosynthesis of long-chain FAs from building blocks of acetyl-CoA and malonyl-CoA using NADPH (8). FASN signaling is a regulator of several signaling pathways during tumorigenesis and immune escape and thus can be considered as a target for combination with cancer immunotherapy (7). It has been demonstrated that FASN expression negatively correlates with infiltrating levels of macrophages, neutrophils, CD8+ T cells, CD4+ T cells and DCs in cancer. Cioccoloni G et al. showed that orlistat (an inhibitor of FASN) impairs PD-L1 expression and decreases cell growth in the human T-cell leukemia cell line (9). In bladder cancer, FASN has shown value as an immunotherapy indicator and regulator, especially in ICI (Immune checkpoint inhibitor) therapy. Furthermore, FASN is overexpressed in a wide variety of human cancers and their pre-neoplastic lesions, such as prostate, breast, colon, endometrium, and pancreas, and is associated with tumor aggressiveness and poor prognosis. This makes FASN a useful diagnostic tumor biomarker (10). However, to date, there is little clear evidence of the relationship between serum LDH and FASN levels and the clinicopathologic parameters in bladder cancer patients. In this study, we attempted to reveal possible associations between serum LDH and FASN levels and a variety of clinicopathological factors in patients with bladder cancer.
Methods
Study population: The present study included 100 cases with a histopathological diagnosis of bladder cancer, and 50 non-smoking sex-age- matched healthy individuals as controls. Patients with history of additional neoplasms, autoimmune diseases, evidence of infectious diseases during the past three months, and who had received chemotherapy or radiation before sample collection, or if hemolysis was detected in their serum samples were excluded. To determine the tumor stage and grade, we used The American Joint Committee on Cancer (AJCC, 8th edition, 2017) TNM classification and the WHO International Society of Urological Pathology (WHO-ISUP) classification, respectively. All study individuals gave written informed consent before participation. This study was approved by the Research Ethics Committee of Shiraz University of Medical Sciences (IR. SUMS. REC.1398. 540).
Enzyme-linked immunosorbent assay (ELISA): 3 ml of the peripheral blood sample was taken from patients and healthy individuals; the sera were separated from whole blood by centrifugation and stored at -80◦C until further analysis. Serum concentrations of LDH and FASN were assessed by human-specific sandwich ELISA kits from BT Laboratory (Shanghai, China) according to the manufacturer’s procedures and using the standard concentrations of LDH and FASN, provided by BT laboratory, and presented as U/L and pg/ml, respectively.
Statistical analysis: We used Statistical Package for Social Sciences (SPSS, Version 22.0 for Windows [IBM Corporation, USA]) for the statistical analysis. The Shapiro-Wilk and Kolmogorov-Smirnov tests were used to check the normality of the data. Suitable parametric or non-parametric tests were used for comparison between groups. The relationship of serum LDH and serum FASN levels with various clinicopathologic parameters were analyzed by chi-square test. To assess the value of LDH and FASN in the discrimination of bladder cancer patients from the normal population, the area under the ROC curve (AUC) was determined as well as its specificity and sensitivity were measured. We selected the upper limit of normal levels as the cut-off values of the FASN and LDH, which were 15pg/ml and 245U/L, respectively. Higher than the cut-off values were considered as a high level of FASN and LDH. Variables with normal distribution were reported as mean±SD; otherwise, as median, and interquartile range (IQR). P-values were considered statistically significant when they were ≤ 0.05.
Results
Out of 100 cases diagnosed with bladder cancer, 73 (73%) were males, and 27 (27%) were females. The age range was between 34 and 91 years in patients, with a mean age of 67.65± 13.02. In the control group, the majority of participants were males (N= 35, 70.0%), and the age range was between 38 and 90 years with a mean age of 67.13± 11.58.
There were no remarkable differences between patients and controls with respect to mean age (P= 0.85) and gender (P= 0.73). Most patients had high-grade tumors (64 patients), and most tumors were less than 3 cm in size. The baseline clinicopathologic and demographic parameters of patients and their associations with LDH and FASN serum levels are shown in table 1.The median serum levels of LDH in patients and the control group were [336.13 (271.00 to 410.35) U/L vs. 248.18 (235.25 to 270.30) U/L; P= 0.01, respectively], which showed a statistically remarkable difference (figure 1). Regarding FASN, its median serum levels in bladder cancer patients and the control group were [21.11 (16.40 to 23.31) pg/ml vs. 12.52 [9.12 to 16.81) pg/ml; P= 0.03, respectively] indicating substantial differences between patients and control group (figure 2).
Table 1.
Clinicopathologic and demographic features of the studied bladder cancer patients
| variable | LDH | P value | FASN | P-value | |||
|---|---|---|---|---|---|---|---|
|
Normal
(≤ 245 U/L) |
High
(> 245 U/L) |
Normal
(≤15 pg/ml) |
High
(>15 pg/ml) |
||||
| Age | ≤50 (N=10) |
2 (20.0%) | 8 (80.0%) | 0.48 | 3 (30.0%) | 7 (70.0%) | 0.83 |
| >50 (N=90) |
33 (36.6%) | 57 (63.4%) | 35 (38.8%) | 55 (61.2%) | |||
| Gender | Male (N=73) |
25 (34%) | 48 (66%) | 0.29 | 38 (52.1%) | 35 (47.9%) | 0.07 |
| Female (N=27) |
13 (48.1%) | 14 (51.9%) | 8 (29.6%) | 19(70.4%) | |||
| Smoking | Yes (N=33) |
13 (39.3%) | 20 (60.7%) | 1.0 | 10 (30.3%) | 23 (69.7%) | 0.015 |
| No (N=67) |
25 (37.3%) | 42 (62.7%) | 39 (58.2%) | 28 (41.8%) | |||
| Tumor size | ≤ 3cm (N=70) |
33 (47.2%) | 37 (52.8%) | 0.89 | 42 (60.0%) | 28 (40.0%) | 0.48 |
| > 3cm (N=30) |
13 (43.3%) | 17 (56.7%) | 15 (50.0%) | 15 (50.0%) | |||
| Tumor Grade | PUNLMP* (N=18) |
8 (44.4%) | 10 (55.5%) | 0.61 | 11(61.2%) | 7 (38.8%) | 0.36 |
| Low grade (N=18) |
7 (38.8%) | 11 (61.2%) | 8 (45.4%) | 10 (54.6%) | |||
| High grade (N=64) |
24 (37.5%) | 40 (62.5%) | 30 (47.0%) | 34 (53.0%) | |||
| Tumor Stage | I-II (N=23) |
16 (69.5%) | 7 (30.5%) | 0.007 | 18 (78.5%) | 5 (21.5%) | 0.006 |
| III-IV (N=77) |
27 (35.0%) | 50 (65.0%) | 33(42.8%) | 44 (57.1%) | |||
|
Lympho-vascular
invasion |
No (N=55) |
40 (72.7%) | 15 (27.3%) | < 0.001 | 33 (60.0%) | 22 (40.0%) | 0.07 |
| Yes (N=45) |
15 (33.3%) | 30 (66.6%) | 18 (40.0%) | 27 (60.0%) | |||
* Fatty acid synthase (FASN), Lactate dehydrogenase (LDH), Papillary urothelial neoplasms of low malignant potential (PUNLMP)
Figure 1.
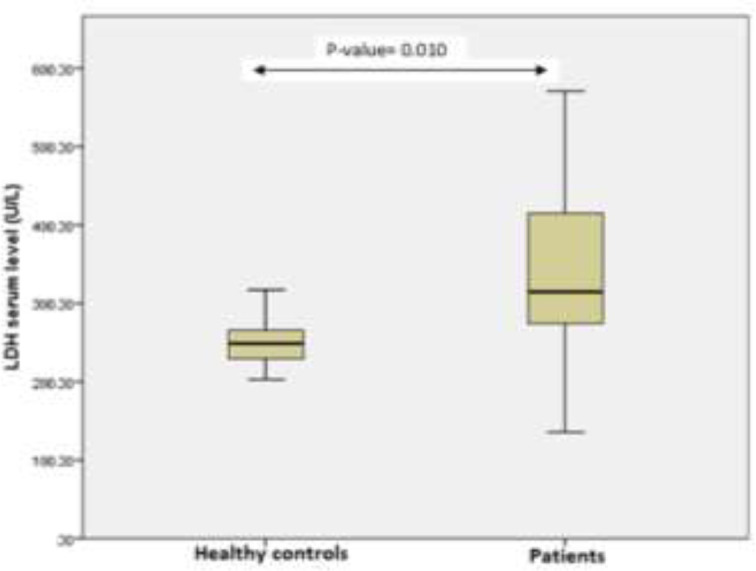
Changes in serum LDH levels in bladder cancer patients in comparison to control subjects
Figure 2.
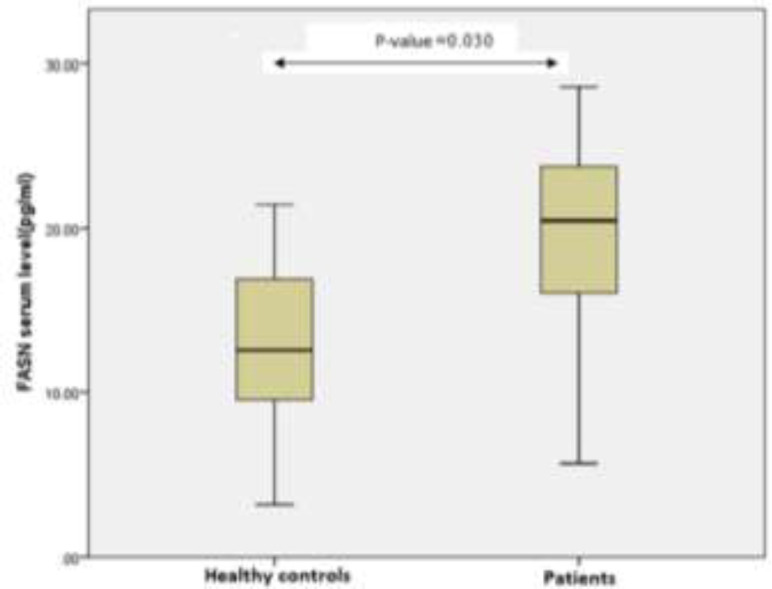
Changes in serum FASN levels in bladder cancer patients in comparison to control subjects
In this study, patients with bladder cancer were also classified into 4 groups: the normal level of LDH (≤ 245 U/L), high level of LDH (> 245 U/L), normal level of FASN (≤ 15pg/ml), and high level of FASN (>15 pg/ml), then correlations of the LDH and FASN serum levels and clinicopathological parameters were investigated. No statistically significant relationship was observed between serum levels of LDH and FASN with tumor grade (P= 0.61, P=0.36, respectively) or tumor size (P= 0.89, P= 0.48, respectively). According to the stage, patients with advanced stages (III-IV) of the tumor had remarkably higher LDH and FASN serum levels (P= 0.007 and P= 0.006, respectively) compared to early stages (I-II) (table 1). Also, elevated serum levels of LDH were associated with increased lympho-vascular invasion (P= 0.0001).
In patients with a history of smoking, the serum level of FASN was remarkably higher in comparison with non-smokers (P= 0.015), although there was no relationship between LDH serum level and smoking (P= 1.0). There was no remarkable association between gender and serum level of LDH (P= 0.29) or FASN (P= 0.07). The same was true for age (P= 0.48, P= 0.83, respectively). Serum samples from 50 healthy controls and 100 bladder cancer patients were used for the receiver operating characteristic (ROC) curve analysis to evaluate the sensitivity and specificity of LDH and FASN in the detection of bladder cancer. The ROC curve resulted in an AUC of 0.783 (95% CI, 0.681 to 0.885) for LDH (figure 3) and an AUC of 0.856 (95% CI, 0.778 to 0.935) for FASN (figure 4).
Figure 3.
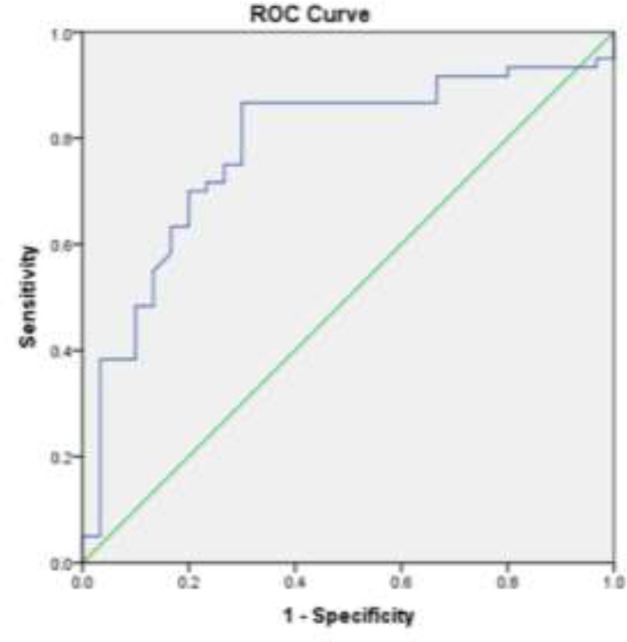
Area under the ROC curve analysis to evaluate the value of LDH in bladder cancer patients
Figure 4.
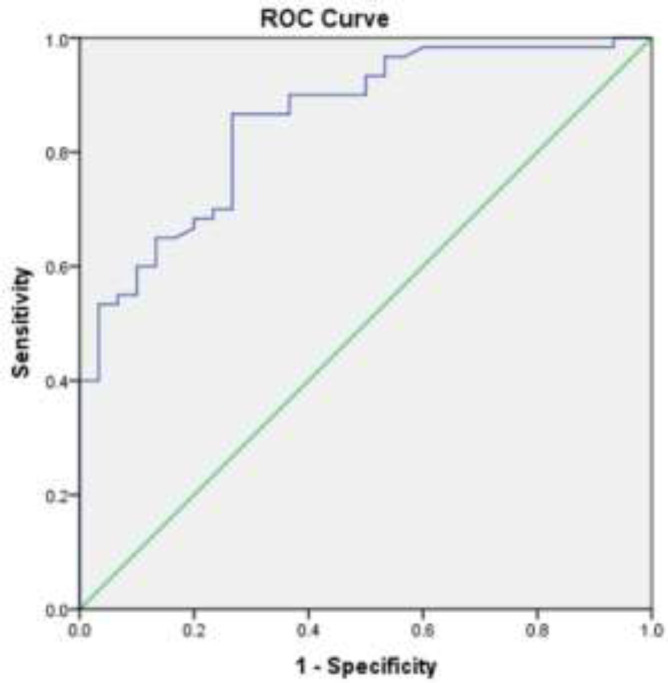
Area under the ROC curve analysis to evaluate the value of FASN in bladder cancer patients
Discussion
In the present study, we found that serum levels of FASN and LDH dramatically increase in bladder cancer patients in comparison to healthy individuals. Furthermore, in tumor stage and grade analysis, it was observed that patients with higher stages of bladder cancer have higher serum levels of LDH and FASN. In line with our results, several studies have confirmed that elevated serum levels of FASN and LDH were correlated with clinicopathologic characteristics and poor prognosis in several types of malignancies including gastric cancer, breast cancer, prostate cancer, and pancreatic cancer (11-13). The role of LDH in urinary tract cancers has been investigated in some previous studies. Yuan D et al. (14) proved that LDHA acts as an oncogene, and its up-regulation in bladder cancer resulted in increased glucose uptake and lactate production that promotes aerobic glycolysis, cell proliferation and invasion. Zhang XK et al. (15) found that elevated serum level of LDH was associated with lymph node metastasis, vascular invasion, tumor necrosis, advanced pathological stage, and subsequent bladder tumor in upper urinary tract urothelial carcinoma (UUTUC), which was consistent with our results showing that patients with high serum level of LDH had increased lymphovascular invasion and more aggressive pathological phenotype. Although several studies confirmed LDH to be a good prognostic predictor in cancers, the exact mechanism is still unclear. LDH plays a major role in the glycolytic pathway and metabolizes the conversion of pyruvate to lactate combined with the NADH and NAD+ conversion. Therefore, a high LDH level may reflect the Warburg effect or the oncogenic aerobic glycolysis, which can induce the malignant transformation and cancer cells survival (15).
Furthermore, the acidic microenvironment and high lactate generated by active tumor cell metabolism could easily accelerate the proliferation and metastasis of tumor cells as well as macrophage-mediated angiogenesis. They also decrease T cell infiltration and cytotoxic function in CD8+ T cells. It has been revealed that the aggressive angiogenesis might be related to the tumor cells metastasis due to the fact that elevated LDH causes overexpression of hypoxia-inducible factor which stimulates vascular endothelial growth factor (16). These underlying mechanisms could explain why patients with higher serum LDH have a worse prognosis. The serum level of LDH is a straightforward marker that is simple to use in the clinic. It also appears to be a helpful marker in therapeutic management decisions as well as a good indicator of the efficacy and efficiency of anti-cancer therapy (16). Hannisdal E et al. showed a strong association between levels of serum LDH and survival after definitive radiotherapy in bladder carcinoma patients (17). Furthermore, LDH has recently been recognized as a possible target in the fight against cancer. For example, Oxamate is a well-known LDHA inhibitor that acts as a single agent or in combination with pembrolizumab or phenformin, has high potential to reduce the progression of non-small cell lung cancer (NSCLC) and brain tumors, respectively (18).
Tumor metabolism is a chief mechanism in carcinogenesis initiation and development. Several studies have confirmed that the metabolism of glucose is essential for cancer development and invasion. A few studies, however, have focused on the role of lipid metabolism in carcinogenesis and cancer progression. FASN is a pivotal enzyme in lipid metabolism. It is low in normal tissue except for liver and adipose tissues, and its role when it is overexpressed in various cancers such as ovarian, breast, and prostate cancers (19-21) is undeniable. FASN was first recognized as an oncogenic antigen 519 (OA519) in breast cancer patients, which was accompanied by a significantly poor prognosis (22). FASN was also demonstrated to be associated with cancer progression, occurrence, metastasis, and chemotherapeutic resistance in different types of cancer; therefore, it becomes a focus of current research on the discovery of novel therapeutic and diagnostic targets for cancer. In terms of urological malignancies, FASN overexpression and its correlation with tumor aggressiveness and poor cancer-specific survival are evident in patients with kidney and prostate cancer (22, 23).
Jiang et al. (24) have reported FASN overexpression in urothelial carcinoma of bladder and inhibition of FASN suppressed phosphorylated AKT (p-AKT) and induced apoptosis in bladder cancer. In another study, Visca et al. (25) indicated that FASN expression is associated with tumor recurrence and advanced stage in bladder cancer. While the mechanism of FASN overexpression-induced malignant transformation or cell proliferation in malignant tumors is not completely understood, several mechanisms, including activation of PI3K / AKT and ERK1/2 signaling pathways, increased expression of epidermal growth factor receptor (EGFR) and modulation and nuclear maturation of the sterol transcription factor regulatory element-binding protein 1C have been described. The development of lipid inhibitors for anti-cancer therapy has garnered renewed interest in recent years (26). There is a therapeutic window for intervention because pharmacological suppression of FASN has demonstrated to be effective in a variety of malignant cells both in vitro and in vivo (27). A significant relationship between smoking (a major risk factor for bladder cancer) and increased FASN serum levels was detected in the present study. Noelle Ma et al., (28) in a study, proved that smoking and nicotine exposure during pregnancy and lactation increase hepatic FASN expression and lead to increased levels of hepatic and circulating triglycerides in postnatal life in rats. They found that FASN expression was enhanced by increased levels of the nuclear receptor liver X receptor (LXRα) protein and binding to the LXRE element of the FASN promoter in postnatal day 180 (PND 180) male offspring of Wistar rats. However, in the Long et al.’s study (29), no significant relationship was observed between smoking and increased serum FASN levels in colorectal cancer patients.
The data suggest that serum FASN and LDH may be useful biomarkers for the prognosis and detection of bladder cancer. Many studies have shown the role of these two factors in the development, prognosis, and metastasis of various cancers. Since the serum level of LDH and FASN can be easily determined in the clinic, which is economical and convenient to perform, they might be a good and useful marker that is practical in the clinical management and diagnosis of bladder cancer, although further studies are needed to determine the utility of LDH and FASN as early detection biomarkers in bladder cancer.
Acknowledgments
We would like to thank Shiraz University of Medical Sciences and Shiraz Institute for cancer research for financial support.
Funding:
This work was supported by a grant from Shiraz University of Medical Sciences Grant no.: 98-01-16-19580 and in part by Shiraz Institute for Cancer Research Grant no.: ICR-100-502.
Ethics approval:
This study was approved by the Research Ethics Committee of Shiraz University of Medical Sciences (IR. SUMS. REC.1398. 540).
Conflict of Interests:
The authors declare no conflict of interest.
Authors’ contribution:
Research conception and design: Mohammad Javad Fattahi, Fatemeh Sedaghat. Data acquisition: Ali Ariafar, Zahra Shiravani, Mahyar Malekzadeh. Statistical analysis: Fatemeh Sedaghat. Data analysis and interpretation: Mohammad Javad Fattahi, Fatemeh Sedaghat, Mohammad Reza Haghshenas. Drafting of the manuscript: Mohammad Javad Fattahi, Fatemeh Sedaghat, and Mohammad Reza Haghshenas. Critical revision of the manuscript: Mohammad Javad Fattahi, Fatemeh Sedaghat, Mohammad Reza Haghshenas, Ali Ariafar. Supervision: Mohammad Javad Fattahi. Administrative, technical, or material support: Mahyar Malekzadeh, Shima Madani. Approval of the final manuscript: Mohammad Javad Fattahi, Fatemeh Sedaghat.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Sanli O, Dobruch J, Knowles MA, et al. Bladder cancer. Nat Rev Dis Primers. 2017;3:17022. doi: 10.1038/nrdp.2017.22. [DOI] [PubMed] [Google Scholar]
- 3.Yousef PG, Gabril MY. An update on the molecular pathology of urinary bladder tumors. Pathol Res Pract. 2018;214:1–6. doi: 10.1016/j.prp.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Gallo M, Sapio L, Spina A, et al. Lactic dehydrogenase and cancer: an overview. Front Biosci (Landmark Ed) 2015;20:1234–49. doi: 10.2741/4368. [DOI] [PubMed] [Google Scholar]
- 5.Chirasani SR, Leukel P, Gottfried E, et al. Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. Int J Cancer. 2013;132:843–53. doi: 10.1002/ijc.27712. [DOI] [PubMed] [Google Scholar]
- 6.Tan P, Chen J, Xie N, et al. Is preoperative serum lactate dehydrogenase useful in predicting the outcomes of patients with upper tract urothelial carcinoma? Cancer Med. 2018;7:5096–5106. doi: 10.1002/cam4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang L, Fang X, Wang H, Li D, Wang X. Ovarian Cancer-Intrinsic Fatty Acid Synthase Prevents Anti-tumor Immunity by Disrupting Tumor-Infiltrating Dendritic Cells. Front Immunol. 2018;9:2927. doi: 10.3389/fimmu.2018.02927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo CY, Ann DK. When fats commit crimes: fatty acid metabolism, cancer stemness and therapeutic resistance. Cancer Commun (Lond) 2018;38:47. doi: 10.1186/s40880-018-0317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cioccoloni G, Aquino A, Notarnicola M, et al. Fatty acid synthase inhibitor orlistat impairs cell growth and down-regulates PD-L1 expression of a human T-cell leukemia line. J Chemother. 2020;32:30–40. doi: 10.1080/1120009X.2019.1694761. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Q, Feng D, Wang Z, et al. Fatty acid synthase is the key regulator of fatty acid metabolism and is related to immunotherapy in bladder cancer. Front Immunol. 2022;13:836939. doi: 10.3389/fimmu.2022.836939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JE, Cook RJ, Lipton A, Coleman RE. Serum lactate dehydrogenase is prognostic for survival in patients with bone metastases from breast cancer: a retrospective analysis in bisphosphonate-treated patients. Clin Cancer Res. 2012;18:6348–55. doi: 10.1158/1078-0432.CCR-12-1397. [DOI] [PubMed] [Google Scholar]
- 12.Ito T, Sato K, Maekawa H, et al. Elevated levels of serum fatty acid synthase in patients with gastric carcinoma. Oncol Lett. 2014;7:616–20. doi: 10.3892/ol.2014.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fazli HR, Moradzadeh M, Mehrbakhsh Z, et al. Diagnostic significance of serum fatty acid synthase in patients with pancreatic cancer. Middle East J Dig Dis. 2021;13:115–20. doi: 10.34172/mejdd.2021.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan D, Zheng S, Wang L, et al. MiR-200c inhibits bladder cancer progression by targeting lactate dehydrogenase A. Oncotarget. 2017;8:67663–9. doi: 10.18632/oncotarget.18801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang XK, Zhang ZL, Lu X, et al. Prognostic significance of preoperative serum lactate dehydrogenase in upper urinary tract urothelial carcinoma. Clin Genitourin Cancer. 2016;14:341–5. doi: 10.1016/j.clgc.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Van Wilpe S, Koornstra R, Den Brok M, et al. Lactate dehydrogenase: a marker of diminished antitumor immunity. Oncoimmunology. 2020;9:1731942. doi: 10.1080/2162402X.2020.1731942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannisdal E, Fosså SD, Høst H. Blood tests and prognosis in bladder carcinomas treated with definitive radiotherapy. Radiother Oncol. 1993;27:117–22. doi: 10.1016/0167-8140(93)90131-q. [DOI] [PubMed] [Google Scholar]
- 18.Qiao T, Xiong Y, Feng Y, et al. Inhibition of LDH-A by Oxamate enhances the efficacy of Anti-PD-1 treatment in an NSCLC humanized mouse model. Front Oncol. 2021;11:632364. doi: 10.3389/fonc.2021.632364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bessadóttir M, Skúladóttir E, Gowan S, et al. Effects of anti-proliferative lichen metabolite, protolichesterinic acid on fatty acid synthase, cell signalling and drug response in breast cancer cells. Phytomedicine. 2014;21:1717–24. doi: 10.1016/j.phymed.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Sadowski MC, Pouwer RH, Gunter JH, et al. The fatty acid synthase inhibitor triclosan: repurposing an anti-microbial agent for targeting prostate cancer. Oncotarget. 2014;5:9362–81. doi: 10.18632/oncotarget.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veigel D, Wagner R, Stübiger G, et al. Fatty acid synthase is a metabolic marker of cell proliferation rather than malignancy in ovarian cancer and its precursor cells. Int J Cancer. 2015;136:2078–90. doi: 10.1002/ijc.29261. [DOI] [PubMed] [Google Scholar]
- 22.Epstein JI, Carmichael M, Partin AW. OA-519 (fatty acid synthase) as an independent predictor of pathologic state in adenocarcinoma of the prostate. Urology. 1995;45:81–6. doi: 10.1016/s0090-4295(95)96904-7. [DOI] [PubMed] [Google Scholar]
- 23.Horiguchi A, Asano T, Asano T, et al. Fatty acid synthase over expression is an indicator of tumor aggressiveness and poor prognosis in renal cell carcinoma. J Urol. 2008;180:1137–40. doi: 10.1016/j.juro.2008.04.135. [DOI] [PubMed] [Google Scholar]
- 24.Jiang B, Li EH, Lu YY, et al. Inhibition of fatty-acid synthase suppresses P-AKT and induces apoptosis in bladder cancer. Urology. 2012;80:484. e9–15. doi: 10.1016/j.urology.2012.02.046. [DOI] [PubMed] [Google Scholar]
- 25.Visca P, Sebastiani V, Pizer ES, et al. Immunohistochemical expression and prognostic significance of FAS and GLUT1 in bladder carcinoma. Anticancer Res. 2003;23:335–9. [PubMed] [Google Scholar]
- 26.Flavin R, Peluso S, Nguyen PL, Loda M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6:551–562. doi: 10.2217/fon.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ventura R, Mordec K, Waszczuk J, et al. Inhibition of de novo palmitate synthesis by fatty acid synthase induces apoptosis in tumor cells by remodeling cell membranes, inhibiting signaling pathways, and reprogramming gene expression. EBioMedicine. 2015;2:808–824. doi: 10.1016/j.ebiom.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma N, Nicholson CJ, Wong M, Holloway AC, Hardy DB. Fetal and neonatal exposure to nicotine leads to augmented hepatic and circulating triglycerides in adult male offspring due to increased expression of fatty acid synthase. Toxicol Appl Pharmacol. 2014;275:1–11. doi: 10.1016/j.taap.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Long QQ, Yi YX, Qiu J, Xu CJ, Huang PL. Fatty acid synthase (FASN) levels in serum of colorectal cancer patients: correlation with clinical outcomes. Tumour Biol. 2014;35:3855–9. doi: 10.1007/s13277-013-1510-8. [DOI] [PubMed] [Google Scholar]


