Abstract
Background:
Bleeding during nose surgery is very important, because it can cause a problem in the surgeon's field of view and lead to an increase in the probability of surgical complications. The aim of this study was to compare the local effects of tranexamic acid and phenylephrine on bleeding in rhinoplasty.
Methods:
The present study is a double-blind clinical trial in which 98 patients who were candidates for rhinoplasty Shiraz were randomly divided into 49 groups of phenylephrine and tranexamic acid. In the first group, 1 cc of phenylephrine and in the second group, 1 cc of tranexamic acid was used locally and then the variables in the two groups were compared. The current study was approved by the Iranian Clinical Trial Registration Center with code IRCT20201205049602N1.
Results:
The average blood pressure and heart rate in the phenylephrine group first increased and then decreased, but in the tranexamic acid group decreased from the beginning to 30 minutes. Based on the results of the test, there was a difference between the two groups in terms of the amount of bleeding, and a statistically significant relationship was observed. The average bleeding volume was lower in the phenylephrine group (p<0.0001).
Conclusion:
Results of this study showed that the amount of bleeding in the phenylephrine group was lower than that of tranexamic acid. It is recommended to use phenylephrine in rhinoplasty surgery to reduce bleeding and improve the surgeon's vision.
Key Words: Tranexamic acid, Phenylephrine, Rhinoplasty, Bleeding
One of the most common cosmetic surgeries is rhinoplasty. In 2020, 352,555 people underwent this surgery in America (1). This statistic has higher figures in Asia and especially in Iran, to the point where Iran has been named as the capital of rhinoplasty surgery in the world (1-3). Rhinoplasty is an elective surgery that people incur costs and surgery due to beauty, so correct rhinoplasty and reducing its short-term and long-term complications is very important because the smallest mistake can cause a dissatisfaction with an elective surgery and need to repeated surgeries (4-7). Similar to other surgeries, rhinoplasty has short and long-term side effects such as infection, cartilage side effects, soft tissue related side effects, remaining surgical scars, deformities caused by surgery, etc. and the reduction of these complications leads to greater satisfaction and reduction of patient problems. The most common and early complication is bleeding during and after surgery (8).
Bleeding is one of the most annoying complications in rhinoplasty surgery, which causes an increase in surgery time, a decrease in the surgeon's vision and uncertainty about the outcome of the surgery, anemia and the need for blood transfusions and hemodynamic problems during surgery, long-term edema of the eyelids and an increase in recovery time also probability of death (9).
Therefore, one of the necessary interventions in rhinoplasty is to reduce bleeding during and after surgery (10). Various studies have been conducted to reduce nosebleeds by using different types of drugs, such as tranexamic acid, phenylephrine, labetalol, nitroglycerin, lidocaine, clonidine, epinephrine, dexamethasone, etc. (11, 12). Among these drugs, two very common drugs to reduce bleeding are tranexamic acid and phenylephrine. Tranexamic acid is an anti-fibrinolytic drug that is commonly used to reduce rhinoplasty bleeding and its use has been emphasized in many studies (13, 14). The use of phenylephrine (alpha-1 adrenergic receptor agonist) as a vasoconstrictor in reducing and stopping nosebleeds has been going on for a long time (15).
But what is important is to decide on the use of a drug that has the least side effects and leads to the best performance, especially reducing the volume of bleeding (16). Despite the fact that tranexamic acid is recognized by the World Health Organization (WHO) as an essential drug, it is cheap and available in many hospitals (9), many questions regarding other effects and clinical complications such as anti-inflammatory response to cardiac bypass pulmonary, the risk of thromboembolism, adverse neurological effects (seizures), as well as disease and death caused by the systemic administration of tranexamic acid are unanswered (8), But in the local application of the drug, the concern for the occurrence of these side effects is minimized (17).
Therefore, considering the unavoidable side effects of the systemic use of tranexamic acid to reduce bleeding and the importance of the amount of bleeding in selective surgeries such as rhinoplasty, and considering the few studies that have been conducted in the field of topical application of tranexamic acid to reduce bleeding during rhinoplasty, also Since no study has been conducted to compare the effectiveness of topical phenylephrine and topical tranexamic acid in rhinoplasty, the comparison of more common drugs to manage bleeding can be one of the research priorities, and its results will greatly contribute to the faster recovery of patients. This study was conducted with the aim of comparing the local effect of tranexamic acid and phenylephrine on the amount of bleeding in rhinoplasty.
Methods
Study type: The current study is a double-blind clinical trial that investigated the effect of topical tranexamic acid compared to topical phenylephrine in controlling rhinoplasty surgery bleeding.
Research population: The research population included 20 to 30-year-old rhinoplasty candidates under general anesthesia at Shahid Ayatollah Dastgheib Hospital in Shiraz in 2021.
Research area: The research area was Shahid Ayatollah Dastgheib Hospital in Shiraz. Shiraz, the capital of Fars province, is located in the south of Iran. Sample size using a similar study (18) and considering the probability of success of phenylephrine (28.3%) and tranexamic acid (66.7%) to improve bleeding, the sample size was estimated to be 98 49 people in each group) people. (The first type error and the power of the study were considered equal to 0.01 and 90%, respectively.
Research sample: The research sample included patients 20 to 30 years old who were candidates for rhinoplasty, under general anesthesia, referring to Shahid Ayatollah Dastgheib Hospital in Shiraz.
The inclusion criteria included patients who were candidates for rhinoplasty, inclined to participate in the intervention, and had full consciousness at the time of informed consent, and the exclusion criteria included known sensitivity to phenylephrine or tranexamic acid, blood pressure above 140/90, tachycardia, history of disorders. coagulation such as hemophilia and venous thrombosis; Cerebral stroke, embolism, taking warfarin, liver, kidney, heart diseases and reluctance to continue cooperation and withdraw from the study.
Sampling: Randomization block method was used for random assignment. Since the final sample size was 98 people, 7 blocks of 14 cells were used. The sequence of blocks was determined randomly. At first, the researcher wrote the name of each block (1 to 7) on a separate paper and put it in the envelopes. In the next step, using a simple random sampling method, he chose one of the envelopes (for example, envelope 3), so the entry of people into the study starts from block number 3. There are 7 A cells and 7 B cells in each block. According to the agreement predetermined by the principal investigator, those placed in cell A will receive Phenylephrine treatment and those placed in cell B will receive Tranexamic acid treatment. In the end, 49 people were in Phenylephrine group and 49 people were in Tranexamic acid group.
Blinding: The patient was unconscious and was not aware of his assignment to any of the studied groups. The preparation of the mesh containing phenylephrine and tranexamic acid was done by an independent colleague, and the doctor and the person evaluating the results were not aware of the assignment of the studied subjects to the studied groups.
Intervention: During the implementation of the study, the patients were divided into two intervention groups. In the first group, 1 cc of phenylephrine (Phenylephrine Hcl, Nasal Drop, 0.5%) was drawn with a syringe and it was poured on the mesh and packed for five minutes immediately after intubation and before making a surgical incision inside the nasal cavity and also in the second group, 1 cc of tranexamic acid injection solution (Tranexamic Acid, Injection Solution 500mg/5ml) with a drawn syringe and it was poured on the mesh and packed for five minutes immediately after intubation and before making a surgical incision inside the nasal cavity. In this study, general anesthesia was used for all patients, and the anesthesia team and anesthetic methods and drugs were the same in both groups, and midazolam, fentanyl, atracurium, sodium thiopental (STP), propofol, and remifentanil were used to anesthetize the patient.
Research tool: The data collection tool included a checklist made by the researcher, which included four parts in this study. The first part is related to the demographic information of the patient (age, gender), the second part includes the amount of bleeding during surgery (the amount of blood in the suction minus the amount of washing liquid). The third part was related to the surgeon's satisfaction with the amount of bleeding in surgery and the fourth part included a checklist to record the patient's hemodynamic symptoms (systolic blood pressure (SBP) and diastole (DBP) and heart rate). Four main outcomes were evaluated including intraoperative bleeding, systolic blood pressure, diastolic blood pressure and heart rate.
Statistical analysis: Quantitative data were described with mean (standard deviation) and qualitative with number (percentage). To compare quantitative variables between two groups, t-test and qualitative variables were used, chi-square test.
Friedman and Cochran's tests as well as analysis of variance with repeated observations were used to check the changes in each group. To compare the trend of changes and after removing the effect of other variables, generalized estimating equation (GEE) was used. A statistical significance level of 0.05 was considered and all statistical analyses were performed with STATA Version 14 software.
Ethical considerations: The results of the present study were the results of a Master's thesis at Mazandaran University of Medical Sciences, which was approved by the Ethics Committee of this university with code IR.MAZUMS.REC.1399.518. Also, the current study was approved by the Iranian Clinical Trial Registration Center with code IRCT20201205049602N1.
Results
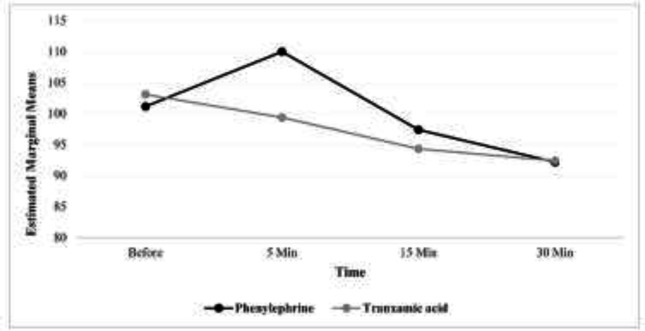
In this clinical trial study, 98 people in two groups of phenylephrine and tranexamic acid treatment, 49 people in each group participated and were examined. 69 (70.4%) people of the study participants were women and the rest were men. The surgery time of the studied subjects varied from 1.1 to 2.25 hours and the mean (standard deviation) of this time was equal to 1.41 (0.25) hours. The proportion of women in the phenylephrine group was higher than the tranexamic acid group, and this difference was statistically significant (P=0.020). The duration of surgery was similar in both groups. The outcomes investigated in systolic blood pressure and their comparison using the GEE model showed that in both groups the trend of changes was decreasing (the presence of time effect, p<0.001).
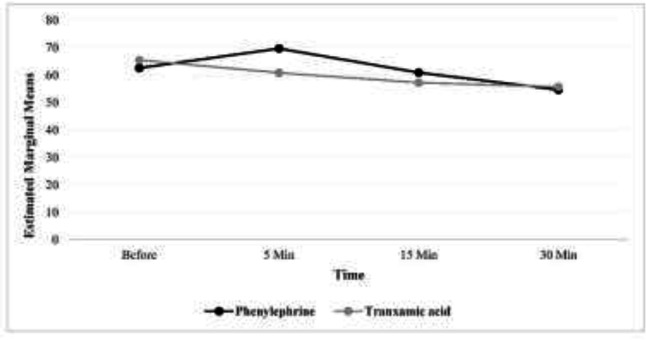
In other words, while in the phenylephrine group, the systolic blood pressure had an upward trend at the beginning and 5 minutes after the surgery, but then it started to decrease, however, in the tranexamic acid group, from the beginning of the time after the surgery to 30 minutes after that, this trend has been completely downward. To compare the systolic blood pressure of the two groups after controlling for the effect of gender, in the GEE model, it was found that there is no statistically significant difference between the two groups (P=0.780). The outcomes investigated in diastolic blood pressure and their comparison using the GEE model, showed that in both groups the trend of changes was decreasing (the presence of time effect, p<0.001).
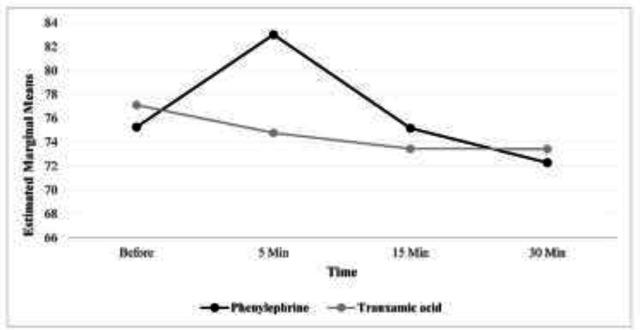
In other words, while in the phenylephrine group, diastolic blood pressure had an upward trend at the beginning and 5 minutes after surgery but later, it started a downward trend, but in the group of tranexamic acid, from the beginning of the time after surgery to 30 minutes after that, this trend was completely downward. The outcomes examined in heart rate showed that in both groups the trend of changes was decreasing (the presence of time effect, p<0.001). In other words, while in the phenylephrine group, the heart rate increased at the beginning and after 5 minutes after the surgery, but then it decreased, but in the tranexamic acid group, from the beginning of time after surgery to 30 minutes later, this trend was completely downward. After controlling the effect of gender, in the GEE model, it was found that there is no statistically significant difference between the two groups (P=0.920) (table 1).
Table 1.
The mean (standard deviation) of the investigated outcomes and their comparison using the GEE model
| Outcome | Groups | Time | Effect | |||||
|---|---|---|---|---|---|---|---|---|
| Before | 5 Min | 15 Min | 30 Min | Time (within group) | Between group † | Interaction ‡ | ||
| Systolic blood pressure (mm Hg) | Phenylephrine | 101.12 (16.11) | 109.98 (14.18) | 97.39 (15.52) | 92.16 (14.25) | P<0.001 | P=0.780 | P<0.001 |
| Tranxamic acid | 103.08 (14.42) | 99.37 (15.62) | 94.31 (14.93) | 92.39 (14.83) | P<0.001 | |||
| Diastolic blood pressure (mm Hg) | Phenylephrine | 62.59 (14.68) | 69.67 (14.34) | 60.92 (14.12) | 54.64 (12.64) | P<0.001 | P=0.650 | P<0.001 |
| Tranxamic acid | 65.47 (13.93) | 60.82 (14.69) | 57.26 (14.38) | 55.71 (13.93) | P<0.001 | |||
| Heart rate | Phenylephrine | 75.27 (10.53) | 83.02 (10.65) | 75.18 (11.08) | 72.29 (11.05) | P<0.001 | P=0.920 | P<0.001 |
| Tranxamic acid | 77.12 (11.34) | 74.78 (12.09) | 73.45 (11.77) | 73.43 (11.77) | P<0.001 | |||
† After adjustment for sex in GEE model, ‡ Interaction between time and Drug
Figures 1, 2 and 3 respectively show the average systolic blood pressure, diastolic blood pressure and heart rate at different times in the two treatment groups. The mean volume of bleeding in the group of tranexamic acid was more than that of the group of phenylephrine and the observed difference was statistically significant (p<0.001) (table 2).
Figure 1.
Average systolic blood pressure at different times in two groups
Figure 2.
Average diastolic blood pressure at different times in two groups
Figure 3.
Average heart rate at different times in two treatment groups
Table 2.
Comparison of bleeding volume in two treatment groups
| Outcome | Groups | N | Mean±SD * | P-value |
|---|---|---|---|---|
| Bleeding volume (cc) | Phenylephrine | 49 | 101.12±34.60 | <0.001 |
| Tranxamic acid | 49 | 150.82±45.80 |
* Standard Deviation
In terms of the satisfaction of the surgeon and the need for suction to drain the blood from the surgical site, the proportion of people who had moderate bleeding and needed suction was more in the group of tranexamic acid than in the group of phenylephrine, and the observed difference was also statistically significant(p<0.001) (table 3).
Table 3.
Comparison of the surgeon's satisfaction level of bleeding in two treatment groups
| Medicine type | Surgeon's consent | N (%) | P-value |
|---|---|---|---|
| Phenylephrine | Mild/ Sometimes suction was needed | 17 (34.7) | <0.001 |
| Mild/often suction was needed | 32 (65.3) | ||
| Moderate bleeding | 0 (0.0) | ||
| Tranxamic acid | Mild/ Sometimes suction was needed | 4 (8.2) | |
| Mild/often suction was needed | 36 (73.5) | ||
| Moderate bleeding | 9 (18.4) |
Discussion
This study was conducted with the aim of comparing the local use of phenylephrine and tranexamic acid in reducing rhinoplasty bleeding. The results of this study indicate that there is no significant difference (comparatively) in the use of two drugs tranexamic acid and phenylephrine in changes in systolic and diastolic blood pressure and heart rate during rhinoplasty surgery. But the systolic and diastolic blood pressure and heart rate in the use of phenylephrine first increased (on average 10 mm of mercury in blood pressure and less than 15 beats in heart rate) and then decreased, but there has been a continuous decline in tranexamic acid. The results obtained in the field of increased blood pressure and heart rate in the use of phenylephrine due to vasoconstriction are not far from mind. Although the results of this study do not statistically indicate the use of tranexamic acid compared to phenylephrine, but considering the initial increase in blood pressure with the use of phenylephrine, it is recommended that in patients with high blood pressure (without considering other issues such as bleeding volume and surgeon's satisfaction) tranexamic acid should be used as the drug of choice. Tranexamic acid is a synthetic lysine analogue antifibrinolytic that competitively prevents the dissolution and destruction of fibrin clots by plasmin with the lysine block on plasminogen, thus reducing bleeding (19). Phenylephrine is an alpha-1 adrenergic receptor agonist that reduces bleeding by contracting the smooth muscles of peripheral vessels (18). Bixi Gao states that the use of tranexamic acid is more appropriate in reducing hematoma and bleeding in patients with moderate and severe hypertension, which is consistent with the current study (20). Hartley C Atkinson has also stated that the use of phenylephrine in patients causing an initial increase in blood pressure and then its decrease (21), although this transient increase does not affect the clinical course of the patient according to the study of Bethany Stavert (22). In Kazemi’s study, contrary to the current study, does not consider the use of phenylephrine to cause changes in blood pressure and heart rate (23).The need for more studies on the use of these two drugs in patients with high blood pressure is evident.
But But the most important issue in local and short surgeries such as rhinoplasty surgery is the amount of bleeding and the satisfaction of the surgeon. The results of this study indicate the appropriate effect of phenylephrine on reducing the amount of bleeding, the need for suction, and increasing the surgeon's satisfaction with the reduction of bleeding and better visibility compared to tranexamic acid. We recommend that phenylephrine be used in rhinoplasty surgery to reduce bleeding and increase the surgeon's vision. Francis X. Guyette, in a study aimed at reducing bleeding in 927 patients with pre-hospital injuries, did not consider tranexamic acid to be a suitable drug to reduce the mortality rate due to bleeding, which is not consistent with the present study (24).
On the other hand, Amini stated that, in patients with nosebleeds, after taking anticoagulant drugs, tranexamic acid drug had a better effect and in a shorter period of time than phenylephrine (25). Another study by Atabaki et al. regarding hemostasis in nosebleeds with two drugs tranexamic acid and phenylephrine reported that after 10 minutes, a statistically significant relationship was observed between the two groups, which is consistent with the present study (18). The researcher believes that there is a need for more studies on the time of using tranexamic acid and the type of drug used (intravenous, oral, topical), which is according to the findings of Amy Brenner 2019. He has stated that the time of using tranexamic acid is very important in reducing bleeding to the extent that it can make this drug ineffective (26). One of the major limitations of the present study is that the long-term effect or complications associated with phenylephrine or tranexamic acid beyond 30 minutes and these effects may remain unknown. It is recommended to use phenylephrine in rhinoplasty surgery to reduce bleeding and improve the surgeon's vision. More studies are needed in patients with high blood pressure and the use of non-local drugs.
Acknowledgments
We thank all operating room personnel, doctors, patients for participating in this study.
Funding:
This article is extracted from the results of the master's thesis of student of surgical technology approved at Mazandaran University of Medical Sciences with the ethics code IR.MAZUMS.REC.1399.518 and the IRCT20201205049602N1.
Conflict of Interests:
The authors declare no conflict of interest.
Authors’ contribution:
N. SH and H. A: study design, conceptualization and drafting of the article; A.A: Methodology and statistical analysis; E. K, E. N. F and H. D: critical revision and writing of the draft of the article. All authors cooperated in the stages of the study and read and approved the present article.
Availability of data and materials:
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethical consideration:
In this study, the 31 codes approved by the Ethics Committee of the research of the Ministry of Health and Medical Education of Iran was taken into consideration by the researchers and followed-up.
This study was implemented after receiving the code of ethics from the ethics committee and permission from the university officials, and before entering the patients into the study, the process and purpose of the study were explained to the patients and written informed consent was obtained. The opinions of the patients were respected and if the participating patients were not willing to cooperate after some time in the middle of the project, they were excluded from the study.
According to the ethical principles, the researchers are obliged to inform the participants of the results after the end of the project. If the researchers realize that there are risks for the participants during the project, they will prevent it from continuing.
Consent for publication:
Not applicable.
References
- 1.American Society of Plastic Surgeons (ASPS) Plastic surgery statistics report in 2020 Accessed Jan 25, 2022. Available from: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf.
- 2.Kalantar Motamedi MH, Ebrahimi A, Shams A, Nejadsarvari N. Health and social Problems of rhinoplasty in Iran. World J Plast Surg. 2016;5:75–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Loghmani S, Loghmani S, Baghi H, Hoghoughi MA, Dalvi F. Demographic characteristics of patients undergoing rhinoplasty: A single center two-time-period comparison. World J Plast Surg. 2017;6:275–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Blasberg EA, Golden J, Rubin S, Spiegel JH. Board certification and surgeon's fee for aesthetic rhinoplasty. Facial Plast Surg. 2022;38:188–92. doi: 10.1055/s-0041-1729631. [DOI] [PubMed] [Google Scholar]
- 5.Heilbronn C, Cragun D, Wong BJF. Complications in rhinoplasty: A literature review and comparison with a survey of consent forms. Facial Plast Surg Aesthet Med. 2020;22:50–6. doi: 10.1089/fpsam.2019.29007.won. [DOI] [PubMed] [Google Scholar]
- 6.Lao WW, Hsieh TY, Ramirez AE. Differences and similarities between Eastern and Western rhinoplasty: Features and proposed algorithms. Ann Plast Surg. 2021;86:S259–64. doi: 10.1097/SAP.0000000000002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rettinger G. Risks and complications in rhinoplasty. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2007;6 Doc08. [PMC free article] [PubMed] [Google Scholar]
- 8.Eytan DF, Wang TD. Complications in rhinoplasty. Clin Plast Surg. 2022;49:179–89. doi: 10.1016/j.cps.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 9.de Vasconcellos SJA, do Nascimento-Júnior EM, de Aguiar Menezes MV, et al. Preoperative Tranexamic acid for treatment of bleeding, edema, and ecchymosis in patients undergoing rhinoplasty: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144:816–23. doi: 10.1001/jamaoto.2018.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGuire C, Nurmsoo S, Samargandi OA, Bezuhly M. Role of Tranexamic acid in Reducing intraoperative blood loss and postoperative edema and ecchymosis in primary elective rhinoplasty: A systematic review and meta-analysis. JAMA Facial Plast Surg. 2019;21:191–8. doi: 10.1001/jamafacial.2018.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhaddad ST, Khanna AK, Mascha EJ, Abdelmalak BB. Phenylephrine as an alternative to cocaine for nasal vasoconstriction before nasal surgery: A randomised trial. Indian J Anaesth. 2013;57:163–9. doi: 10.4103/0019-5049.111844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motazedian G, Sohrabpour M, Jahromi MS, Ghaedi M. Bleeding management in rhinoplasty surgery: A systematic review study on clinical trial studies conducted in Iran. Int J Med Investig. 2021;10:32–40. [Google Scholar]
- 13.Elena Scarafoni E. A systematic review of Tranexamic acid in plastic surgery: What's new? Plast Reconstr Surg Glob Open. 2021;9:e3172. doi: 10.1097/GOX.0000000000003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph J, Martinez-Devesa P, Bellorini J, Burton MJ. Tranexamic acid for patients with nasal haemorrhage (epistaxis) Cochrane Database Syst Rev. 2018;12:CD004328. doi: 10.1002/14651858.CD004328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellew SD, Johnson KL, Nichols MD, Kummer T. Effect of intranasal vasoconstrictors on blood pressure: A randomized, double-blind, placebo-controlled trial. J Emerg Med. 2018;55:455–64. doi: 10.1016/j.jemermed.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uner OC, Kuru HI, Cinbis RG, Tastan O, Cicek AE. DeepSide: A deep learning approach for drug side effect prediction. IEEE/ACM Trans Comput Biol Bioinform. 2023;20:330–9. doi: 10.1109/TCBB.2022.3141103. [DOI] [PubMed] [Google Scholar]
- 17.Ausen K, Fossmark R, Spigset O, Pleym H. Safety and efficacy of local Tranexamic acid for the prevention of surgical bleeding in soft-tissue surgery: A review of the literature and recommendations for plastic surgery. Plast Reconstr Surg. 2022;149:774–87. doi: 10.1097/PRS.0000000000008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atabaki P, Samarei R, Aribi MS, Soheili A, Mehryar HR. A comparative study on the effect of topical phenylephrine with topical tranexamic acid in management of epistaxis. Nurs Midw J. 2017;15:488–96. [Google Scholar]
- 19.Ker K, Roberts I. Tranexamic acid for surgical bleeding. BMJ. 2014;349:g4934. doi: 10.1136/bmj.g4934. [DOI] [PubMed] [Google Scholar]
- 20.Gao B, Xue T, Rong X, et al. Tranexamic acid inhibits hematoma expansion in intracerebral hemorrhage and traumatic brain injury Does blood pressure play a potential role? A meta-analysis from randmized controlled rrials. J Stroke Cerebrovasc Dis. 2021;30:105436. doi: 10.1016/j.jstrokecerebrovasdis.2020.105436. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson HC, Potts AL, Anderson BJ. Potential cardiovascular adverse events when phenylephrine is combined with paracetamol: simulation and narrative review. Eur J Clin Pharmacol. 2015;71:931–8. doi: 10.1007/s00228-015-1876-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stavert B, McGuinness MB, Harper CA, Guymer RH, Finger RP. Cardiovascular adverse effects of phenylephrine eyedrops: A systematic review and meta-analysis. JAMA Ophthalmol. 2015;133:647–52. doi: 10.1001/jamaophthalmol.2015.0325. [DOI] [PubMed] [Google Scholar]
- 23.Kazemi A, McLaren JW, Sit AJ. Effect of topical phenylephrine 2 5% on episcleral venous pressure in normal human eyes. Invest Ophthalmol Vis Sci. 2021;62 doi: 10.1167/iovs.62.13.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyette FX, Brown JB, Zenati MS, et al. Tranexamic acid during prehospital transport in patients at risk for hemorrhage after injury: A double-blind, placebo-controlled, randomized clinical trial. JAMA Surg. 2020;156:11–20. doi: 10.1001/jamasurg.2020.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amini K, Arabzadeh A, Jahed S, Amini P. Topical Tranexamic acid versus phenylephrine-lidocaine for the treatment of anterior epistaxis in patients taking aspirin or clopidogrel; a randomized clinical trial. Arch Acad Emerg Med. 2021;9:e6. doi: 10.22037/aaem.v9i1.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner A, Ker K, Shakur-Still H, Roberts I. Tranexamic acid for post-partum haemorrhage: What, who and when. Best Pract Res Clin Obstet Gynaecol. 2019;61:66–74. doi: 10.1016/j.bpobgyn.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.