Abstract
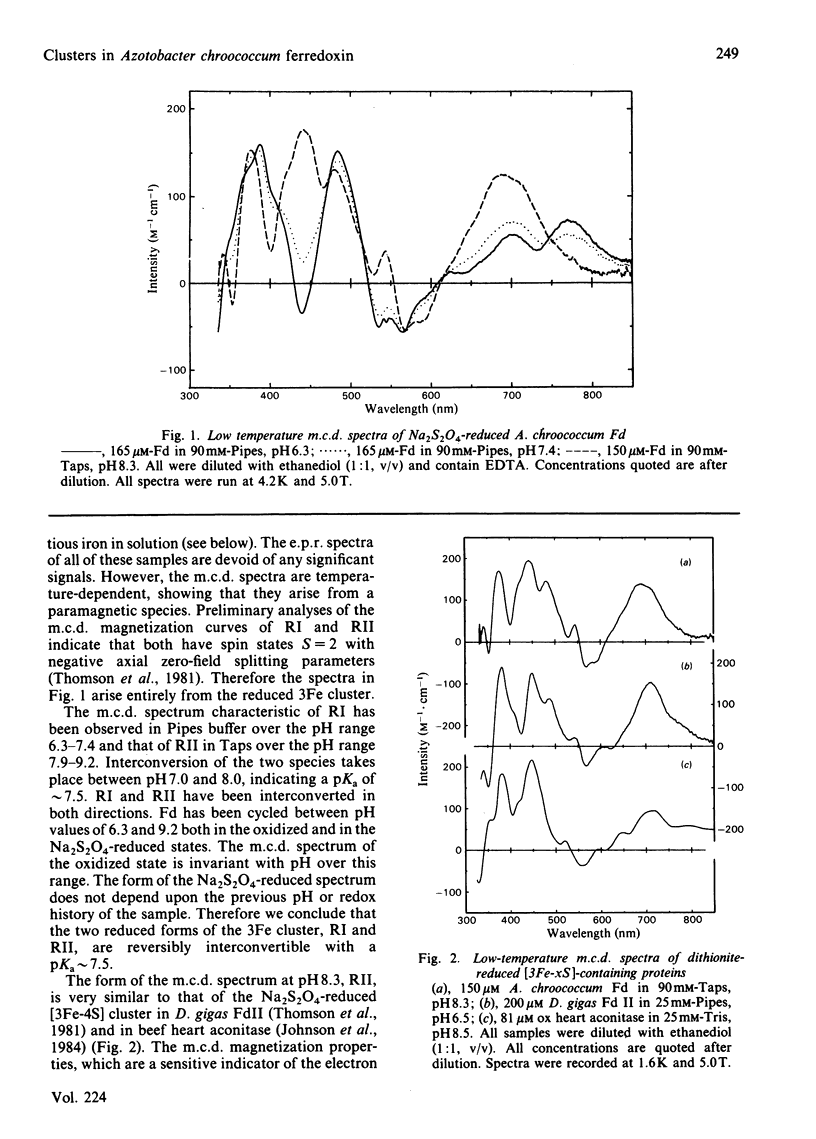
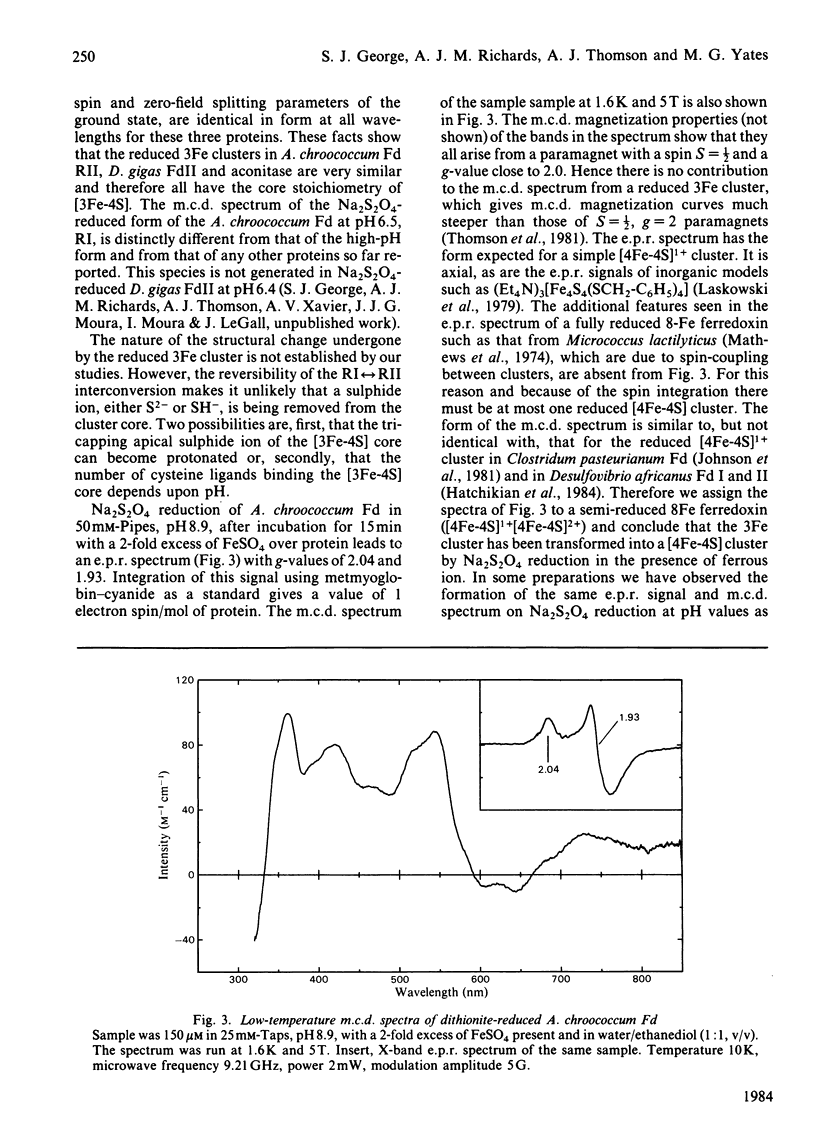
Ferredoxin from Azotobacter chroococcum has been studied by low-temperature magnetic-circular-dichroism and electron-paramagnetic-resonance spectroscopy. When aerobically isolated ferredoxin contains a [3Fe-4S] and [4Fe-4S] cluster. Anaerobic treatment with dithionite in the presence of ethanediol reduces the [3Fe-4S] cluster to give two spectroscopically distinct forms RI and RII which are reversibly interconvertible with a pKa approximately 7.5. The higher-pH form, RII, has a high affinity for ferrous ion and converts readily to a [4Fe-4S]1+ cluster, scavenging iron from the medium. The presence of the iron chelator EDTA inhibits this conversion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonio M. R., Averill B. A., Moura I., Moura J. J., Orme-Johnson W. H., Teo B. K., Xavier A. V. Core dimensions in the 3Fe cluster of Desulfovibrio gigas ferredoxin II by extended X-ray absorption fine structure spectroscopy. J Biol Chem. 1982 Jun 25;257(12):6646–6649. [PubMed] [Google Scholar]
- Beinert H., Emptage M. H., Dreyer J. L., Scott R. A., Hahn J. E., Hodgson K. O., Thomson A. J. Iron-sulfur stoichiometry and structure of iron-sulfur clusters in three-iron proteins: evidence for [3Fe-4S] clusters. Proc Natl Acad Sci U S A. 1983 Jan;80(2):393–396. doi: 10.1073/pnas.80.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H., Thomson A. J. Three-iron clusters in iron-sulfur proteins. Arch Biochem Biophys. 1983 Apr 15;222(2):333–361. doi: 10.1016/0003-9861(83)90531-3. [DOI] [PubMed] [Google Scholar]
- Emptage M. H., Kent T. A., Huynh B. H., Rawlings J., Orme-Johnson W. H., Münck E. On the nature of the iron-sulfur centers in a ferredoxin from Azotobacter vinelandii. Mössbauer studies and cluster displacement experiments. J Biol Chem. 1980 Mar 10;255(5):1793–1796. [PubMed] [Google Scholar]
- Ghosh D., Furey W., Jr, O'Donnell S., Stout C. D. Structure of a 7Fe ferredoxin from Azotobacter vinelandii. J Biol Chem. 1981 May 10;256(9):4185–4192. [PubMed] [Google Scholar]
- Ghosh D., O'Donnell S., Furey W., Jr, Robbins A. H., Stout C. D. Iron-sulfur clusters and protein structure of Azotobacter ferredoxin at 2.0 A resolution. J Mol Biol. 1982 Jun 15;158(1):73–109. doi: 10.1016/0022-2836(82)90451-x. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Thomson A. J., Richards A. J., Peterson J., Robinson A. E., Ramsay R. R., Singer T. P. Characterization of the Fe-S cluster in aconitase using low temperature magnetic circular dichroism spectroscopy. J Biol Chem. 1984 Feb 25;259(4):2274–2282. [PubMed] [Google Scholar]
- Johnson M. K., Thomson A. J., Robinson A. E., Rao K. K., Hall D. O. Low-temperature magnetic circular dichroism spectra and magnetisation curves of 4Fe clusters in iron-sulphur proteins from Chromatium and Clostridium pasteurianum. Biochim Biophys Acta. 1981 Feb 27;667(2):433–451. doi: 10.1016/0005-2795(81)90209-9. [DOI] [PubMed] [Google Scholar]
- Kennedy M. C., Emptage M. H., Dreyer J. L., Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983 Sep 25;258(18):11098–11105. [PubMed] [Google Scholar]
- Kent T. A., Dreyer J. L., Kennedy M. C., Huynh B. H., Emptage M. H., Beinert H., Münck E. Mössbauer studies of beef heart aconitase: evidence for facile interconversions of iron-sulfur clusters. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1096–1100. doi: 10.1073/pnas.79.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews R., Charlton S., Sands R. H., Palmer G. On the nature of the spin coupling between the iron-sulfur clusters in the eight-iron ferredoxins. J Biol Chem. 1974 Jul 10;249(13):4326–4328. [PubMed] [Google Scholar]
- Morgan T. V., Stephens P. J., Devlin F., Stout C. D., Melis K. A., Burgess B. K. Spectroscopic studies of ferricyanide oxidation of Azotobacter vinelandii ferredoxin I. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1931–1935. doi: 10.1073/pnas.81.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shethna Y. I. Non-heme iron (iron-sulfur) proteins of Azotobacter vinelandii. Biochim Biophys Acta. 1970 Apr 7;205(1):58–62. doi: 10.1016/0005-2728(70)90061-7. [DOI] [PubMed] [Google Scholar]
- Sweeney W. V., Rabinowitz J. C., Yoch D. C. High and low reduction potential 4Fe-4S clusters in Azotobacter vinelandii (4Fe-4S) 2ferredoxin I. Influence of the polypeptide on the reduction potentials. J Biol Chem. 1975 Oct 10;250(19):7842–7847. [PubMed] [Google Scholar]
- Thomson A. J., Robinson A. E., Johnson M. K., Moura J. J., Moura I., Xavier A. V., Legall J. The three-iron cluster in a ferredoxin from Desulphovibrio gigas. A low-temperature magnetic circular dichroism study. Biochim Biophys Acta. 1981 Aug 28;670(1):93–100. doi: 10.1016/0005-2795(81)90053-2. [DOI] [PubMed] [Google Scholar]
- Williams-Smith D. L., Bray R. C., Barber M. J., Tsopanakis A. D., Vincent S. P. Changes in apparent pH on freezing aqueous buffer solutions and their relevance to biochemical electron-paramagnetic-resonance spectroscopy. Biochem J. 1977 Dec 1;167(3):593–600. doi: 10.1042/bj1670593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M. G. Effect of non-haem iron proteins and cytochrome C from Azotobacter upon the activity and oxygen sensitivity of Azobacter nitrogenase. FEBS Lett. 1970 Jun 27;8(5):281–285. doi: 10.1016/0014-5793(70)80287-3. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. Two biologically active ferredoxins from the aerobic nitrogen-fixing bacteriu, Azotobacter vinelandii. J Biol Chem. 1972 Jul 25;247(14):4514–4520. [PubMed] [Google Scholar]
- Yoch D. C., Benemann J. R., Valentine R. C., Arnon D. I. The electron transport system in nitrogen fixation by Azotobacter. II. Isolation and function of a new type of ferredoxin. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1404–1410. doi: 10.1073/pnas.64.4.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]


