Abstract
Plants must balance light capture for photosynthesis with protection from potentially harmful ultraviolet (UV) radiation. Photoprotection is mediated by concerted action of photoreceptors, but the underlying molecular mechanisms are not fully understood. In this study, we provide evidence that UV RESISTANCE LOCUS 8 (UVR8) UV-B, phytochrome red, and cryptochrome blue-light photoreceptors converge on the induction of FERULIC ACID 5-HYDROXYLASE 1 (FAH1) that encodes a key enzyme in the phenylpropanoid biosynthesis pathway, leading to the accumulation of UV-absorbing sinapate esters in Arabidopsis (Arabidopsis thaliana). FAH1 induction depends on the basic leucine zipper transcription factors ELONGATED HYPOCOTYL 5 (HY5) and HY5 HOMOLOG that function downstream of all 3 photoreceptors. Noticeably, mutants with hyperactive UVR8 signaling rescue fah1 UV sensitivity. Targeted metabolite profiling suggests that this phenotypic rescue is due to the accumulation of UV-absorbing metabolites derived from precursors of sinapate synthesis, namely, coumaroyl glucose and feruloyl glucose. Our genetic dissection of the phenylpropanoid pathway combined with metabolomic and physiological analyses show that both sinapate esters and flavonoids contribute to photoprotection with sinapates playing a major role for UV screening. Our findings indicate that photoreceptor-mediated regulation of FAH1 and subsequent accumulation of sinapate “sunscreen” compounds are key protective mechanisms to mitigate damage, preserve photosynthetic performance, and ensure plant survival under UV.
Efficient acclimatory photoprotection of the photosynthetic machinery from UV-B–mediated damage requires photoreceptor-mediated accumulation of sinapate esters.
Introduction
Light fuels photosynthesis and affects plant growth, development, and metabolism throughout their life cycle. To optimize growth and development according to ambient light conditions, plants employ a set of photoreceptors, including the red/far-red–sensory phytochromes (phyA–phyE); the blue light–perceiving cryptochromes (cry1, cry2); and the ultraviolet-B (UV-B)/short-wavelength UV-A radiation photoreceptor UV RESISTANCE LOCUS 8 (UVR8; Galvão and Fankhauser 2015; Allorent and Petroutsos 2017; Demarsy et al. 2018; Rai et al. 2020; Podolec et al. 2021a). Upon light exposure, phytochrome, cryptochrome, and UVR8 photoreceptors inhibit the E3 ubiquitin ligase CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1), thereby allowing stabilization of key transcription factors involved in photomorphogenic responses such as the basic leucine zipper (bZIP) transcription factors ELONGATED HYPOCOTYL 5 (HY5) and HY5 HOMOLOG (HYH) (Osterlund et al. 2000; Holm et al. 2002; Favory et al. 2009; Binkert et al. 2014; Yin et al. 2015; Hoecker 2017; Podolec and Ulm 2018; Lau et al. 2019; Ponnu et al. 2019).
UVR8 exists as a homodimer in its ground state (Favory et al. 2009; Rizzini et al. 2011). Upon UV-B photon absorption, UVR8 monomerizes and accumulates in the nucleus in a COP1-dependent manner (Kaiserli and Jenkins 2007; Rizzini et al. 2011; Yin et al. 2016; Fang et al. 2022). The 2 WD40-repeat proteins REPRESSOR OF UV-B PHOTOMORPHOGENESIS 1 (RUP1) and RUP2 act as negative feedback regulators of the UVR8 photocycle by facilitating UVR8 re-dimerization and thereby repressing UV-B signaling (Gruber et al. 2010; Heijde and Ulm 2013; Wang et al. 2023). In agreement, rup1 rup2 mutants show enhanced UV-B responsiveness and acclimation (Gruber et al. 2010). Interestingly, the uvr8-17D mutant, which expresses the constitutively monomeric UVR8G101S photoreceptor variant, phenotypically resembles rup1 rup2, reinforcing the importance of UVR8 re-dimerization to optimally balance UV-B–induced photomorphogenesis with plant growth and development (Podolec et al. 2021b).
Despite its necessity for photosynthesis, light can also constitute an environmental stressor for the photosynthetic machinery when photosynthetic capacity is overwhelmed, as well as due to its intrinsic, potentially damaging UV-B component (Jansen et al. 1998; Li et al. 2009; Demarsy et al. 2018). UV-B–induced damage to photosystem II (PSII) occurs primarily in the water-oxidizing manganese cluster of the oxygen evolving complex and at the D1 and D2 proteins (Takahashi et al. 2010; Takahashi and Badger 2011; Demarsy et al. 2018). Photoreceptors can promote photoprotection, a set of mechanisms through which plants alleviate the negative effects of light on cell integrity and particularly on the photosynthetic machinery at PSII (Jung and Niyogi 2008; Allorent and Petroutsos 2017; Demarsy et al. 2018). Evidence in Arabidopsis (Arabidopsis thaliana) and Chlamydomonas (Chlamydomonas reinhardtii) suggests that UVR8 controls an acclimatory response that maintains optimal photosynthetic efficiency under elevated UV-B (Davey et al. 2012; Tilbrook et al. 2016). Recent studies indicate that UVR8 acts in concert with other photoreceptors, mainly cry1, to ensure survival under UV-containing sunlight (Morales et al. 2013; Rai et al. 2019; Rai et al. 2020; Tissot and Ulm 2020; Stockenhuber et al. 2024), but the underlying mechanism has remained enigmatic.
UVR8 signaling leads to transcriptional regulation of genes involved in photomorphogenic responses, including those resulting in UV acclimation and tolerance (Kliebenstein et al. 2002; Brown et al. 2005; Oravecz et al. 2006; Favory et al. 2009; Robson et al. 2019; Podolec et al. 2021a; Rai et al. 2021). Phenolic “sunscreen” metabolites that filter out UV and prevent damage of underlying tissues are important for UV tolerance of land plants (Caldwell et al. 1983; Landry et al. 1995; Sheahan 1996; Kliebenstein et al. 2002; Stracke et al. 2010; Neugart et al. 2021; Shi and Liu 2021; González Moreno et al. 2022; Procko et al. 2022). “Sunscreen” metabolites in flowering plants mainly include flavonol glycosides and sinapate esters (Tevini et al. 1991; Sheahan 1996; Burchard et al. 2000), with biosynthesis of flavonol glycosides in particular being known to be UV-B inducible (Li et al. 1993; Landry et al. 1995; Shirley et al. 1995; Stracke et al. 2010; Clayton et al. 2018; Neugart et al. 2019; Nichelmann and Pescheck 2021; Shi and Liu 2021). Flavonols are widely considered as central to UV-B–induced UV photoprotection (e.g. Wellmann 1975; Caldwell et al. 1983; Ryan et al. 2001; Winkel-Shirley 2002; Agati and Tattini 2010; Vogt 2010; Kusano et al. 2011; Jenkins 2014; Nichelmann and Pescheck 2021; Podolec et al. 2021a; Shi and Liu 2021). By contrast, sinapate esters are generally thought to be constitutively present and provide basal UV tolerance (Hectors et al. 2007; Götz et al. 2010; Nichelmann and Pescheck 2021), although UVR8-mediated sinapate accumulation has been reported to contribute to pathogen resistance (Demkura and Ballaré 2012).
Sinapate esters belong to the group of hydroxycinnamic acids (HCAs), a widely occurring class of phenylpropanoids in the plant kingdom. The 3 major sinapate esters in Arabidopsis are sinapoyl glucose, sinapoyl malate, and sinapoyl choline (Fraser and Chapple 2011). A key enzyme essential for the biosynthesis of sinapate, the precursor of sinapate esters, is ferulic acid-5-hydroxylase (F5H), a cytochrome P450 (CYP84A1) encoded by the FAH1 locus in Arabidopsis (Lorenzen et al. 1996; Ruegger et al. 1999). fah1 mutants are hypersensitive to UV stress, further supporting that sinapate esters play a major role in basal, constitutive UV protection (Landry et al. 1995).
Here, we show that UVR8-mediated UV acclimation alleviates UV-B–induced damage to the photosynthetic machinery by mitigating the damage at PSII. This photoprotection mechanism particularly relies on induced F5H activity and subsequent accumulation of sinapate esters that reduce the penetration of UV into photosynthetic tissue. We further show that hyperactive UV-B signaling in uvr8-17D and rup1 rup2 can suppress the UV stress hypersensitivity phenotype of fah1, likely due to enhanced accumulation of UV-absorbing metabolites derived from sinapate precursors, which arise due to an impaired sinapate biosynthesis pathway in fah1. Finally, our data suggest that phytochrome, cryptochrome, and UVR8 photoreceptors orchestrate photoprotection against UV via combined induction of FAH1 and sinapate ester accumulation.
Results
UVR8-mediated UV-B acclimation involves photoprotection of the photosynthetic machinery
UVR8 activation promotes tolerance to UV stress (Kliebenstein et al. 2002; Favory et al. 2009; Podolec et al. 2021a). To quantitatively assess the effect of UVR8 activation and UVR8-mediated acclimation on UV stress–induced photoinhibition, we measured chlorophyll fluorescence in wild-type and UVR8 signaling mutants exposed to different UV-B treatments, and determined Fv/Fm ratios representing the maximum quantum yield of PSII (Baker 2008; Murchie and Lawson 2013). First, 7-d-old light-grown seedlings of Col wild-type, rup1 rup2, and uvr8 plants were exposed (acclimated, +/−) or not (−/−) to UV-B for 3 d with supplementary narrowband UV-B (0.08 mW cm−2; Fig. 1A). At Day 10, acclimated and non-acclimated plants were exposed to 2 h of broadband UV stress irradiation (non-acclimated and stressed −/+; acclimated and stressed +/+; 2.2 mW cm−2). Col wild type, rup1 rup2, and uvr8 grown in white light (−/−) all displayed an optimal Fv/Fm value of about 0.83 (Fig. 1, B and C), characteristic of unstressed Arabidopsis plants (Baker 2008; Murchie and Lawson 2013). UV stress on non-acclimated plants (−/+) caused a large decrease of Fv/Fm to ∼0.42 in all 3 genotypes, indicating photoinhibition due to severe damage to PSII (Fig. 1, B and C), further supporting that UVR8 signaling does not contribute to acute UV stress responses (González Besteiro et al. 2011). UV-B acclimation alone (+/−) did not affect Fv/Fm in wild-type Col and rup1 rup2 but led to a decrease in Fv/Fm in the uvr8 mutant (Fig. 1, B and C), indicating that already acclimatory levels of UV-B can damage PSII in the absence of the UVR8 photoreceptor, further demonstrating the crucial role of UVR8 for UV-B acclimation and UV stress tolerance. Moreover, results of UV stress treatment after acclimation (+/+) directly supported a UVR8-dependent photoprotective effect of UV-B acclimation: in Col wild type, acclimation reduced the extent of photoinhibition by the UV stress treatment, and this photoprotection was further enhanced in rup1 rup2 but was absent in uvr8 (Fig. 1, B and C; comparing +/+ with −/+). Whereas hyh acclimated like wild type, hy5 and hy5 hyh showed reduced photoprotection, with hy5 hyh exhibiting UV stress effects comparable to uvr8 (Supplementary Fig. S1A). By contrast, uvr8-17D, expressing UVR8G101S with enhanced UVR8 activity, showed enhanced UV photoprotection (Supplementary Fig. S1B) like that seen for rup1 rup2 (Fig. 1, B and C). Interestingly, although enhanced UV photoprotection after acclimation was also detectable in uvr8-17D hy5 and uvr8-17D hyh (Supplementary Fig. S1B), no acclimatory effect could be detected in uvr8-17D hy5 hyh (Supplementary Fig. S1B), supporting that HY5 and HYH are key transcription factors mediating UVR8-dependent UV photoprotection.
Figure 1.
UVR8-dependent UV-B acclimation enhances photoprotection. A) Scheme for UV-B treatment and Fv/Fm sampling. WL (black bar), white light (20 µmol m−2 s−1); WL + UV-B (blue bar; acclimation), WL with supplemental narrowband UV-B (0.08 mW cm−2); UV STRESS (gray bar and light blue bar), treatment under broadband UV lamps (2.2 mW cm−2). B) False-color image representing Fv/Fm values of 10-d-old wild-type (Col), uvr8-6 (uvr8), and rup1 rup2 seedlings treated according to the scheme in A). C)Fv/Fm measurements of 10-d-old wild-type (Col), uvr8-6 (uvr8), and rup1 rup2 seedlings. Values of independent experiments and means ± sd are shown (n = 3). Shared letters indicate no statistically significant difference between the means (P > 0.05), as determined by two-way ANOVAs followed by Tukey's test for multiple comparisons. D) Immunoblot analysis of D1 and RbcL (Rubisco large subunit; loading control) levels in 10-d-old wild-type (Col), uvr8-6, and rup1 rup2 seedlings grown and treated as indicated. A to D) −/−, not acclimated, not stressed; −/+, not acclimated, stressed; +/−, acclimated, not stressed; +/+, acclimated, stressed.
To further characterize the effect of UV-B on PSII, we analyzed the levels of D1, a PSII core protein and major target of UV-B damage (Booij-James et al. 2000; Takahashi and Badger 2011; Davey et al. 2012; Tilbrook et al. 2016). UV stress strongly reduced D1 levels across each of wild-type Col, uvr8, and rup1 rup2 (−/+ vs. −/−, Fig. 1D). Acclimatory UV-B (+/−) reduced D1 levels slightly in Col wild type and more severely in uvr8, whereas no reduction was apparent in rup1 rup2 (Fig. 1D), further suggesting that UVR8 is required for photoprotection of the photosynthetic machinery even under acclimatory doses of UV-B. In addition, and in agreement with the Fv/Fm data, UV-B acclimation mitigated the UV-stress effect on D1 levels in Col wild type and rup1 rup2, but not in uvr8 where D1 levels were already low under acclimatory UV-B conditions (+/− and +/+, Fig. 1D). We have thus established experimental conditions to evaluate UV acclimation and UV stress tolerance efficiently and quantitatively using Fv/Fm measurements, and, together, our data show that UVR8-mediated acclimation results in photoprotection, preventing PSII damage and D1 degradation, thus maintaining photosynthetic performance under UV.
UVR8 signaling induces FAH1 expression and accumulation of sinapate esters that underlies photoprotective acclimation
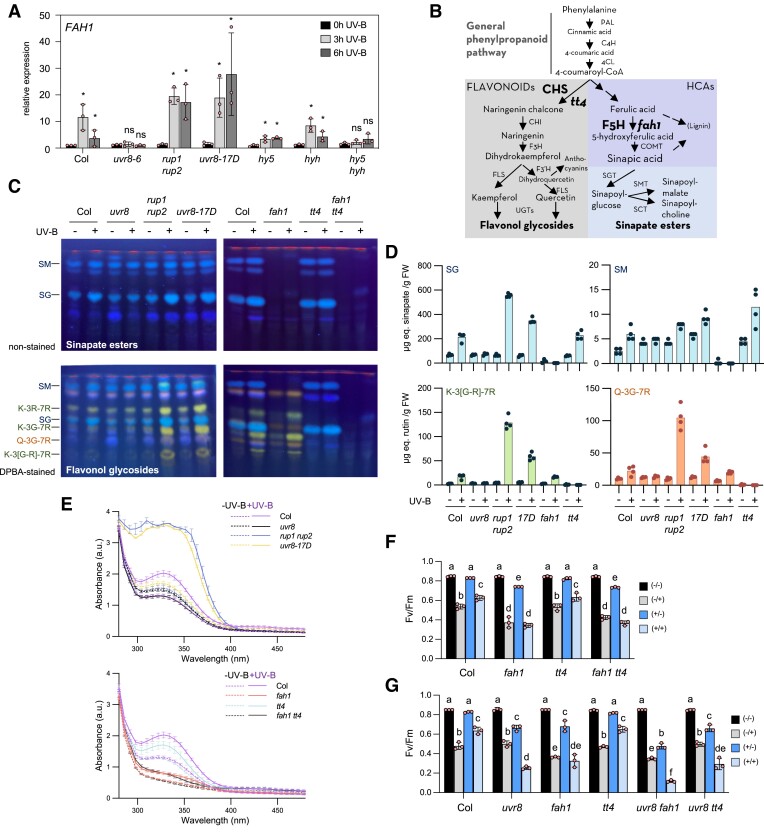
To better understand the underlying mechanism of UVR8-mediated photoprotection, we investigated the contribution of inducible phenylpropanoid “sunscreen” metabolites. UVR8 is well known to promote the accumulation of flavonol glycosides and anthocyanins by upregulating the expression of genes encoding key biosynthetic enzymes, including chalcone synthase (CHS) that catalyzes the first committed step in the flavonoid biosynthesis pathway (Kliebenstein et al. 2002; Brown et al. 2005; Favory et al. 2009; Stracke et al. 2010). Sinapate esters, on the other hand, are so far generally considered to provide constitutive UV protection (Götz et al. 2010; Rai et al. 2019; Nichelmann and Pescheck 2021). Interestingly, genome-wide expression analyses indicated that FAH1, encoding a key enzyme in sinapate biosynthesis and its derivatives sinapate esters, is induced upon UVR8 activation (Favory et al. 2009; Tavridou et al. 2020a). We further analyzed FAH1 expression in 7-d-old seedlings exposed to 3- and 6-h narrowband UV-B. FAH1 expression indeed increased upon UV-B exposure in wild type and to a reduced extent in hy5 and hyh single mutants, whereas expression induction was abolished in uvr8 and hy5 hyh (Fig. 2A). rup1 rup2 and uvr8-17D showed slightly elevated and sustained upregulation of FAH1 mRNA levels (Fig. 2A), which was dependent on HY5 and HYH as indicated by an abolished response in uvr8-17D hy5 hyh (Supplementary Fig. S2A). Next to HY5 and HYH, the PRODUCTION OF FLAVONOL GLYCOSIDES (PFG) family of R2R3-MYB transcription factors has a pivotal function in the regulation of genes involved in the phenylpropanoid biosynthetic pathway leading to flavonol synthesis and accumulation (Stracke et al. 2007). In contrast to CHS regulation, however, FAH1 expression was not dependent on PFG1/MYB12, PFG2/MYB11, and PFG3/MYB111 (Supplementary Fig. S2, B and C; Stracke et al. 2010). Collectively, our data show that FAH1 expression is induced under UV-B, and this response is dependent on UVR8 as well as HY5 and HYH.
Figure 2.
UVR8 signaling induces FAH1 expression and accumulation of sinapate esters underlying UV-B acclimation. A) RT-qPCR analysis of FAH1 gene expression changes in response to 3- and 6-h supplemental UV-B (0.08 mW cm−2) in 7-d-old light-grown (20 µmol m−2 s−1) uvr8-6 (uvr8), rup1 rup2, uvr8-17D, hy5, hyh, and hy5 hyh seedlings compared to wild type (Col). Values of independent measurements and means ± sd are shown (n = 3). The asterisk (*) indicates significant difference between the means of UV treated (3 or 6 h) and not UV treated (0 h; P < 0.05), as determined by two-way ANOVAs on log2-transformed values. B) Simplified schematic of the phenylpropanoid biosynthetic pathway toward sinapate esters and flavonol glycosides in Arabidopsis. CHS and F5H are highlighted as the key enzymes for synthesis of flavonol glycosides and sinapate esters, respectively, with tt4 and fah1 as the respective corresponding mutants. PAL, phenylalanine ammonium lyase; C4H, cinnamate-4-hydroxylase; 4CL, 4-coumarate:CoA ligase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; FLS, flavonol synthase; F3′H, flavonoid 3′-hydroxylase; UGTs, UDP-dependent glycosyltransferases; F5H, ferulic acid 5-hydroxylase; COMT, caffeic acid O-methyltransferase; SGT, sinapate–UDP-glucose glucosyltransferase; SMT, sinapoylglucose:malate sinapoyltransferase; SCT, sinapoylglucose:choline sinapoyltransferase. C) HPTLC analysis of sinapate ester (upper panels) and flavonol glycoside (lower panels) levels in 10-d-old seedlings of wild type (Col), uvr8-6 (uvr8), rup1 rup2, uvr8-17D, fah1-101 (fah1), tt4, and fah1-101 tt4 (fah1 tt4) grown for 7 d in white light (20 µmol m−2 s−1) and exposed to supplemental UV-B (0.08 mW cm−2) for 3 d (UV-B: +) or not (UV-B: −). SM, sinapoyl malate; K-3R-7R, kaempferol-3-O-rhamnoside-7-O-rhamnoside; SG, sinapoyl glucose; K-3G-7R, kaempferol-3-O-glucoside-7-O-rhamnoside; Q-3G-7R, quercetin-3-O-glucoside-7-O-rhamnoside; K-3[G-R]-7R, kaempferol 3-O-[rhamnosyl-glucoside]-7-O-rhamnoside. D) LC-MS–based targeted quantification of the secondary metabolites sinapoyl glucose (SG), sinapoyl malate (SM), kaempferol 3-O-[rhamnosyl-glucoside]-7-O-rhamnoside (K-3[G-R]-7R), and quercetin-3-O-glucoside-7-O-rhamnoside (Q-3G-7R) in 10-d-old seedlings of wild type (Col), uvr8-6 (uvr8), rup1 rup2, uvr8-17D (17D), fah1-101 (fah1), and tt4 grown for 7 d in white light (20 µmol m−2 s−1) and exposed to supplemental UV-B (0.08 mW cm−2) for 3 d (UV-B: +), or not (UV-B: −). Values of independent samples and means are shown (n = 4). μg eq. sinapate, μg equivalent of sinapate; μg eq. rutin, μg equivalent of rutin; FW, fresh weight. E) Absorption spectra (280 to 480 nm) of methanolic extracts (2:1, v/FW) from 10-d-old seedlings of wild type (Col), uvr8-6 (uvr8), rup1 rup2, uvr8-17D, fah1-101 (fah1), tt4, and fah1-101 tt4 (fah1 tt4) grown for 7 d in white light (20 µmol m−2 s−1) and exposed to supplemental UV-B (0.08 mW cm−2) for 3 d (+UV, continuous lines) or not (−UV, dashed lines). Means ± sem are shown (n = 3). FW, fresh weight; a.u., arbitrary units. F and G)Fv/Fm measurements of 10-d-old seedlings of F) wild type (Col), fah1-101 (fah1), tt4, and fah1-101 tt4 (fah1 tt4) and G) wild type (Col), uvr8-6 (uvr8), fah1-101 (fah1), tt4, uvr8-6 fah1-101 (uvr8 fah1), and uvr8-6 tt4 (uvr8 tt4) grown as described in Fig. 1A. Values of independent experiments and means ± sd are shown (n = 3). Shared letters indicate no statistically significant difference between the means (P > 0.05), as determined by two-way ANOVAs followed by Tukey's test for multiple comparisons. −/−, not acclimated, not stressed; −/+, not acclimated, stressed; +/−, acclimated, not stressed; +/+, acclimated, stressed.
We next analyzed UVR8-dependent activation of the phenylpropanoid biosynthetic pathway at the metabolic level (Fig. 2B). Methanolic extracts from UV-B–treated plants were analyzed by high-performance thin-layer chromatography (HPTLC) and quantified by ultrahigh-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS; see Supplementary Tables S1 to S3 for quantification of 4 HCA esters and 4 flavonoid compounds). For HPTLC, sinapate esters were visualized by their blue autofluorescence under UV-A, whereas flavonol glycosides were visualized under UV-A after staining of the HPTLC plate with diphenylboric acid 2-aminoethylester (DPBA; Stracke et al. 2007, 2010). In agreement with the FAH1 expression data, we observed increased accumulation of sinapate esters (e.g. sinapoyl glucose, SG) in wild type exposed to UV-B (Fig. 2, C [upper panel] and D; Supplementary Table S1). Increased sinapate esters were detectable in addition to the well-known accumulation of flavonol glycosides (i.e. kaempferol and quercetin glycosides) under UV-B (Fig. 2, C [lower panel] and D; Supplementary Table S1). Increase in these metabolites under UV-B was dependent on UVR8 as well as HY5 and HYH, as indicated by an absence of UV-B–induced accumulation in uvr8 and hy5 hyh (Fig. 2D; Supplementary Fig. S2D and Table S1). uvr8-17D and rup1 rup2 showed elevated accumulation of flavonol glycosides and sinapate esters (Fig. 2, C and D), whereas this response was reduced in uvr8-17D hy5 and absent in uvr8-17D hy5 hyh (Supplementary Fig. S2D).
Next, we used a series of mutants, including fah1 and the CHS knockout mutant transparent testa 4 (tt4), to dissect the contribution of different phenylpropanoid metabolites to UV photoprotection (Fig. 2B). As expected, fah1 mutants did not accumulate sinapate esters (e.g. sinapoyl glucose and sinapoyl malate) but accumulated wild-type levels of flavonol glycosides, whereas tt4 mutants did not accumulate flavonol glycosides but accumulated wild-type levels of sinapate esters (Fig. 2, B to D). Absorption spectra of methanolic extracts showed that UV-absorbing compounds accumulated under UV-B in wild type and even more so in rup1 rup2 and uvr8-17D, but not in uvr8 and hy5 hyh mutants (Fig. 2E; Supplementary Fig. S2E). Extracts from the fah1 mutant showed very low UV absorption capacity, which only slightly increased following UV-B acclimation (Fig. 2E). However, extracts from the tt4 mutant exhibited better UV absorption, which also further increased upon UV-B acclimation (Fig. 2E). In agreement, Fv/Fm measurements showed that fah1 mutants, in contrast to tt4, are hypersensitive to UV stress with no improvement following acclimatory UV-B pretreatment (Fig. 2, F and G; Supplementary Fig. S2, F and G). Moreover, tt4 displayed Fv/Fm values comparable to those of wild type, and the fah1 tt4 double mutant was not more sensitive to UV stress than the fah1 single mutant (Fig. 2F). Altogether, our data suggest that sinapate esters play a dominant role as both basal and UV-B–induced “sunscreen”, thereby providing UV photoprotection in Arabidopsis seedlings.
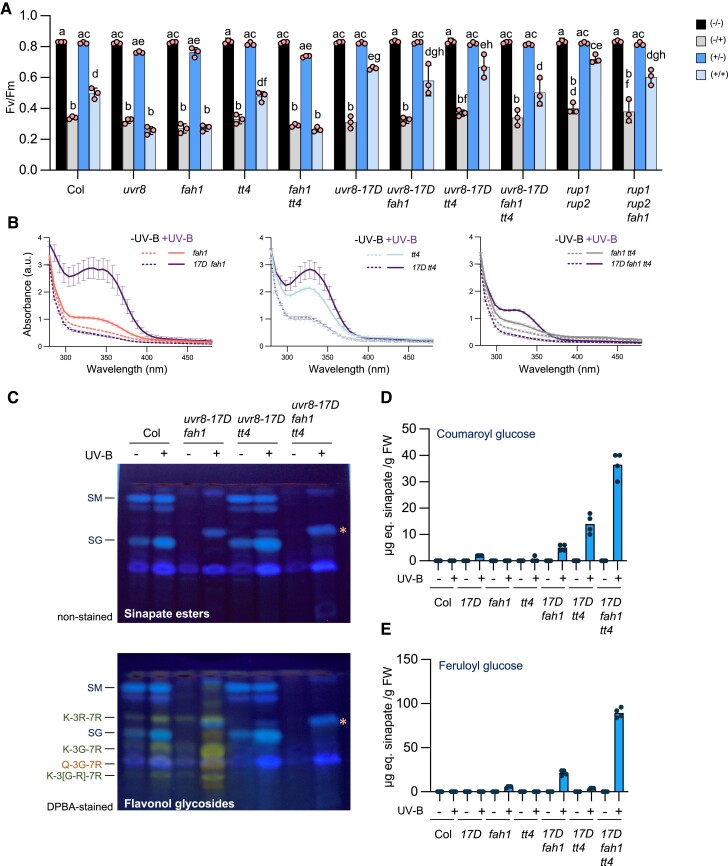
Enhanced UVR8 signaling rescues photoprotection in fah1 mutants, associated with the accumulation of HCA derivatives coumaroyl glucose and feruloyl glucose
We observed that uvr8 fah1, but not uvr8 tt4, was more sensitive to UV stress than both uvr8 and fah1 single mutants (Fig. 2G), suggesting that FAH1 and UVR8 may also contribute independently to UV photoprotection. Therefore, we tested whether enhanced UVR8 signaling can suppress fah1 UV stress hypersensitivity. Indeed, compared to the fah1 single mutant, enhanced UVR8 activity in uvr8-17D fah1 and rup1 rup2 fah1 strongly improved photoprotection specifically under UV-B acclimation conditions (+/+, Fig. 3A). Strikingly, the triple mutant uvr8-17D fah1 tt4 was able to acclimate and induce photoprotection under UV-B comparable to wild type (Fig. 3A). Thus, enhanced UVR8 signaling provided by the UVR8G101S variant expressed in uvr8-17D can induce a FAH1- and CHS-independent photoprotection mechanism. However, the absorbance spectra of uvr8-17D fah1 and uvr8-17D fah1 tt4 methanolic extracts indicated that elevated UV-absorbing metabolites were present despite the absence of FAH1 and CHS (Fig. 3, B and C). This suggests that enhanced UVR8 signaling in the uvr8-17D background is associated with UV-B acclimation even in the absence of sinapoyl malate, sinapoyl glucose, and flavonol glycosides (Fig. 3C; Supplementary Table S2). Intriguingly, HPTLC analysis of methanolic extracts from UV-B–acclimated uvr8-17D fah1 and uvr8-17D fah1 tt4 revealed an additional, prominent band under nonstained conditions (highlighted with an asterisk in Fig. 3C). This band was also detectable, although weakly, in UV-B–acclimated extracts of fah1, fah1 tt4, and uvr8-17D tt4 (Figs. 2C and 3C). We isolated the band in question directly from a HPTLC plate carrying extracts of UV-B–treated uvr8-17D fah1 tt4, as well as a wild-type control from the corresponding position on the HPTLC plate. UHPLC-MS/MS was then used and identified its constituents as the HCA derivatives coumaroyl glucose and feruloyl glucose (Supplementary Fig. S3). These 2 metabolites were indeed able to absorb UV (Supplementary Fig. S3, A and B). Targeted LC-MS analysis confirmed strong accumulation of coumaroyl glucose in UV-B–acclimated seedlings expressing hyperactive UVR8G101S (uvr8-17D, uvr8-17D fah1, uvr8-17D tt4, and, particularly, uvr8-17D fah1 tt4; Fig. 3D; see also Supplementary Table S2 for quantification of other HCA esters and flavonol glycosides), whereas feruloyl glucose seemed to accumulate only in UV-B–acclimated seedlings wherein FAH1 was absent (fah1, uvr8-17D fah1, and, particularly, uvr8-17D fah1 tt4; Fig. 3E; and Supplementary Table S2). Thus, UV-B acclimation and UV tolerance in uvr8-17D fah1 tt4 is specifically associated with the accumulation of UV-absorbing coumaroyl glucose and feruloyl glucose. In addition, this may contribute to the hypersensitivity of uvr8 fah1 compared to fah1 (Fig. 2G), as the induction of the feruloyl and coumaroyl glucose is UV-B–mediated and therefore absent in a uvr8 mutant (Supplementary Table S2).
Figure 3.
Enhanced UVR8 signaling rescues fah1 photoprotection and promotes accumulation of unique HCAs. A)Fv/Fm measurements of 10-d-old seedlings of wild type (Col), uvr8-6 (uvr8), fah1-101(fah1), tt4, fah1-101 tt4 (fah1 tt4), uvr8-17D, uvr8-17D fah1-101 (uvr8-17D fah1), uvr8-17D tt4, uvr8-17D fah1-101 tt4 (uvr8-17d fah1 tt4), rup1 rup2 and rup1 rup2 fah1-101 (rup1 rup2 fah1) grown as described in Fig. 1A. Values of independent experiments and means ± sd are shown (n = 3). Shared letters indicate no statistically significant difference between the means (P > 0.05), as determined by two-way ANOVAs followed by Tukey's test for multiple comparisons. −/−, not acclimated, not stressed; −/+, not acclimated, stressed; +/−, acclimated, not stressed; +/+, acclimated, stressed. FW, fresh weight; a.u., arbitrary units. B) Absorption spectra (280 to 480 nm) of methanolic extracts (2:1, v/FW) from 10-d-old seedlings of uvr8-17D (17D), uvr8-17D fah1-101 (17D fah1), tt4, uvr8-17D tt4 (17D tt4), and uvr8-17D fah1-101 tt4 (17d fah1 tt4) grown for 7 d in white light and exposed to supplemental UV-B for 3 d (+UV-B, continuous lines) or not (−UV-B, dashed lines). Data of fah1 tt4 are identical to the ones in Fig. 2E (samples were analyzed in parallel). Values of independent measurements and means ± sem are shown (n = 3). FW, fresh weight; a.u., arbitrary units. C) HPTLC analysis of sinapate esters (upper panels) and flavonol glycosides (lower panels) levels in 10-d-old seedlings of wild type (Col), uvr8-17D fah1-101, uvr8-17D tt4, and uvr8-17D fah1-101 tt4 grown for 7 d in white light (20 µmol m−2 s−1) and exposed to supplemental UV-B (0.08 mW cm−2) for 3 d (UV-B: +) or not (UV-B: −). SM, sinapoyl malate; K-3R-7R, kaempferol-3-O-rhamnoside-7-O-rhamnoside; SG, sinapoyl glucose; K-3G-7R, kaempferol-3-O-glucoside-7-O-rhamnoside; Q-3G-7R, quercetin-3-O-glucoside-7-O-rhamnoside; K-3[G-R]-7R, kaempferol 3-O-[rhamnosyl-glucoside]-7-O-rhamnoside. The asterisk (*) indicates coumaroyl glucose and feruloyl glucose, as identified by LC-MS/MS (see Supplementary Fig. S3). D and E) Quantification of D) coumaroyl glucose and E) feruloyl glucose by LC-MS–based analysis of methanolic extracts from 10-d-old seedlings of wild type (Col), uvr8-17D (17D), fah1-101 (fah1), tt4, uvr8-17D fah1-101, uvr8-17D tt4, and uvr8-17D fah1-101 tt4 grown for 7 d in white light (20 µmol m−2 s−1) and exposed to supplemental UV-B (0.08 mW cm−2) for 3 d (UV-B: +) or not (UV-B: −). Values of independent samples and means are shown (n = 4). μg eq. sinapate, μg equivalent of sinapate; FW, fresh weight.
FAH1 plays a major role for plant performance in presence of UV-B
Our data indicate that FAH1 and sinapate ester accumulation are key for photoprotection against UV stress at the seedling stage. We thus further examined plant growth and survival for fah1 and tt4 mutants under UV. On soil, in the absence of UV-B, uvr8, fah1, tt4, and fah1 tt4 grew and developed similarly to wild type (Fig. 4A). However, under supplemental UV-B, uvr8, fah1, and fah1 tt4 were affected and showed strongly impeded growth and damaged PSII in comparison to wild type and tt4, which were less impaired (Fig. 4, A and B), supporting that induced FAH1 and sinapate ester accumulation play a major role for photoprotection against UV-B. TT4 seemed to play a less important but clear role in development under UV-B. Indeed, the fah1 tt4 rosettes were smaller than fah1 rosettes after 4 wk under supplemental UV-B, and the Fv/Fm ratio was lower (Fig. 4, A and B). In agreement with the Fv/Fm data of seedlings, UVR8G101S–mediated hyperactivation of UV-B signaling in uvr8-17D fah1 and uvr8-17D fah1 tt4 suppressed the UV stress hypersensitivity of fah1 and fah1 tt4, resulting in less photoinhibition (Fig. 4B). It is of note, however, that reduced growth of uvr8-17D lines under UV-B is due to enhanced UV-B–induced photomorphogenesis (Podolec et al. 2021b) and is not representing a UV-B stress phenotype—in contrast to uvr8, fah1, and fah1 tt4 (Fig. 4B).
Figure 4.
FAH1 plays a major role for photoprotection in soil-grown plants. A) Photographs of 4-wk-old wild-type (Col), uvr8-6 (uvr8), fah1-101 (fah1), tt4, fah1-101 tt4 (fah1 tt4), uvr8-17D, uvr8-17D fah1-101 (uvr8-17D fah1), uvr8-17D tt4, and uvr8-17D fah1-101 tt4 (uvr8-17D fah1 tt4) plants grown under long-day conditions under 100 µmol m−2 s−1 of white light with supplemental UV-B (+UV-B, 0.3 mW cm−2, lower panels) or not (-UV-B, upper panels). Bars = 4 cm. B) False-color image representing Fv/Fm values of wild-type (Col), uvr8-6 (uvr8), fah1-101(fah1), tt4, fah1-101 tt4 (fah1 tt4), uvr8-17D, uvr8-17D fah1-101 (uvr8-17D fah1), uvr8-17D tt4, and uvr8-17D fah1-101 tt4 (uvr8-17D fah1 tt4) plants grown under long-day conditions under 100 µmol m−2 s−1 of white light with supplemental UV-B (+UV-B, 0.3 mW cm−2, lower panels) or not (-UV-B, upper panels). C to H) Accumulation of selected HCA esters and flavonol glycosides in soil-grown plants. LC-MS–based targeted metabolite quantifications of C) sinapoyl glucose (SG), D) sinapoyl malate (SM), E) kaempferol 3-O-[rhamnosyl-glucoside]-7-O-rhamnoside (K-3[G-R]-7R), F) quercetin-3-O-glucoside-7-O-rhamnoside (Q-3G-7R), G) coumaroyl glucose, and H) feruloyl glucose in 3-wk-old wild type (Col), uvr8-6 (uvr8), uvr8-17D (17D), rup1 rup2, fah1-101 (fah1), tt4, fah1-101 tt4 (fah1 tt4), uvr8-17D fah1-101 (17D fah1), uvr8-17D tt4 (17D tt4), and uvr8-17D fah1-101 tt4 (17D fah1 tt4) grown on soil under long-day conditions under 100 µmol m−2 s−1 of white light in the absence of UV-B and exposed (+) or not (−) to supplemental UV-B (0.3 mW cm−2) for 24 h. Values of independent samples and means ± sem are shown (n = 4). μg eq. sinapate, μg equivalent of sinapate; μg eq. rutin, μg equivalent of rutin; FW, fresh weight.
Targeted metabolite profiling of methanolic extracts from 3-wk-old soil-grown plants confirmed UVR8-dependent accumulation of sinapate esters, particularly sinapoyl glucose in wild-type and tt4 plants, and its absence in fah1 mutants (Fig. 4, C and D; Supplementary Table S3 for quantification of other HCA esters and flavonol glycosides). Conversely, UVR8-dependent accumulation of flavonol glycosides was detectable in fah1 but not in tt4 mutants (Fig. 4, E and F). Interestingly, as seen in in vitro–grown seedlings (Fig. 3), the increased UV tolerance of uvr8-17D fah1 and uvr8-17D fah1 tt4 versus that of fah1 and fah1 tt4 was associated with the over-accumulation of coumaroyl glucose and feruloyl glucose compared to wild type (Fig. 4, G and H). We conclude that UVR8-induced accumulation of sinapate esters, or derivatives of its precursors in absence of F5H activity in fah1 mutants, plays a major role in UV-B acclimation in soil-grown plants, and together with flavonols ensure plant survival under UV.
Photoreceptor signaling pathways converge on FAH1 expression and act in concert for UV photoprotection
Phytochrome and cryptochrome signaling contribute to UV tolerance (Rai et al. 2019; Tissot and Ulm 2020; Stockenhuber et al. 2024). In agreement, under prolonged UV exposure, uvr8 mutants are more UV sensitive than wild type, but uvr8 cry1 and uvr8 phyB are even more UV sensitive than uvr8 mutants (Fig. 5A; Tissot and Ulm 2020). By contrast, cry1 phyB are less affected in the presence of UV-B (Fig. 5A; Tissot and Ulm 2020). Strikingly, the uvr8 cry1 phyB triple mutant was even more UV sensitive than uvr8 cry1 and uvr8 phyB double mutants (Fig. 5A). Indeed, uvr8 cry1 phyB survived under white light but died after about 6 wk under white light supplemented with UV under conditions where even uvr8 cry1 and uvr8 phyB survived (Fig. 5A). In agreement, analysis of non-acclimated plants under UV stress confirmed that cry1 and phyB play a role in basal UV protection, as Fv/Fm values for non-acclimated cry1, uvr8 phyB, uvr8 cry1, cry1 phyB, and uvr8 cry1 phyB under UV stress were lower than those for wild type (Fig. 5B). In agreement, visible light–induced FAH1 expression was impaired in cry1 and phyB mutant backgrounds (Fig. 5C). These findings indicate that photoreceptors for blue light, red light, and UV-B cooperatively regulate UV photoprotection, activating FAH1 expression and accumulating key UV-absorbing compounds.
Figure 5.
Cryptochromes and phytochromes redundantly contribute to UVR8-mediated FAH1 expression and UV-b tolerance. A) Photographs of 6-wk-old wild-type (Ler), uvr8-1 (uvr8), phyB-5 (phyB), hy4-2.23N (cry1), uvr8-1 phyB-5 (uvr8 phyB), uvr8-1 hy4-2.23N (uvr8 cry1), hy4-2.23N phyB-5 (cry1 phyB), and uvr8-1 hy4-2.23N phyB-5 (uvr8 cry1 phyB) plants grown under short-day conditions with 100 µmol m−2 s−1 of white light with supplemental UV-B (+UV, 0.06 mW cm−2) or not (−UV). Bars = 5 cm. B)Fv/Fm measurements of 7-d-old seedlings of wild-type (Ler), uvr8-1 (uvr8), phyB-5 (phyB), hy4-2.23N (cry1), uvr8-1 phyB-5 (uvr8 phyB), uvr8-1 hy4-2.23N (uvr8 cry1), hy4-2.23N phyB-5 (cry1 phyB), and uvr8-1 hy4-2.23N phyB-5 (uvr8 cry1 phyB) plants grown under long-day conditions with 100 µmol m−2 s−1 of white light without supplemental UV-B and exposed (−/+) or not (−/−) to broadband UV-B for 2 h starting at ZT4. Values of independent experiments and means ± sd are shown (n = 3). Asterisks (*) indicate statistically significant difference between the means (P < 0.05), as determined by two-way ANOVAs followed by Tukey's test for multiple comparisons. −/−, not acclimated, not stressed; −/+, not acclimated, stressed. C) RT-qPCR of FAH1 expression in 7-d-old seedlings of wild-type (Ler), uvr8-1 (uvr8), uvr8-1 phyB-5 (uvr8 phyB), uvr8-1 hy4-2.23N (uvr8 cry1), hy4-2.23N phyB-5 (cry1 phyB), and uvr8-1 hy4-2.23N phyB-5 (uvr8 cry1 phyB) grown for 4 d under long-day conditions with 100 µmol m−2 s−1 of white light without supplemental UV-B and dark-adapted for 3 d, then exposed for 3 h to white light (100 µmol m−2 s−1) or kept in darkness (Dark). Values of independent measurements and means ± sd are shown (n = 3). Shared letters indicate no statistically significant difference between the means (P > 0.05), as determined by two-way ANOVAs followed by Tukey's test for multiple comparisons.
Discussion
UVR8 and cryptochromes act together to ensure plant survival when grown in the field in the presence of UV (Rai et al. 2019; Stockenhuber et al. 2024). However, the extent to which different photoprotective mechanisms activated upon light perception contribute to UV tolerance is not well understood. Our findings suggest that photoreceptor-mediated regulation of biosynthesis of UV-absorbing sinapate esters is a key response to ensure plant survival under UV.
Sinapate esters belong to the family of phenylpropanoids which comprises specialized metabolites with broad functions including roles in pollinator attraction, cuticle composition, pathogen defense, reactive oxygen species (ROS) scavenging and photoprotection against UV and high light (Sheahan 1996; Rozema et al. 2009; Erb and Kliebenstein 2020; Ferreyra et al. 2021). The phenylpropanoid pathway diverges at 4-coumaroyl-CoA, providing flavonoids (including flavonols and anthocyanins) and HCAs like sinapate, as well as its conjugated forms (Fraser and Chapple 2011). Flavonoids are generally known to be photoreceptor-inducible and are thus the focus of many studies in the field, whereas sinapate esters are usually considered constitutively present and often neglected in the literature on UV acclimation (Lois 1994; Kliebenstein et al. 2002; Morales et al. 2010; Stracke et al. 2010; Kusano et al. 2011; Neugart et al. 2019; Rai et al. 2019; Podolec et al. 2021a), despite the fact that they are highly efficient to absorb across a broad range of UV wavelength (Dean et al. 2014). However, UVR8-induced sinapate ester accumulation was described in response to UV-A (Brelsford et al. 2019) and in the context of UV-B–induced defense responses against the necrotrophic fungal pathogen Botrytis cinerea (Demkura and Ballaré 2012). Moreover, transgenic enhancement of sinapate synthesis was also found to enhance the resistance to diamondback moth (Plutella xylostella) herbivory (McInnes et al. 2023). Our data show that, in addition to the induction of flavonoid biosynthesis, UVR8, cryptochromes, and phytochromes indeed activate FAH1 expression through the HY5 and HYH transcription factors, promoting sinapate ester accumulation and photoprotection. HY5 functions often in concert with BBX factors, such as BBX20–22, BBX29, and BBX31, that were shown to promote accumulation of soluble phenylpropanoid derivatives and may thus contribute to UV-B acclimation (Yadav et al. 2019; Bursch et al. 2020; Xu 2020; Podolec et al. 2022; Medina-Fraga et al. 2023). Whether FAH1 expression can be modulated by other transcriptional regulators associated with UVR8 signaling remains to be determined (Liang et al. 2018, 2019, 2020; Qian et al. 2020; Tavridou et al. 2020a, 2020b; Yang et al. 2020; Podolec et al. 2021a). Moreover, other transcription factors presently not linked to UVR8 signaling may additionally modulate the response leading to sinapate esters at different levels. For example, MYB4 represses expression of C4H, encoding cinnamate 4-hydroxylase, and myb4 mutants show enhanced levels of sinapate esters and enhanced tolerance to UV-B stress (Jin et al. 2000). Downregulation of MYB4 in response to UV-B may thus contribute to sinapate ester accumulation and enhanced UV-absorbing capability (Jin et al. 2000).
We used chlorophyll fluorescence measurement as a noninvasive approach and calculated the Fv/Fm to assess PSII integrity, thereby providing an estimation of PSII damage. As we measured the Fv/Fm directly after UV stress treatment, the observed decrease likely reflects the direct damage caused by UV on D1 and the oxygen evolving center (Takahashi et al. 2010). Absorption spectra and physiological analyses of WT and fah1 seedlings and rosette-stage plants show that sinapate esters provide UV-screening properties in plant extracts, make a major contribution to direct photoprotection of the photosynthetic machinery upon UV stress treatment, and play a major role for plant survival under UV in lab conditions. Flavonoids seemed to play a lesser role in UV-B–induced acclimation and UV tolerance under our experimental conditions, importantly at both in vitro seedling and more mature soil-grown plant stages as indicated by less-pronounced UV-induced damage at PSII in tt4 compared to fah1 and much better growth under UV; however, the small rosette phenotype of tt4 and additive effect of tt4 and fah1 mutations after 3 wk under supplemental UV-B indicates that flavonoids contribute to plant performance under UV, in agreement with previous reports (Landry et al. 1995; Stracke et al. 2010). Thus, although flavonoids are diverse, abundant, and highly UV-inducible, the function of HCA-derived compounds seems of even greater importance for UV tolerance, at least under the laboratory conditions used in this work.
As sinapate esters absorb across much of the UV range with high extinction coefficients (Dean et al. 2014) and their absence is associated with a strong decrease in UV absorbance of fah1 mutant extracts, we conclude that the major contribution of sinapoyl glucose accumulation to UV acclimation and tolerance is the increase of UV “sunscreen” capacity. However, we cannot exclude a secondary role of sinapoyl glucose as antioxidant and radical scavenger (Kylli et al. 2008). Moreover, in response to photooxidative stress, fah1 mutants accumulate less anthocyanins, which are visible light–absorbing pigments and ROS scavengers (Maruta et al. 2014; Anderson et al. 2015; Naing and Kim 2021). Although we cannot exclude a contribution of possibly lower anthocyanin levels in fah1 mutants under our conditions, a major contribution is unlikely as the tt4 mutants devoid of anthocyanins and flavonols (Shirley et al. 1995) are much less sensitive than fah1 mutants. However, the antioxidant and/or visible light–absorbing properties of flavonoids may contribute to UV tolerance, alongside the UV “sunscreen” function of sinapate esters (Agati and Tattini 2010; Hideg et al. 2013). The inducibility of the different flavonoids and HCAs and their relative contribution to UV acclimation and tolerance may also change depending on growth conditions and plant developmental stage (see also Supplementary Tables S1 to S3), which could explain variations seen in leaf UV sensitivity depending on their developmental age (Lois 1994). In addition, a synergistic photoprotective effect can be expected by the combination of visible and UV light “sunscreen” properties of flavonoid and HCA derivatives and potentially their concomitant antioxidant activities, particularly under high PAR and UV, when UV may damage the photosynthetic machinery decreasing the capacity to absorb and use efficiently the light energy and thus when visible light may become additionally harmful causing ROS production. However, absence of CHS in tt4 mutants, and thus flavonoids, has no detectable effect on plant phenotype under prolonged UV exposure in a sun simulator, further supporting that HCAs might indeed play a more important role than flavonoid to ensure plant survival also under more natural conditions in presence of UV (Rai et al. 2019). As combinatorial uvr8, cry1, and phyB mutants are affected for FAH1 expression and cannot survive under prolonged UV exposure, we propose that induction of FAH1 and associated sinapate synthesis is a key mechanism for UV tolerance, although we do not exclude that other photoreceptor-induced photoprotective mechanisms contribute to UV tolerance, which may even be of much higher relative importance under other growth conditions. Future experiments in the field will be of great interest and importance to further characterize the contribution of flavonoids and sinapate esters in a more complex light environment and the influence of other environmental factors on light- and UV-induced plant photoprotection.
The development of UV-B protective mechanisms has played a pivotal role in the successful terrestrialization of plants and their colonization of land (Rozema et al. 1997). UV inducibility of sunscreen capacity is broadly spread across the plant kingdom, although the UV-absorbing compounds may differ. The UVR8 photoreceptor is well-conserved across the green lineage (Rizzini et al. 2011; Jenkins 2014; Han et al. 2019; Podolec et al. 2021a; Zhang et al. 2022), with functional homologs of Arabidopsis UVR8 characterized in the single-cell green algae Chlamydomonas (Allorent et al. 2016; Tilbrook et al. 2016); in bryophytes, namely, in the liverwort Marchantia polymorpha and the moss Physcomitrium patens (Soriano et al. 2018; Kondou et al. 2019); and in the flowering plant tomato (Solanum lycopersicum; Li et al. 2018; Liu et al. 2020). UV-B–mediated activation of phenylpropanoid biosynthesis and corresponding gene expression appears to be broadly conserved among land plants (Stracke et al. 2010; Wolf et al. 2010; Schreiner et al. 2017; Clayton et al. 2018; Ferreyra et al. 2021). Phenylpropanoid compounds are not present in bacteria, but the accumulation of UV-absorbing, mycosporin-like amino acids was shown to be UV-B–inducible in cyanobacteria (Rozema et al. 2002; Sinha et al. 2002). Sinapate esters accumulate to a high level in Brassicaceae and have also been detected in other plant species (Milkowski and Strack 2010; Nguyen et al. 2021). A natural variation study reported Arabidopsis accessions that accumulate high levels of phenylacylated flavonols (saiginols, including a flavonol glycoside esterified with sinapoyl) in floral tissues, a trait which was associated with greater UV tolerance and was primarily found in accessions prevalent in regions with high UV-B irradiance (Tohge et al. 2016; Tohge and Fernie 2017). Evolution may have selected for a diversification of phenylpropanoid-derived compounds toward better adaptation to the light environment (Tohge et al. 2013; de Vries et al. 2021). Such revelations also suggest that genetic variation can be used to increase plant UV tolerance. By combining mutations that enhance UVR8 signaling with those that block synthesis of flavonoids and sinapates, we generated plants that accumulated high levels of UV-absorbing HCAs, namely, coumaroyl glucose and feruloyl glucose. An improved understanding of the breadth and relative importance of UV-absorbing metabolites as “sunscreens”, as well as regulation of their synthesis, offers potential opportunities for plant engineering toward better UV tolerance.
Materials and methods
Plant materials
The Arabidopsis (A. thaliana) accessions Columbia (Col-0, herein Col) or Landsberg erecta (Ler-0, herein Ler) were used as wild-type controls in all experiments, as indicated. The following published mutants were used in this study: uvr8-6 (Favory et al. 2009); uvr8-17D (Podolec et al. 2021b); rup1-1 rup2-1 (Gruber et al. 2010); hy5-215 (Oyama et al. 1997); hyh and hy5-215 hyh (Zoulias et al. 2020); fah1-2 (Chapple et al. 1992); fah1-101 (Maruta et al. 2014); tt4-11 (Bowerman et al. 2012); phyA-211 (Nagatani et al. 1993); phyB-9, phyA-211, and phyB-9 (Enderle et al. 2017); myb12, myb111, myb12 myb111, and myb11 myb12 myb111 (Stracke et al. 2007) are all in the Col background, whereas uvr8-1 (Kliebenstein et al. 2002), fah1-7 (Meyer et al. 1996), phyB-5 (Reed et al. 1994), hy4-2.23N (Koornneef et al. 1980), and phyB-5 hy4-2.23N (Tissot and Ulm 2020) are in the Ler background. The fah1-101 tt4-11, uvr8-6 fah1-101, uvr8-6 tt4-11, uvr8-17D fah1-101, and uvr8-17D tt4-11 double mutants were generated by crossing the respective single mutants; uvr8-17D fah1-101 tt4-11 was generated by crossing uvr8-17D fah1-101 and uvr8-17D tt4-11; uvr8-17D hy5-215, uvr8-17D hyh, and uvr8-17D hy5-215 hyh were generated by crossing uvr8-17D and hy5-215 hyh; and uvr8-1 hy4-2.23N phyB-5 was generated by crossing uvr8-1 hy4-2.23N and uvr8-1 phyB-5. Genotyping of hy5-215, rup1-1, rup2-1, and uvr8-6 (Gruber et al. 2010), uvr8-17D (Podolec et al. 2021b), and phyB-5 (Neff and Chory 1998) was performed as previously published. Supplementary Table S4 lists the primers used for genotyping all other mutants used to generate combinatorial mutants.
Growth conditions and light treatments
For experiments under aseptic conditions in vitro, Arabidopsis seeds were surface-sterilized with chlorine gas and sown on half-strength MS basal salt medium (Duchefa) containing 0.8% (w/v) agar (Applichem) supplemented with 1% (w/v) sucrose. After 2-d stratification at 4 °C, seedlings were grown in white light, or seeds were exposed to 6-h white light (60 µmol m−2 s−1) before transferring them into darkness at 22 °C for experiments involving etiolated seedlings. Except otherwise indicated, seedlings were grown in continuous white light (white-light fluorescent tubes Osram L18W/30, 20 µmol m−2 s−1), supplemented with narrowband UV-B tubes (Philips TL20W/01RS, 0.08 mW cm−2). For UV stress treatment, plates were irradiated under broadband UV-B tubes (Philips TL20W/12RS; 2.2 mW cm−2). For UV stress treatment on photoreceptor mutants (Fig. 5B), seedlings were grown under aseptic conditions in long-day conditions with a 16-h day/8-h night cycle (22 °C/18 °C) in a Percival growth chamber (CLF Climatics) with white-light fluorescent tubes Philips Alto II F17T8/TL841 (100 µmol m−2 s−1) for 7 d, and UV stress treatment was performed at ZT4, for 2 h. Each experiment was done using 3 replicate plates per condition, each containing all genotypes tested in parallel. Control (−UV-B, under lid and a Schott WG368 long-pass filter, for 10 d) and treatment (+UV-B, under lid, and a Schott WG368 long-pass filter for 7 d and then under lid and a Schott WG305 long-pass filter for 3 d) plates were positioned side-by-side in the same light field, given white-light and UV-B irradiances were measured under the lid and filter (WG305 or WG368), as will be reaching the respective plants.
For RT-qPCR–based FAH1 expression analysis of photoreceptor mutants under aseptic conditions, seedlings were grown under long-day conditions with a 16-h day/8-h night cycle (22 °C/18 °C) in a Percival growth chamber (CLF Climatics) with white-light fluorescent tubes Philips Alto II F17T8/TL841 (100 µmol m−2 s−1) for 4 d, dark-adapted for 3 d, and kept in darkness or exposed to white light for 3 h.
For UV tolerance assays on soil, plants were either grown under long-day conditions with a 16-h day/8-h night cycle (22 °C/18 °C) in a Percival growth chamber (CLF Climatics) with white-light fluorescent tubes Philips Alto II F17T8/TL841 (100 µmol m−2 s−1) supplemented, or not, with Philips TL40/W01RS narrowband UV-B tubes (0.3 mW cm−2; for phenylpropanoid mutants analysis), or under short-day conditions with a 8-h day/16-h night cycle (22 °C/18 °C; 100 µmol m−2 s−1) supplemented, or not, with Philips TL40/W01RS narrowband UV-B tubes (0.06 mW cm−2; for the analysis of photoreceptor mutants).
Fluence rates of visible light were measured with a LI-250 Light Meter (LI-COR Biosciences). UV-B irradiances were measured with a VLX-3W UV Light Meter equipped with a CX-312 sensor (Vilber Lourmat; see also Supplementary Fig. S4 for spectra of the different light sources used, as measured with an Ocean Optics QE65000 spectrometer, and Supplementary Fig. S5 for graphical representation of the experimental setups).
Immunoblot analysis
Total protein extracts from seedlings were prepared as previously described (Samol et al. 2012). In brief, samples were harvested, frozen in liquid nitrogen, and ground, and proteins were extracted by incubating in lysis buffer (100 mM Tris–HCl pH 7.7, 2% (w/v) SDS, 50 mM NaF, and Protease Inhibitor Cocktail [Sigma-Aldrich]) for 30 min at 37 °C. After 15-min centrifugation at 4 °C at 15,000 × g, the clear supernatants were collected, and total protein concentration was measured with bicinchoninic acid solution (BCA, Sigma-Aldrich) following manufacturer's instructions. Ten micrograms of protein of each sample was separated by SDS-PAGE gel electrophoresis (15% w/v polyacrylamide gels) and transferred to PVDF membranes (Roth) for 7 min at 20 V using the iBlot 2 Dry Blotting System (Thermo Fisher Scientific), before blocking in TBS-T with 5% (w/v) nonfat dry milk for 1 h. Polyclonal anti-D1 (AS05084A, C-terminal, Agrisera) and anti-RbcL (AS03037, Agrisera) were used as primary antibodies. Horseradish peroxidase (HRP)–conjugated anti-rabbit immunoglobulins (Dako A/S) were used as secondary antibodies. Signal was revealed using the ECL Select Western Blotting Detection Reagent (GE Healthcare) and analyzed using an Amersham Imager 680 camera system (GE Healthcare).
Reverse transcription quantitative PCR (RT-qPCR)
Plant total RNA was isolated with the Plant RNeasy kit (Qiagen) including DNase treatment according to the manufacture's standard protocol. Synthesis of cDNA was performed using the Taqman Reverse Transcription Reagent kit (Thermo Fisher Scientific). Each RT-qPCR reaction contained cDNA (equivalent of 3 ng RNA) synthetized with a 1:1 mixture of oligo(dT) primers and random hexamers. RT-qPCR was performed using a PowerUp SYBR Green Master Mix (Thermo Fisher Scientific) on a QuantStudioTM 5 Real-Time PCR System (ThermoFisher Scientific). The gene-specific primers used were 5ʹ-ATG ATG GGG ATG TTG TCG AT-3ʹ and 5ʹ-CGT CCA TGA TGA TTG CTT TG-3ʹ for FAH1 (AT4G36220) and 5′-AGC TGA TGG ACC TGC AGG CAT CTT GGC-3′ and 5′- TGC ATG TGA CGT TTC CGA ATT GTC GAC-3′ for CHS (AT5G13930). The ΔΔCT method (Livak and Schmittgen 2001) was used to calculate expression values, with PP2AA3 (PROTEIN PHOSPHATASE 2A SUBUNIT A3, AT1G13320) as a reference gene (Czechowski et al. 2005). All expression values were normalized against an untreated wild type, which was set to 1. Each experiment was performed by combining 3 independent biological replicates.
F v/Fm measurements
A PSI (Photon Systems Instruments) FluorCam 800 MF was used to assay the maximum quantum efficiency of PSII (Fv/Fm) by measuring plant chlorophyll fluorescence. Before measurements, plates (10 to 20 seedlings per genotype per plate) were incubated in darkness for at least 5 min, or plants grown on soil (5 plants per pots) were incubated in darkness for 20 min, which was confirmed to be sufficient to reach steady state under the plant growth conditions used (as recommended by Murchie and Lawson 2013). Maximum fluorescence in the dark (Fm) was measured using saturating pulses of 2000 µmol m−2 s−1 (white light, 400 to 720 nm) for 960 ms, followed by orange-red light (620 nm) detection pulses (10 µs). Fv/Fm was calculated as Fv/Fm = (Fm − Fo)/Fm, where Fm is the maximal fluorescence and Fo is the minimal fluorescence in the dark-adapted state (Baker 2008).
Extraction of phenolic compounds, HPTLC, and measurements of absorption spectra
HPTLC was used to analyze the phenolic compound profile, as described previously (Stracke et al. 2007, 2010). Phenolic compounds were extracted from 50 mg of fresh plant material homogenized using a Silamat S6 (Ivodar Vivadent) in 100 μL of 80% (v/v) methanol, incubated for 10 min at 70 °C at 800 g, and centrifuged for 10 min at 12,000 g at room temperature (RT). Forty microliters of supernatant was spotted using capillary tubes on silica-60 HPTLC glass plates (Sigma-Aldrich) used as the stationary phase. Adsorption chromatography was carried out for ∼1 h using ethyl acetate, formic acid, glacial acetic acid, and water (100:12:12:26) as mobile phase in a closed glass tank. The chromatograms were examined under UV-A (365 nm, Fisher, Bioblock Scientific) and photographed to detect sinapate esters. Subsequently, separated phenylpropanoids were stained by spraying the plates with a 1% (w/v) diphenylboric acid 2-aminoethylester (DPBA; Roth) solution in 80% (v/v) methanol, followed by examination under UV-A (365 nm, Fisher, Bioblock Scientific) and documentation by photography. Several HPTLC-separated flavonol glycosides and sinapate esters were labelled according to a previous report that identified their structures by a successive combination of HPLC– photodiode array detection (PDA)–ESI/MS (Stracke et al. 2007).
The same extraction method was used to prepare samples for absorption spectra measurements. After the centrifugation step, 100 µL of clear supernatant was transferred to a transparent 96-well plate. and absorption spectra were measured using a TECAN Spark 10M Multimode Microplate Reader.
Analysis of secondary metabolites by LC-MS/MS
Plant material was manually ground in a mortar under liquid nitrogen. Fifty milligrams of powder was transferred into 1.5 mL tube, mixed with 5 volumes of 80% (v/v) methanol containing 0.1% (v/v) formic acid, and further homogenized using a TissueLyser II (Qiagen) and glass beads (diameter 2 to 3 mm) for 3 min at 30 Hz. Samples were then centrifuged for 3 min at 12,000 × g; clear supernatants were collected and transferred into HPLC vials for analysis. The targeted analysis of phenolic compounds was performed using UHPLC–PDA–high-resolution mass spectrometry (UHPLC–PDA–HRMS). The system comprised an Acquity UPLC coupled to an eLambda PDA and a Synapt G2 QTOF (Waters) and was entirely controlled by MassLynx 4.1. The separation was performed on an Acquity UPLC CSH C18 column (100 × 2.1 mm, 1.7 µm, Waters) at a flow rate of 0.4 mL/min. A gradient of water + 0.05% (v/v) formic acid (Phase A) and acetonitrile + 0.05% (v/v) formic acid (pPase B) was applied as follows: 2% to 30% B in 6 min, 30% to 100% B in 3.5 min, hold at 100% (v/v) B for 1.5 min, and re-equilibration at 2% (v/v) B for 4 min. The PDA range was 190 to 600 nm and its acquisition frequency 5 Hz. The QTOF mass spectrometer was operated in electrospray negative ionization in full scan mode (scan range 50 to 1200 Da). Four flavonoids, 4 sinapoyl derivatives, coumaroyl glucose, and feruloyl glucose were profiled (Supplementary Tables S1 to S3). Sinapoyl derivatives and other HCAs were quantified as sinapate equivalents and flavonoids as rutin equivalents, all using external calibrations (Glauser et al. 2012; Moreira et al. 2018). Data were processed in TargetLynx XS (Waters).
For the analysis of bands extracted from a HPTLC silica plate, the selected band was scraped off from the HPTLC plate and silica powder was resuspended in 80% (v/v) methanol and 0.1% (v/v) formic acid. Samples were vortexed 3 times for 10 s and kept overnight at 4 °C in the dark. Then, samples were centrifuged 5 min at 12,000 × g, and supernatants were collected and transferred into HPLC vials for analysis. The untargeted analysis of HPTLC fractions was performed using a similar setup with small modifications. The separation was performed on an Acquity UPLC HSS T3 column (100 × 2.1 mm, 1.8 µm, Waters). The mass spectrometer was again operated in electrospray negative ionization, but data-independent acquisition using the so-called MSe mode was used. The obtained chromatograms were visually inspected for differences, and peaks of interest were identified based on their UV and MS/MS spectra. For the interpretation of MS/MS spectra, a combination of spectral matching using the RIKEN spectral database ReSpect and in silico fragmentation using Sirius 5.5.1 including the CSI:FingerID and Canopus suite was used.
Statistical analyses
Statistical analyses were performed using GraphPad Software Prism 9 software (San Diego, California). Statistical significance of the differences between means was determined using two-way ANOVA followed by Tukey's test for multiple comparisons, except for Fig. 2A where log2 transformation of the values was performed before the ANOVA.
Accession numbers
Sequence data from this work can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: AT5G13930 (CHS/TT4), AT4G08920 (CRY1), AT1G04400 (CRY2), AT4G36220 (FAH1), AT5G11260 (HY5), AT3G17609 (HYH), AT3G62610 (MYB11), AT2G47460 (MYB12), AT5g49330 (MYB111), AT1G09570 (PHYA), AT2G18790 (PHYB), AT5G52250 (RUP1), AT5G23730 (RUP2), and AT5G63860 (UVR8).
Supplementary Material
Acknowledgments
We would like to thank Michel Goldschmidt-Clermont for helpful comments on the manuscript; Yamama Naciri, José Manuel Nunes, and Tom Walker for advice on statistics; Henrik Johansson for kindly providing hy5-215 hyh mutant seeds; and the Nottingham Arabidopsis Stock Centre (NASC) for providing various other seed material.
Contributor Information
Manuela Leonardelli, Department of Plant Sciences, Section of Biology, Faculty of Sciences, University of Geneva, CH-1211 Geneva, Switzerland.
Nicolas Tissot, Department of Plant Sciences, Section of Biology, Faculty of Sciences, University of Geneva, CH-1211 Geneva, Switzerland.
Roman Podolec, Department of Plant Sciences, Section of Biology, Faculty of Sciences, University of Geneva, CH-1211 Geneva, Switzerland; Institute of Genetics and Genomics in Geneva (iGE3), University of Geneva, CH-1211 Geneva, Switzerland.
Florence Ares-Orpel, Department of Plant Sciences, Section of Biology, Faculty of Sciences, University of Geneva, CH-1211 Geneva, Switzerland.
Gaétan Glauser, Neuchâtel Platform of Analytical Chemistry, University of Neuchâtel, CH-2000 Neuchâtel, Switzerland.
Roman Ulm, Department of Plant Sciences, Section of Biology, Faculty of Sciences, University of Geneva, CH-1211 Geneva, Switzerland; Institute of Genetics and Genomics in Geneva (iGE3), University of Geneva, CH-1211 Geneva, Switzerland.
Emilie Demarsy, Department of Plant Sciences, Section of Biology, Faculty of Sciences, University of Geneva, CH-1211 Geneva, Switzerland.
Author contributions
M.L., E.D., and R.U. conceived and designed the research. M.L., F.A-O., and E.D. performed the experiments. G.G. performed and contributed metabolomics data. N.T. and R.P. generated and contributed genetic material. M.L., E.D., and R.U. analyzed the data and wrote the paper. All authors reviewed and approved the submitted manuscript.
Supplementary data
The following materials are available in the online version of this article.
Supplementary Figure S1 . Enhanced photoprotection through UVR8-mediated UV-B acclimation depends on HY5 and HYH.
Supplementary Figure S2 . UVR8 signaling induction of FAH1 expression and accumulation of sinapate esters depend on HY5 and HYH.
Supplementary Figure S3 . Enhanced UVR8 signaling promotes accumulation of coumaroyl glucose and feruloyl glucose.
Supplementary Figure S4 . Spectra of the different light sources used in the study.
Supplementary Figure S5 . Graphical representation of the experimental setups used in this study.
Supplementary Table S1 . Quantification of HCAs and flavonol glycosides in 10-d-old seedlings grown in vitro (related to Fig. 2).
Supplementary Table S2 . Quantification of HCAs and flavonol glycosides in 10-d-old seedlings grown in vitro (related to Fig. 3).
Supplementary Table S3 . Quantification of HCAs and flavonol glycosides in soil-grown plants (related to Supplementary Fig. S3).
Supplementary Table S4 . Genotyping information.
Funding
This work was supported by the University of Geneva and the Swiss National Science Foundation (grants 31003A_175774 and 310030_207716 to R.U.). R.P. was supported by an Institute of Genetics and Genomics in Geneva iGE3 PhD Salary Award.
Data availability
The data underlying this article are available in this article and its online supplementary material.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Agati G, Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010:186(4):786–793. 10.1111/j.1469-8137.2010.03269.x [DOI] [PubMed] [Google Scholar]
- Allorent G, Lefebvre-Legendre L, Chappuis R, Kuntz M, Truong TB, Niyogi KK, Ulm R, Goldschmidt-Clermont M. UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 2016:113(51):14864–14869. 10.1073/pnas.1607695114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allorent G, Petroutsos D. Photoreceptor-dependent regulation of photoprotection. Curr Opin Plant Biol. 2017:37:102–108. 10.1016/j.pbi.2017.03.016 [DOI] [PubMed] [Google Scholar]
- Anderson NA, Bonawitz ND, Nyffeler K, Chapple C. Loss of FERULATE 5-HYDROXYLASE leads to mediator-dependent inhibition of soluble phenylpropanoid biosynthesis in Arabidopsis. Plant Physiol. 2015:169(3):1557–1567. 10.1104/pp.15.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008:59(1):89–113. 10.1146/annurev.arplant.59.032607.092759 [DOI] [PubMed] [Google Scholar]
- Binkert M, Kozma-Bognar L, Terecskei K, De Veylder L, Nagy F, Ulm R. UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter. Plant Cell. 2014:26(10):4200–4213. 10.1105/tpc.114.130716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij-James IS, Dube SK, Jansen MAK, Edelman M, Mattoo AK. Ultraviolet-B radiation impacts light-mediated turnover of the photosystem II reaction center heterodimer in Arabidopsis mutants altered in phenolic metabolism. Plant Physiol. 2000:124(3):1275–1284. 10.1104/pp.124.3.1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerman PA, Ramirez MV, Price MB, Helm RF, Winkel BS. Analysis of T-DNA alleles of flavonoid biosynthesis genes in Arabidopsis ecotype Columbia. BMC Res Notes. 2012:5(1):485. 10.1186/1756-0500-5-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelsford CC, Morales LO, Nezval J, Kotilainen TK, Hartikainen SM, Aphalo PJ, Robson TM. Do UV-A radiation and blue light during growth prime leaves to cope with acute high light in photoreceptor mutants of Arabidopsis thaliana? Physiol Plant. 2019:165(3):537–554. 10.1111/ppl.12749 [DOI] [PubMed] [Google Scholar]
- Brown BA, Cloix C, Jiang GH, Kaiserli E, Herzyk P, Kliebenstein DJ, Jenkins GI. A UV-B-specific signaling component orchestrates plant UV protection. Proc Natl Acad Sci U S A. 2005:102(50):18225–18230. 10.1073/pnas.0507187102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard P, Bilger W, Weissenbock G. Contribution of hydroxycinnamates and flavonoids to epidermal shielding of UV-A and UV-B radiation in developing rye primary leaves as assessed by ultraviolet-induced chlorophyll fluorescence measurements. Plant Cell Environ. 2000:23(12):1373–1380. 10.1046/j.1365-3040.2000.00633.x [DOI] [Google Scholar]
- Bursch K, Toledo-Ortiz G, Pireyre M, Lohr M, Braatz C, Johansson H. Identification of BBX proteins as rate-limiting cofactors of HY5. Nat Plants. 2020:6(8):921–928. 10.1038/s41477-020-0725-0 [DOI] [PubMed] [Google Scholar]
- Caldwell MM, Robberecht R, Flint SD. Internal filters: prospects for UV-acclimation in higher plants. Physiol Plant. 1983:58(3):445–450. 10.1111/j.1399-3054.1983.tb04206.x [DOI] [Google Scholar]
- Chapple CC, Vogt T, Ellis BE, Somerville CR. An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell. 1992:4(11):1413–1424. 10.1105/tpc.4.11.1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton WA, Albert NW, Thrimawithana AH, McGhie TK, Deroles SC, Schwinn KE, Warren BA, McLachlan ARG, Bowman JL, Jordan BR, et al. UVR8-mediated induction of flavonoid biosynthesis for UVB tolerance is conserved between the liverwort Marchantia polymorpha and flowering plants. Plant J. 2018:96(3):503–517. 10.1111/tpj.14044 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005:139(1):5–17. 10.1104/pp.105.063743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MP, Susanti NI, Wargent JJ, Findlay JE, Paul Quick W, Paul ND, Jenkins GI. The UV-B photoreceptor UVR8 promotes photosynthetic efficiency in Arabidopsis thaliana exposed to elevated levels of UV-B. Photosynth Res. 2012:114(2):121–131. 10.1007/s11120-012-9785-y [DOI] [PubMed] [Google Scholar]
- de Vries S, Fürst-Jansen JMR, Irisarri I, Dhabalia Ashok A, Ischebeck T, Feussner K, Abreu IN, Petersen M, Feussner I, de Vries J. The evolution of the phenylpropanoid pathway entailed pronounced radiations and divergences of enzyme families. Plant J. 2021:107(4):975–1002. 10.1111/tpj.15387 [DOI] [PubMed] [Google Scholar]
- Dean JC, Kusaka R, Walsh PS, Allais F, Zwier TS. Plant sunscreens in the UV-B: ultraviolet spectroscopy of jet-cooled sinapoyl malate, sinapic acid, and sinapate ester derivatives. J Am Chem Soc. 2014:136(42):14780–14795. 10.1021/ja5059026 [DOI] [PubMed] [Google Scholar]
- Demarsy E, Goldschmidt-Clermont M, Ulm R. Coping with ‘dark sides of the sun’ through photoreceptor signaling. Trends Plant Sci. 2018:23(3):260–271. 10.1016/j.tplants.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Demkura PV, Ballaré CL. UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation. Mol Plant. 2012:5(3):642–652. 10.1093/mp/sss025 [DOI] [PubMed] [Google Scholar]
- Enderle B, Sheerin DJ, Paik I, Kathare PK, Schwenk P, Klose C, Ulbrich MH, Huq E, Hiltbrunner A. PCH1 and PCHL promote photomorphogenesis in plants by controlling phytochrome B dark reversion. Nat Commun. 2017:8(1):2221. 10.1038/s41467-017-02311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Kliebenstein DJ. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol. 2020:184(1):39–52. 10.1104/pp.20.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Lin L, Zhang Q, Lu M, Skvortsova MY, Podolec R, Zhang Q, Pi J, Zhang C, Ulm R, et al. Mechanisms of UV-B light-induced photoreceptor UVR8 nuclear localization dynamics. New Phytol. 2022:236(5):1824–1837. 10.1111/nph.18468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favory JJ, Stec A, Gruber H, Rizzini L, Oravecz A, Funk M, Albert A, Cloix C, Jenkins GI, Oakeley EJ, et al. Interaction of COP1 and UVR8 regulates UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. EMBO J. 2009:28(5):591–601. 10.1038/emboj.2009.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreyra MLF, Serra P, Casati P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol Plant. 2021:173(3):736–749. 10.1111/ppl.13543 [DOI] [PubMed] [Google Scholar]
- Fraser CM, Chapple C. The phenylpropanoid pathway in Arabidopsis. Arabidopsis Book. 2011:9:e0152. 10.1199/tab.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão VC, Fankhauser C. Sensing the light environment in plants: photoreceptors and early signaling steps. Curr Opin Neurobiol. 2015:34:46–53. 10.1016/j.conb.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Glauser G, Schweizer F, Turlings TC, Reymond P. Rapid profiling of intact glucosinolates in Arabidopsis leaves by UHPLC-QTOFMS using a charged surface hybrid column. Phytochem Anal. 2012:23(5):520–528. 10.1002/pca.2350 [DOI] [PubMed] [Google Scholar]
- González Besteiro MA, Bartels S, Albert A, Ulm R. Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J. 2011:68(4):727–737. 10.1111/j.1365-313X.2011.04725.x [DOI] [PubMed] [Google Scholar]
- González Moreno A, de Cózar A, Prieto P, Domínguez E, Heredia A. Radiationless mechanism of UV deactivation by cuticle phenolics in plants. Nat Commun. 2022:13(1):1786. 10.1038/s41467-022-29460-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M, Albert A, Stich S, Heller W, Scherb H, Krins A, Langebartels C, Seidlitz HK, Ernst D. PAR modulation of the UV-dependent levels of flavonoid metabolites in Arabidopsis thaliana (L.) Heynh. leaf rosettes: cumulative effects after a whole vegetative growth period. Protoplasma. 2010:243(1–4):95–103. 10.1007/s00709-009-0064-5 [DOI] [PubMed] [Google Scholar]
- Gruber H, Heijde M, Heller W, Albert A, Seidlitz HK, Ulm R. Negative feedback regulation of UV-B-induced photomorphogenesis and stress acclimation in Arabidopsis. Proc Natl Acad Sci U S A. 2010:107(46):20132–20137. 10.1073/pnas.0914532107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Chang X, Zhang Z, Chen H, He H, Zhong B, Deng XW. Origin and evolution of core components responsible for monitoring light environment changes during plant terrestrialization. Mol Plant. 2019:12(6):847–862. 10.1016/j.molp.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Hectors K, Prinsen E, De Coen W, Jansen MAK, Guisez Y. Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation show specific changes in morphology and gene expression in the absence of stress symptoms. New Phytol. 2007:175(2):255–270. 10.1111/j.1469-8137.2007.02092.x [DOI] [PubMed] [Google Scholar]
- Heijde M, Ulm R. Reversion of the Arabidopsis UV-B photoreceptor UVR8 to the homodimeric ground state. Proc Natl Acad Sci U S A. 2013:110(3):1113–1118. 10.1073/pnas.1214237110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideg E, Jansen MA, Strid A. UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci. 2013:18(2):107–115. 10.1016/j.tplants.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Hoecker U. The activities of the E3 ubiquitin ligase COP1/SPA, a key repressor in light signaling. Curr Opin Plant Biol. 2017:37:63–69. 10.1016/j.pbi.2017.03.015 [DOI] [PubMed] [Google Scholar]
- Holm M, Ma LG, Qu LJ, Deng XW. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 2002:16(10):1247–1259. 10.1101/gad.969702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MAK, Gaba V, Greenberg BM. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci. 1998:3(4):131–135. 10.1016/S1360-1385(98)01215-1 [DOI] [Google Scholar]
- Jenkins GI. The UV-B photoreceptor UVR8: from structure to physiology. Plant Cell. 2014:26(1):21–37. 10.1105/tpc.113.119446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 2000:19(22):6150–6161. 10.1093/emboj/19.22.6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HS, Niyogi KK. Molecular analysis of photoprotection of photosynthesis. In: Demmig-Adams B, Adams WW, Mattoo AK, editors. Photoprotection, photoinhibition, gene regulation, and environment. Dordrecht: Springer; 2008. p. 127–143. [Google Scholar]
- Kaiserli E, Jenkins GI. UV-B promotes rapid nuclear translocation of the Arabidopsis UV-B specific signaling component UVR8 and activates its function in the nucleus. Plant Cell. 2007:19(8):2662–2673. 10.1105/tpc.107.053330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Lim JE, Landry LG, Last RL. Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol. 2002:130(1):234–243. 10.1104/pp.005041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondou Y, Miyagi Y, Morito T, Fujihira K, Miyauchi W, Moriyama A, Terasawa T, Ishida S, Iwabuchi K, Kubo H, et al. Physiological function of photoreceptor UVR8 in UV-B tolerance in the liverwort Marchantia polymorpha. Planta. 2019:249(5):1349–1364. 10.1007/s00425-019-03090-w [DOI] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.). Heynh. Z Pflanzenphysiol. 1980:100(2):147–160. 10.1016/S0044-328X(80)80208-X [DOI] [Google Scholar]
- Kusano M, Tohge T, Fukushima A, Kobayashi M, Hayashi N, Otsuki H, Kondou Y, Goto H, Kawashima M, Matsuda F, et al. Metabolomics reveals comprehensive reprogramming involving two independent metabolic responses of Arabidopsis to UV-B light. Plant J. 2011:67(2):354–369. 10.1111/j.1365-313X.2011.04599.x [DOI] [PubMed] [Google Scholar]
- Kylli P, Nousiainen P, Biely P, Sipilä J, Tenkanen M, Heinonen M. Antioxidant potential of hydroxycinnamic acid glycoside esters. J Agric Food Chem. 2008:56(12):4797–4805. 10.1021/jf800317v [DOI] [PubMed] [Google Scholar]
- Landry LG, Chapple CC, Last RL. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol. 1995:109(4):1159–1166. 10.1104/pp.109.4.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K, Podolec R, Chappuis R, Ulm R, Hothorn M. Plant photoreceptors and their signaling components compete for COP1 binding via VP peptide motifs. EMBO J. 2019:38(18):e102140. 10.15252/embj.2019102140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li Y, Deng H, Sun X, Wang A, Tang X, Gao Y, Zhang N, Wang L, Yang S, et al. Tomato UV-B receptor SlUVR8 mediates plant acclimation to UV-B radiation and enhances fruit chloroplast development via regulating SlGLK2. Sci Rep. 2018:8(1):6097. 10.1038/s41598-018-24309-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ou-Lee TM, Raba R, Amundson RG, Last RL. Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell. 1993:5(2):171–179. 10.2307/3869583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wakao S, Fischer BB, Niyogi KK. Sensing and responding to excess light. Annu Rev Plant Biol. 2009:60(1):239–260. 10.1146/annurev.arplant.58.032806.103844 [DOI] [PubMed] [Google Scholar]
- Liang T, Mei S, Shi C, Yang Y, Peng Y, Ma L, Wang F, Li X, Huang X, Yin Y, et al. UVR8 interacts with BES1 and BIM1 to regulate transcription and photomorphogenesis in Arabidopsis. Dev Cell. 2018:44(4):512–523.e5. 10.1016/j.devcel.2017.12.028 [DOI] [PubMed] [Google Scholar]
- Liang T, Shi C, Peng Y, Tan H, Xin P, Yang Y, Wang F, Li X, Chu J, Huang J, et al. Brassinosteroid-activated BRI1-EMS-SUPPRESSOR 1 inhibits flavonoid biosynthesis and coordinates growth and UV-B stress responses in plants. Plant Cell. 2020:32(10):3224–3239. 10.1105/tpc.20.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T, Yang Y, Liu H. Signal transduction mediated by the plant UV-B photoreceptor UVR8. New Phytol. 2019:221(3):1247–1252. 10.1111/nph.15469 [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Q, Yang G, Zhang C, Dong H, Liu Y, Yin R, Lin L. Pivotal roles of tomato photoreceptor SlUVR8 in seedling development and UV-B stress tolerance. Biochem Biophys Res Commun. 2020:522(1):177–183. 10.1016/j.bbrc.2019.11.073 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001:25(4):402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lois R. Accumulation of UV-absorbing flavonoids induced by UV-B radiation in Arabidopsis thaliana L. I. Mechanisms of UV-resistance in Arabidopsis. Planta. 1994:194:498–503. 10.1007/BF00714462 [DOI] [Google Scholar]
- Lorenzen M, Racicot V, Strack D, Chapple C. Sinapic acid ester metabolism in wild type and sinapoylglucose-accumulating mutant of Arabidopsis. Plant Physiol. 1996:112(4):1625–1630. 10.1104/pp.112.4.1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta T, Noshi M, Nakamura M, Matsuda S, Tamoi M, Ishikawa T, Shigeoka S. Ferulic acid 5-hydroxylase 1 is essential for expression of anthocyanin biosynthesis-associated genes and anthocyanin accumulation under photooxidative stress in Arabidopsis. Plant Sci. 2014:219–220:61–68. 10.1016/j.plantsci.2014.01.003 [DOI] [PubMed] [Google Scholar]
- McInnes KJ, van der Hooft JJJ, Sharma A, Herzyk P, Hundleby PAC, Schoonbeek HJ, Amtmann A, Ridout C, Jenkins GI. Overexpression of Brassica napus COMT1 in Arabidopsis heightens UV-B-mediated resistance to Plutella xylostella herbivory. Photochem Photobiol Sci. 2023:22(10):2341–2356. 10.1007/s43630-023-00455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Fraga AL, Chinen LA, Demkura PV, Lichy MZ, Gershenzon J, Ballaré CL, Crocco CD. AtBBX29 integrates photomorphogenesis and defense responses in Arabidopsis. Photochem Photobiol Sci. 2023:22(6):1475–1489. 10.1007/s43630-023-00391-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Cusumano JC, Somerville C, Chapple CC. Ferulate-5-hydroxylase from Arabidopsis thaliana defines a new family of cytochrome P450-dependent monooxygenases. Proc Natl Acad Sci U S A. 1996:93(14):6869–6874. 10.1073/pnas.93.14.6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkowski C, Strack D. Sinapate esters in brassicaceous plants: biochemistry, molecular biology, evolution and metabolic engineering. Planta. 2010:232(1):19–35. 10.1007/s00425-010-1168-z [DOI] [PubMed] [Google Scholar]
- Morales LO, Brosché M, Vainonen J, Jenkins GI, Wargent JJ, Sipari N, Strid A, Lindfors AV, Tegelberg R, Aphalo PJ. Multiple roles for UV RESISTANCE LOCUS8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar ultraviolet radiation. Plant Physiol. 2013:161(2):744–759. 10.1104/pp.112.211375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales LO, Tegelberg R, Brosche M, Keinanen M, Lindfors A, Aphalo PJ. Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol. 2010:30(7):923–934. 10.1093/treephys/tpq051 [DOI] [PubMed] [Google Scholar]
- Moreira X, Castagneyrol B, Abdala-Roberts L, Berny-Mier y Teran JC, Timmermans BGH, Bruun HH, Covelo F, Glauser G, Rasmann S, Tack AJM. Latitudinal variation in plant chemical defences drives latitudinal patterns of leaf herbivory. Ecography. 2018:41(7):1124–1134. 10.1111/ecog.03326 [DOI] [Google Scholar]
- Murchie EH, Lawson T. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot. 2013:64(13):3983–3998. 10.1093/jxb/ert208 [DOI] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J. Lsolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993:102(1):269–277. 10.1104/pp.102.1.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naing AH, Kim CK. Abiotic stress-induced anthocyanins in plants: their role in tolerance to abiotic stresses. Physiol Plant. 2021:172(3):1711–1723. 10.1111/ppl.13373 [DOI] [PubMed] [Google Scholar]
- Neff MM, Chory J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998:118(1):27–35. 10.1104/pp.118.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugart S, Tobler MA, Barnes PW. Different irradiances of UV and PAR in the same ratios alter the flavonoid profiles of Arabidopsis thaliana wild types and UV-signalling pathway mutants. Photochem Photobiol Sci. 2019:18(7):1685–1699. 10.1039/c8pp00496j [DOI] [PubMed] [Google Scholar]
- Neugart S, Tobler MA, Barnes PW. Rapid adjustment in epidermal UV sunscreen: comparison of optical measurement techniques and response to changing solar UV radiation conditions. Physiol Plant. 2021:173(3):725–735. 10.1111/ppl.13517 [DOI] [PubMed] [Google Scholar]
- Nguyen VPT, Stewart JD, Ioannou I, Allais F. Sinapic acid and sinapate esters in Brassica: innate accumulation, biosynthesis, accessibility via chemical synthesis or recovery from biomass, and biological activities. Front Chem. 2021:9:664602. 10.3389/fchem.2021.664602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichelmann L, Pescheck F. Solar UV-B effects on composition and UV screening efficiency of foliar phenolics in Arabidopsis thaliana are augmented by temperature. Physiol Plant. 2021:173(3):762–774. 10.1111/ppl.13554 [DOI] [PubMed] [Google Scholar]
- Oravecz A, Baumann A, Máté Z, Brzezinska A, Molinier J, Oakeley EJ, Adám E, Schäfer E, Nagy F, Ulm R. CONSTITUTIVELY PHOTOMORPHOGENIC1 is required for the UV-B response in Arabidopsis. Plant Cell. 2006:18(8):1975–1990. 10.1105/tpc.105.040097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MT, Hardtke CS, Wei N, Deng XW. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 2000:405(6785):462–466. 10.1038/35013076 [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 1997:11(22):2983–2995. 10.1101/gad.11.22.2983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolec R, Demarsy E, Ulm R. Perception and signaling of ultraviolet-B radiation in plants. Annu Rev Plant Biol. 2021a:72(1):793–822. 10.1146/annurev-arplant-050718-095946 [DOI] [PubMed] [Google Scholar]
- Podolec R, Lau K, Wagnon TB, Hothorn M, Ulm R. A constitutively monomeric UVR8 photoreceptor confers enhanced UV-B photomorphogenesis. Proc Natl Acad Sci U S A. 2021b:118(6):e2017284118. 10.1073/pnas.2017284118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolec R, Ulm R. Photoreceptor-mediated regulation of the COP1/SPA E3 ubiquitin ligase. Curr Opin Plant Biol. 2018:45(Pt A):18–25. 10.1016/j.pbi.2018.04.018 [DOI] [PubMed] [Google Scholar]
- Podolec R, Wagnon TB, Leonardelli M, Johansson H, Ulm R. Arabidopsis B-box transcription factors BBX20-22 promote UVR8 photoreceptor-mediated UV-B responses. Plant J. 2022:111(2):422–439. 10.1111/tpj.15806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnu J, Riedel T, Penner E, Schrader A, Hoecker U. Cryptochrome 2 competes with COP1 substrates to repress COP1 ubiquitin ligase activity during Arabidopsis photomorphogenesis. Proc Natl Acad Sci U S A. 2019:116(52):27133–27141. 10.1073/pnas.1909181116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procko C, Lee T, Borsuk A, Bargmann BOR, Dabi T, Nery JR, Estelle M, Baird L, O'Connor C, Brodersen C, et al. Leaf cell-specific and single-cell transcriptional profiling reveals a role for the palisade layer in UV light protection. Plant Cell. 2022:34(9):3261–3279. 10.1093/plcell/koac167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian C, Chen Z, Liu Q, Mao W, Chen Y, Tian W, Liu Y, Han J, Ouyang X, Huang X. Coordinated transcriptional regulation by the UV-B photoreceptor and multiple transcription factors for plant UV-B responses. Mol Plant. 2020:13(5):777–792. 10.1016/j.molp.2020.02.015 [DOI] [PubMed] [Google Scholar]
- Rai N, Morales LO, Aphalo PJ. Perception of solar UV radiation by plants: photoreceptors and mechanisms. Plant Physiol. 2021:186(3):1382–1396. 10.1093/plphys/kiab162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai N, Neugart S, Yan Y, Wang F, Siipola SM, Lindfors AV, Winkler JB, Albert A, Brosché M, Lehto T, et al. How do cryptochromes and UVR8 interact in natural and simulated sunlight? J Exp Bot. 2019:70(18):4975–4990. 10.1093/jxb/erz236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai N, O'Hara A, Farkas D, Safronov O, Ratanasopa K, Wang F, Lindfors AV, Jenkins GI, Lehto T, Salojärvi J, et al. The photoreceptor UVR8 mediates the perception of both UV-B and UV-A wavelengths up to 350 nm of sunlight with responsivity moderated by cryptochromes. Plant Cell Environ. 2020:43(6):1513–1527. 10.1111/pce.13752 [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994:104(4):1139–1149. 10.1104/pp.104.4.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzini L, Favory JJ, Cloix C, Faggionato D, O'Hara A, Kaiserli E, Baumeister R, Schäfer E, Nagy F, Jenkins GI, et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science. 2011:332(6025):103–106. 10.1126/science.1200660 [DOI] [PubMed] [Google Scholar]
- Robson TM, Aphalo PJ, Banaś AK, Barnes PW, Brelsford CC, Jenkins GI, Kotilainen TK, Łabuz J, Martínez-Abaigar J, Morales LO, et al. A perspective on ecologically relevant plant-UV research and its practical application. Photochem Photobiol Sci. 2019:18(5):970–988. 10.1039/c8pp00526e [DOI] [PubMed] [Google Scholar]
- Rozema J, Blokker P, Mayoral Fuertes MA, Broekman R. UV-B absorbing compounds in present-day and fossil pollen, spores, cuticles, seed coats and wood: evaluation of a proxy for solar UV radiation. Photochem Photobiol Sci. 2009:8(9):1233–1243. 10.1039/b904515e [DOI] [PubMed] [Google Scholar]
- Rozema J, Björn LO, Bornman JF, Gaberščik A, Häder DP, Trošt T, Germ M, Klisch M, Gröniger A, Sinha RP, et al. The role of UV-B radiation in aquatic and terrestrial ecosystems—an experimental and functional analysis of the evolution of UV-absorbing compounds. J Photochem Photobiol B. 2002:66(1):2–12. 10.1016/S1011-1344(01)00269-X [DOI] [PubMed] [Google Scholar]
- Rozema J, van de Staaij J, Björn LO, Caldwell M. UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol. 1997:12(1):22–28. 10.1016/S0169-5347(96)10062-8 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Meyer K, Cusumano JC, Chapple C. Regulation of ferulate-5-hydroxylase expression in Arabidopsis in the context of sinapate ester biosynthesis. Plant Physiol. 1999:119(1):101–110. 10.1104/pp.119.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K, Swinny E, Winefield C, Markham K. Flavonoids and UV photoprotection in Arabidopsis mutants. Z Naturforsch. 2001:56(9–10):745–754. 10.1515/znc-2001-9-1013 [DOI] [PubMed] [Google Scholar]
- Samol I, Shapiguzov A, Ingelsson B, Fucile G, Crèvecoeur M, Vener AV, Rochaix JD, Goldschmidt-Clermont M. Identification of a photosystem II phosphatase involved in light acclimation in Arabidopsis. Plant Cell. 2012:24(6):2596–2609. 10.1105/tpc.112.095703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner M, Wiesner-Reinhold M, Baldermann S, Hanschen FS, Neugart S. UV-B-induced changes in secondary plant metabolites. In: Jordan BR, editor. UV-B radiation and plant life: molecular biology to ecology. Wallingford (Oxfordshire), UK: CABI; 2017. p. 39–57. [Google Scholar]
- Sheahan JJ. Sinapate esters provide greater UV-B attenuation than flavonoids in Arabidopsis thaliana (Brassicaceae). Am J Bot. 1996:83(6):679–686. 10.1002/j.1537-2197.1996.tb12757.x [DOI] [Google Scholar]
- Shi C, Liu H. How plants protect themselves from ultraviolet-B radiation stress. Plant Physiol. 2021:187(3):1096–1103. 10.1093/plphys/kiab245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995:8(5):659–671. 10.1046/j.1365-313X.1995.08050659.x [DOI] [PubMed] [Google Scholar]
- Sinha RP, Sinha JP, Gröniger A, Häder DP. Polychromatic action spectrum for the induction of a mycosporine-like amino acid in a rice-field cyanobacterium, Anabaena sp. J Photochem Photobiol B. 2002:66(1):47–53. 10.1016/S1011-1344(01)00274-3 [DOI] [PubMed] [Google Scholar]
- Soriano G, Cloix C, Heilmann M, Núñez-Olivera E, Martínez-Abaigar J, Jenkins GI. Evolutionary conservation of structure and function of the UVR8 photoreceptor from the liverwort Marchantia polymorpha and the moss Physcomitrella patens. New Phytol. 2018:217(1):151–162. 10.1111/nph.14767 [DOI] [PubMed] [Google Scholar]
- Stockenhuber R, Akiyama R, Tissot N, Milosavljevic S, Yamazaki M, Wyler M, Arongaus AB, Podolec R, Sato Y, Widmer A, et al. UV RESISTANCE LOCUS 8-mediated UV-B response is required alongside CRYPTOCHROME 1 for plant survival in sunlight under field conditions. Plant Cell Physiol. 2024:65(1):35–48. 10.1093/pcp/pcad113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Favory JJ, Gruber H, Bartelniewoehner L, Bartels S, Binkert M, Funk M, Weisshaar B, Ulm R. The Arabidopsis bZIP transcription factor HY5 regulates expression of the PFG1/MYB12 gene in response to light and ultraviolet-B radiation. Plant Cell Environ. 2010:33(1):88–103. 10.1111/j.1365-3040.2009.02061.x [DOI] [PubMed] [Google Scholar]
- Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007:50(4):660–677. 10.1111/j.1365-313X.2007.03078.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Badger MR. Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci. 2011:16(1):53–60. 10.1016/j.tplants.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Milward SE, Yamori W, Evans JR, Hillier W, Badger MR. The solar action spectrum of photosystem II damage. Plant Physiol. 2010:153(3):988–993. 10.1104/pp.110.155747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavridou E, Pireyre M, Ulm R. Degradation of the transcription factors PIF4 and PIF5 under UV-B promotes UVR8-mediated inhibition of hypocotyl growth in Arabidopsis. Plant J. 2020a:101(3):507–517. 10.1111/tpj.14556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavridou E, Schmid-Siegert E, Fankhauser C, Ulm R. UVR8-mediated inhibition of shade avoidance involves HFR1 stabilization in Arabidopsis. PLoS Genet. 2020b:16(5):e1008797. 10.1371/journal.pgen.1008797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevini M, Braun J, Fieser G. The protective function of the epidermal layer of rye seedlings against ultraviolet-B radiation. Photochem Photobiol. 1991:53(3):329–333. 10.1111/j.1751-1097.1991.tb03636.x [DOI] [Google Scholar]
- Tilbrook K, Dubois M, Crocco CD, Yin R, Chappuis R, Allorent G, Schmid-Siegert E, Goldschmidt-Clermont M, Ulm R. UV-B perception and acclimation in Chlamydomonas reinhardtii. Plant Cell. 2016:28(4):966–983. 10.1105/tpc.15.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot N, Ulm R. Cryptochrome-mediated blue-light signalling modulates UVR8 photoreceptor activity and contributes to UV-B tolerance in Arabidopsis. Nat Commun. 2020:11(1):1323. 10.1038/s41467-020-15133-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohge T, Fernie AR. Leveraging natural variance towards enhanced understanding of phytochemical sunscreens. Trends Plant Sci. 2017:22(4):308–315. 10.1016/j.tplants.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Tohge T, Watanabe M, Hoefgen R, Fernie AR. The evolution of phenylpropanoid metabolism in the green lineage. Crit Rev Biochem Mol Biol. 2013:48(2):123–152. 10.3109/10409238.2012.758083 [DOI] [PubMed] [Google Scholar]
- Tohge T, Wendenburg R, Ishihara H, Nakabayashi R, Watanabe M, Sulpice R, Hoefgen R, Takayama H, Saito K, Stitt M, et al. Characterization of a recently evolved flavonol-phenylacyltransferase gene provides signatures of natural light selection in Brassicaceae. Nat Commun. 2016:7(1):12399. 10.1038/ncomms12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt T. Phenylpropanoid biosynthesis. Mol Plant. 2010:3(1):2–20. 10.1093/mp/ssp106 [DOI] [PubMed] [Google Scholar]
- Wang L, Wang Y, Chang H, Ren H, Wu X, Wen J, Guan Z, Ma L, Qiu L, Yan J, et al. RUP2 facilitates UVR8 redimerization via two interfaces. Plant Commun. 2023:4(1):100428. 10.1016/j.xplc.2022.100428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann E. UV dose-dependent induction of enzymes related to flavonoid biosynthesis in cell suspension cultures of parsley. FEBS Lett. 1975:51(1–2):105–107. 10.1016/0014-5793(75)80863-5 [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol. 2002:5(3):218–223. 10.1016/S1369-5266(02)00256-X [DOI] [PubMed] [Google Scholar]
- Wolf L, Rizzini L, Stracke R, Ulm R, Rensing SA. The molecular and physiological responses of Physcomitrella patens to ultraviolet-B radiation. Plant Physiol. 2010:153(3):1123–1134. 10.1104/pp.110.154658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D. COP1 and BBXs-HY5-mediated light signal transduction in plants. New Phytol. 2020:228(6):1748–1753. 10.1111/nph.16296 [DOI] [PubMed] [Google Scholar]
- Yadav A, Bakshi S, Yadukrishnan P, Lingwan M, Dolde U, Wenkel S, Masakapalli SK, Datta S. The B-box-containing microprotein miP1a/BBX31 regulates photomorphogenesis and UV-B protection. Plant Physiol. 2019:179(4):1876–1892. 10.1104/pp.18.01258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhang L, Chen P, Liang T, Li X, Liu H. UV-B photoreceptor UVR8 interacts with MYB73/MYB77 to regulate auxin responses and lateral root development. EMBO J. 2020:39(2):e101928. 10.15252/embj.2019101928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R, Arongaus AB, Binkert M, Ulm R. Two distinct domains of the UVR8 photoreceptor interact with COP1 to initiate UV-B signaling in Arabidopsis. Plant Cell. 2015:27(1):202–213. 10.1105/tpc.114.133868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R, Skvortsova MY, Loubéry S, Ulm R. COP1 is required for UV-B-induced nuclear accumulation of the UVR8 photoreceptor. Proc Natl Acad Sci U S A. 2016:113(30):E4415–E4422. 10.1073/pnas.1607074113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xu C, Zhang S, Shi C, Cheng H, Liu H, Zhong B. Origin and adaptive evolution of UV RESISTANCE LOCUS 8-mediated signaling during plant terrestrialization. Plant Physiol. 2022:188(1):332–346. 10.1093/plphys/kiab486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoulias N, Brown J, Rowe J, Casson SA. HY5 is not integral to light mediated stomatal development in Arabidopsis. PLoS One. 2020:15(1):e0222480. doi: 10.1371/journal.pone.0222480 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in this article and its online supplementary material.