Abstract
Collagen biosynthesis by organ cultures of the hypertrophic zone of calf growth-plate cartilage was studied. It was found that this tissue devotes a large portion of its biosynthetic commitment towards production of a collagen molecule comprising short collagen chains. This collagen is similar to short-chain collagens synthesized by chick-embryo tibiotarsus, rabbit growth-plate cartilage and chick chondrocytes grown in three-dimensional gels. However, in contrast with the collagen synthesized in these three systems, the short-chain collagen synthesized by calf growth-plate hypertrophic cartilage is stabilized by disulphide bonds localized within the pepsin-resistant triple-helical collagenous domains of these molecules.
Full text
PDF

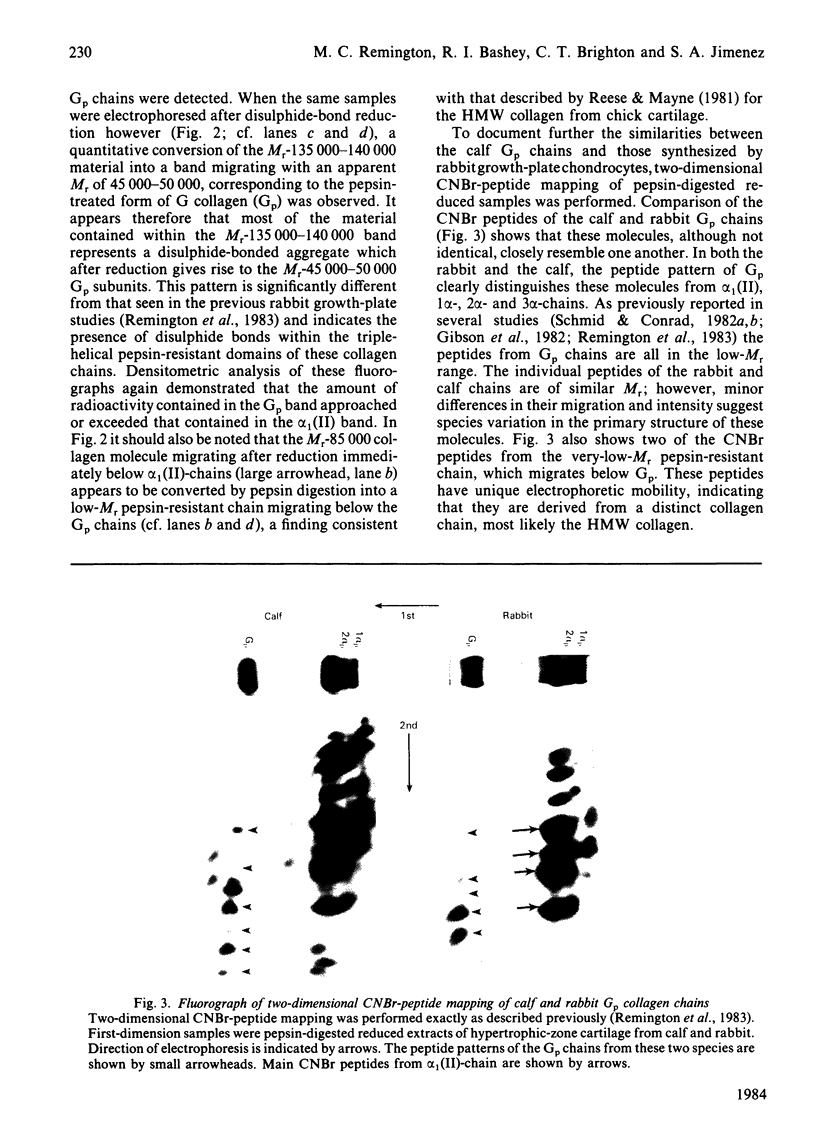
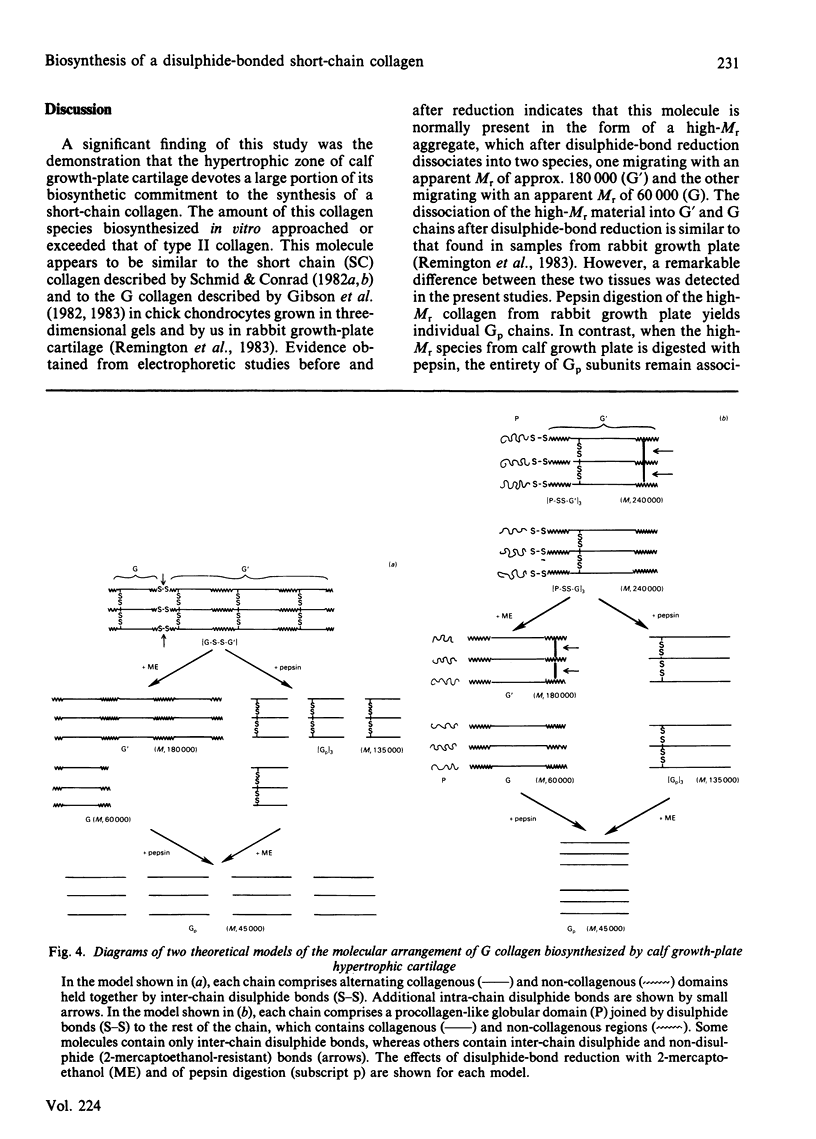


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayad S., Abedin M. Z., Grundy S. M., Weiss J. B. Isolation and characterisation of an unusual collagen from hyaline cartilage and intervertebral disc. FEBS Lett. 1981 Jan 26;123(2):195–199. doi: 10.1016/0014-5793(81)80286-4. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., Byers P. H. Reduced secretion of structurally abnormal type I procollagen in a form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5142–5146. doi: 10.1073/pnas.78.8.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Benya P. D. Two-dimensional CNBr peptide patterns of collagen types I, II and III. Coll Relat Res. 1981;1(1):17–26. doi: 10.1016/s0174-173x(80)80004-5. [DOI] [PubMed] [Google Scholar]
- Burgeson R. E., Hebda P. A., Morris N. P., Hollister D. W. Human cartilage collagens. Comparison of cartilage collagens with human type V collagen. J Biol Chem. 1982 Jul 10;257(13):7852–7856. [PubMed] [Google Scholar]
- Burgeson R. E., Hollister D. W. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- Gibson G. J., Kielty C. M., Garner C., Schor S. L., Grant M. E. Identification and partial characterization of three low-molecular-weight collagenous polypeptides synthesized by chondrocytes cultured within collagen gels in the absence and in the presence of fibronectin. Biochem J. 1983 May 1;211(2):417–426. doi: 10.1042/bj2110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Schor S. L., Grant M. E. Effects of matrix macromolecules on chondrocyte gene expression: synthesis of a low molecular weight collagen species by cells cultured within collagen gels. J Cell Biol. 1982 Jun;93(3):767–774. doi: 10.1083/jcb.93.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielty C. M., Hulmes D. J., Schor S. L., Grant M. E. Embryonic chick cartilage collagens. Differences in the low-Mr species present in sternal cartilage and tibiotarsal articular cartilage. FEBS Lett. 1984 Apr 24;169(2):179–184. doi: 10.1016/0014-5793(84)80314-2. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Mayne R. Minor collagens of chicken hyaline cartilage. Biochemistry. 1981 Sep 15;20(19):5443–5448. doi: 10.1021/bi00522a014. [DOI] [PubMed] [Google Scholar]
- Reese C. A., Wiedemann H., Kühn K., Mayne R. Characterization of a highly soluble collagenous molecule isolated from chicken hyaline cartilage. Biochemistry. 1982 Mar 2;21(5):826–830. doi: 10.1021/bi00534a002. [DOI] [PubMed] [Google Scholar]
- Remington M. C., Bashey R. I., Brighton C. T., Jimenez S. A. Biosynthesis of a low molecular weight collagen by rabbit growth plate cartilage organ cultures. Coll Relat Res. 1983 May;3(3):271–277. doi: 10.1016/s0174-173x(83)80009-0. [DOI] [PubMed] [Google Scholar]
- Schmid T. M., Conrad H. E. A unique low molecular weight collagen secreted by cultured chick embryo chondrocytes. J Biol Chem. 1982 Oct 25;257(20):12444–12450. [PubMed] [Google Scholar]
- Schmid T. M., Conrad H. E. Metabolism of low molecular weight collagen by chondrocytes obtained from histologically distinct zones of the chick embryo tibiotarsus. J Biol Chem. 1982 Oct 25;257(20):12451–12457. [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. A short chain (pro)collagen from aged endochondral chondrocytes. Biochemical characterization. J Biol Chem. 1983 Aug 10;258(15):9504–9509. [PubMed] [Google Scholar]
- Shimokomaki M., Duance V. C., Bailey A. J. Identification of a new disulphide bonded collagen from cartilage. FEBS Lett. 1980 Nov 17;121(1):51–54. doi: 10.1016/0014-5793(80)81265-8. [DOI] [PubMed] [Google Scholar]
- Stambaugh J. E., Brighton C. T. Diffusion in the various zones of the normal and the rachitic growth plate. J Bone Joint Surg Am. 1980 Jul;62(5):740–749. [PubMed] [Google Scholar]