Abstract
Background
Effective management of both acute and post-acute sequelae of SARS-CoV-2 is essential, particularly for type 2 diabetes mellitus (T2DM) patients, who are at increased risk of severe pro-inflammatory responses and complications. Persistent symptoms and residual lung and cardiovascular damage in post-coronavirus disease (COVID-19) individuals highlight the need for comprehensive long-term treatment strategies. Conventional treatments, including Remdesivir and glucocorticoids, have limitations, suggesting that further investigation into Ayurvedic therapies could be beneficial, though controlled trials are currently limited.
Objectives
Evaluate the effectiveness and safety of Ayurveda with the standard of care (SOC) versus SOC in improving symptoms, moderating immune responses (interleukin-6 (IL-6), C-reactive protein (CRP), neutrophil-lymphocyte ratio (NLR), and radiological outcomes in oxygen-dependent, high-risk, non-vaccinated type 2 diabetes COVID-19 patients over 60 days, and thus addressing their heightened vulnerability to severe infections.
Methods
A controlled trial with 50 diabetic COVID-19 patients, aged 18-80, with an NLR of >= 4, primarily on Remdesivir, was assigned to Group 1 (Add-on Ayurveda+SOC, n=30) or Group 2 (SOC, n=20) based on their voluntary choice with follow-up on days 14, 28, and 60. Parametric outcomes in group analysis were assessed with robust regression and non-parametric outcomes with Cochran-Mantel-Haenszel, log-rank test, and chi-square tests at 95% confidence interval (CI).
Results
Group 1 exhibited statistically significant improvements in fever, cough, diarrhea, as well as NLR, IL-6, and CRP by 14 days, and in anosmia, loss of taste, shortness of breath, general weakness, and headache by 60 days. Though the sample size is small, notable improvements can be seen in troponin levels in Group 1 at 28 and 60 days. High-resolution computer tomography COVID-19 reporting and data system (HRCT CO-RADS) scores improved more slowly in Group 2 than in Group 1. Survival rates were 96.4% for Group 1 and 90% for Group 2. Numbers were too small for reliable comparisons at 60 days.
Conclusion
The add-on Ayurveda group showed a better symptomatic response, and faster normalization in inflammatory markers, including IL-6 and NLR by 14 days, and cardiac markers by 28 days. Minimal clinical and no laboratory adverse events were observed. This study supports the need for a randomized, double-blind trial.
Keywords: innate immunity, sannipata jwara, c-reactive protein, cytokine interleukin-6, neutrophils-lymphocyte ratio
Introduction
The implementation of robust and effective multi-disciplinary strategies to address the acute and post-acute sequelae of SARS-CoV-2 infection is needed, as repeated waves are expected over time. Type 2 diabetes mellitus (T2DM) individuals diagnosed with COVID-19 are at an increased risk of experiencing an exacerbated pro-inflammatory response secondary to dysregulated innate immunity and compromised cell-mediated immune response [1]. Such cases often necessitate mechanical ventilation and ICU admission, thereby contributing to higher mortality rates [2]. The situation is complicated by the presence of comorbidities such as hypertension, chronic kidney disease, and coronary artery disease, which are commonly associated with type 2 diabetes [3-5]. Of interest, these patients have elevated neutrophil-to-lymphocyte ratios, indicating inflammation, and importantly, elevated high-sensitivity C-reactive protein and interleukin-6 levels in the initial phases of the infection [6].
Post-acute sequelae following SARS-CoV-2 infection manifest as persistent symptoms and organ damage in individuals who have recovered from the acute phase of COVID-19. These enduring symptoms include but are not limited to, shortness of breath, cognitive dysfunction, anxiety, depression, muscle aches, fever, loss of smell, loss of taste, and specific damage to the heart and kidneys, with a notable focus on lung fibrosis marked by pathological scarring of pulmonary tissues [7]. The HRCT CORADS score can help identify residual lung damage and provide insights into the severity and progression of the disease [8,9]. Multiple studies have demonstrated that individuals without preexisting cardiovascular conditions who contract COVID-19 can exhibit cardiac complications or myocardial injury [10]. Therefore, it is of scientific interest to investigate how various therapies influence long-term cardiac complications in such cases.
Allopathic COVID-19 treatments include Remdesivir, which reduces hospital stays but does not reliably alleviate symptoms and can affect liver and renal function [11-14]. Glucocorticoids lower 28-day mortality for patients on ventilation or oxygen but prolong viral shedding [15-17]. Glucocorticoids do not decrease mortality in those not requiring ventilation or oxygen, and high doses could actually increase mortality compared to standard care [16,17]. Paxlovid reduces hospitalization and mortality, but its effectiveness against variants and severe cases is uncertain [18]. Tocilizumab, an IL-6 receptor inhibitor, reduces mortality and complications for some patients, but not when combined with steroids [19,20]. Convalescent plasma and IVIG have limited efficacy and may pose risks [21].
Ayurveda, a traditional medicine system, adopts a distinct epistemological approach supported by both controlled and uncontrolled evidence demonstrating the effectiveness of using adjuvant Ayurvedic herbs in treating SARS-CoV-2 infections [22-25]. Case reports and case series on integrating Ayurveda in treating diabetic COVID-19 patients exist, but controlled trials are very limited [25-33].
The objective of this study is to address the acute and post-acute sequelae of add-on Ayurveda in non-vaccinated, hospitalized, oxygen-dependent pre-existing Type II diabetes mellitus patients in moderate to severe COVID-19. This intervention is centered around addressing the early accumulation of cytokines, particularly targeting interleukin-6 (IL-6) and C-reactive protein (CRP) as indicators of dysregulated innate immune response. Additionally, it focuses on assessing the neutrophils-lymphocytes ratio (NLR), an indicator of both innate and cell-mediated immune responses. Further, the study explores the effectiveness of the intervention in moderating laboratory markers, lung fibrosis (high-resolution computed tomography [HRCT]), cardiac damage (HS-troponin), renal (renal function tests), and hepatic (liver function tests) through a longer follow-up period.
Materials and methods
Study design
A 60-day, open-label, two-arm, controlled study involving RT-PCR-positive COVID-19 type II diabetes mellitus patients receiving oxygen support but not requiring ICU admission at the time of induction (i.e., moderate severity) was initiated. The study compared Group 1 (Standard of Care (SOC) plus personalized add-on Ayurveda intervention) against Group 2 (SOC only) for up to 60 days. SOC was defined as any treatment that adhered to the "ICMR Guideline on Clinical Management in COVID Treatment Facilities" (used in both groups 1 and 2) [34].
Patients
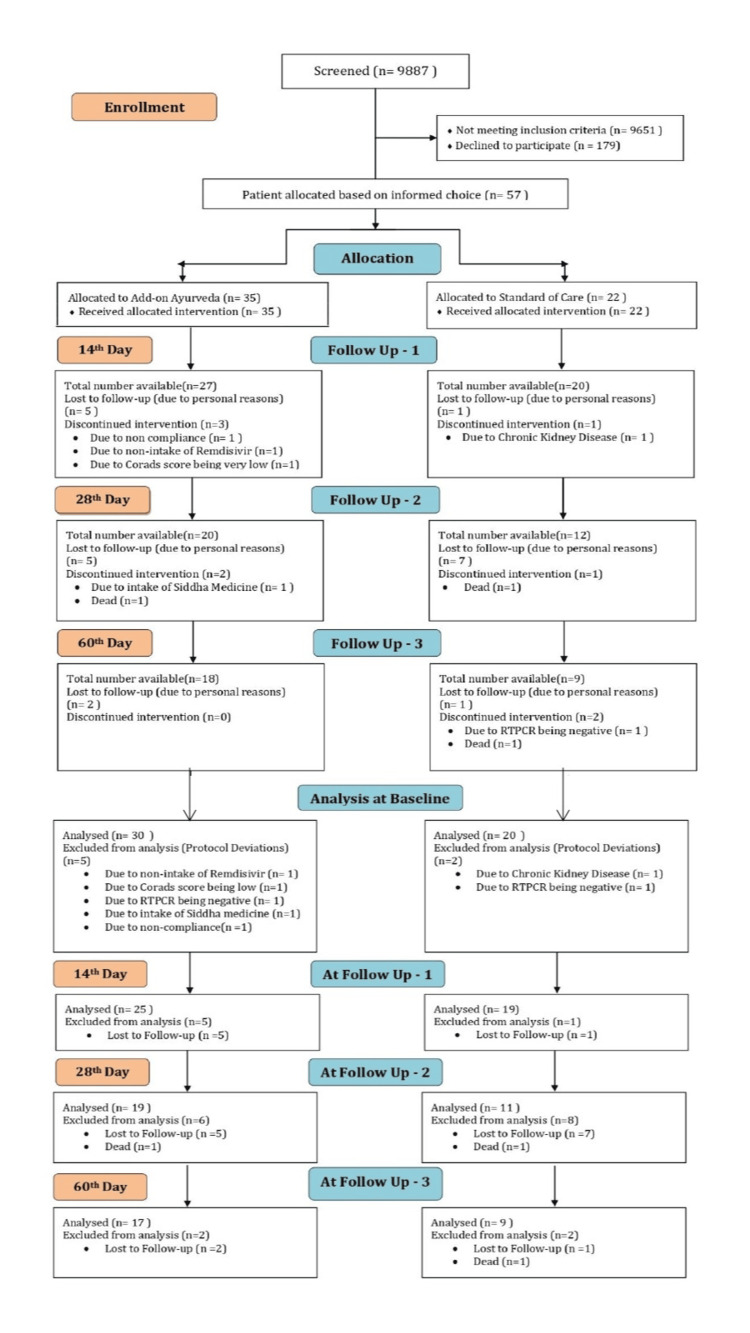
At the special COVID wards of the PSG Institute of Medical Sciences and Research Hospital in Coimbatore, India, 9,887 hospitalized individuals suspected of COVID-19 underwent screening. Among them, 236 unvaccinated diabetic individuals who tested positive for COVID-19 via RT-PCR were invited to participate in the study. The lack of vaccination among these patients was due to either personal choice or challenges in accessing vaccination drives before contracting the virus. The study initially aimed to enroll 100 individuals who met the inclusion and exclusion criteria, commencing in January 2021 on an exploratory basis. However, only 50 unvaccinated participants consented to participate: 30 in Group 1 (received add-on Ayurveda + standard of care) and 20 in Group 2 (received only standard of care). The participants were allocated based on their own voluntary choice into either of the groups. Despite our extensive efforts to achieve the target sample size, recruitment was impeded by the progression of the vaccination drive, decreased severity of infections, and stringent inclusion criteria by January 2022. Including vaccinated subjects in the mid-study could have introduced variability, created subgroups, and obstructed a clear understanding of the primary innate immune response. Thus, this is a controlled but not a randomized study. Group 1 received personalized Ayurvedic treatments, while Group 2 received as per standard of care without Ayurvedic drugs. Both groups were prescribed medications based on symptoms and clinical assessments during follow-up. The rationality behind Ayurvedic and allopathic medications used is furnished in the Appendix (Tables 15-19). The study was registered in the Clinical Trial Registry of India (CTRI Reg No: CTRI/2020/12/029985) and approved by the PSG Institute's Institutional Human Ethics Committee (No: PSG/IHEC/2020/Appr/FB/034). The study period was from December 28, 2020, to January 21, 2022. Ultimately, only 26 out of the 50 unvaccinated participants completed the study due to follow-up challenges, such as transportation issues for patients from other cities, psychological barriers like fear of being identified as a COVID patient or reinfection, a lack of awareness about the importance of follow-up, personal health concerns, and family commitments.
Specific aims
Primary Outcome Measures
To examine the symptomatic response of the patients (fever, anosmia, loss of sense of taste, cough, dyspnea, sore throat, rhinorrhoea, general weakness, headache, irritability, confusion, nausea, and diarrhea) treated with personalized add-on integrative therapy. compared to SOC alone on Baseline, day 14, day 28, and day 60.
To examine the biomarker response (serum IL-6, CRP, NLR) of the patients treated with add-on integrative therapy compared to SOC alone on baseline, day 14, day 28, and day 60.
Secondary Outcome Measures
To examine changes in random blood sugar, hemoglobin, WBC, RBC, absolute neutrophils, absolute lymphocytes, absolute monocytes, platelet count, ALT, AST, ALP, albumin, total bilirubin, direct bilirubin, prothrombin time, serum creatinine, urea, LDH, ferritin, D-dimer, troponin, and HRCT of the lungs.
The detailed schedule of events for primary and secondary outcomes was followed. All lab investigations for patients in the study were performed at the same NABL-accredited laboratory, thereby reducing interlaboratory variability.
Inclusion criteria
The study included participants who were confirmed COVID-19 cases requiring oxygen supplementation but did not need ventilator or ICU support and were aged between 18 and 80 years. Eligible individuals were willing to provide consent and had a pre-existing diagnosis of type II diabetes mellitus. Patients had SpO2 levels between 90-94% on room air or less than 90% with prior approval and a neutrophil-to-lymphocyte ratio (NLR) of 4 or higher. Additionally, participants were supposed to be on Remdesivir medication primarily and were non-vaccinated.
Exclusion criteria
The study excluded individuals with clinically diagnosed COPD, active malignancy, chronic liver or renal diseases, or those who had experienced a myocardial infarction (MI) or cerebrovascular accident (CVA) within the past year. Also excluded were those with type I diabetes mellitus, COVID-19-induced hyperglycemia, or those who were on the faculty or staff of the participating institutions.
Treatment
Throughout the 60-day study period, the interventions were closely monitored and adjusted as needed based on clinical requirements. Both study arms followed the Standard 'ICMR Guideline on Clinical Management in COVID-19'. The Ayurveda interventions in the study group involved intensive drug-based treatments along with dietary and lifestyle modifications, following the principles of classical Ayurveda for distinct stages of Sannipāta jvara (complex fever). During hospitalization, diet and lifestyle conformed to conventional protocols typically adhered to during inpatient care. Ayurveda-based diet and lifestyle suggestions were recommended post-discharge and continued until the 60th day. The personalized intervention was formulated utilizing a host-centric approach, targeting distinct stages of Sannipāta jvara (complicated fever): Nava jvara (acute stage of fever), Pachyamana jvara (phase between ama stage and post-ama stage of fever), Jirna jvara (chronic fever), and some cases of COVID-19 demonstrating features of Punaravartaka jvara (repeated fever). The primary emphasis was on addressing the initial Dhatu-gata jvara lakshana (fever/inflammation in body elements) with the intention of preventing its progression to Dhatu-paka (tissue damage) and mitigating the associated risk of Ojo Kshayam (an immunocompromised state). This strategic approach was aimed at reducing the potential for increased mortality. Specifics of the Ayurveda intervention, based on Agni (gut and cellular metabolism), Vikrithi (disease phenotype), and samprapti (pathogenesis) stages, are outlined in Table 1 and Appendix (Tables 15-18). The medications administered in the study group were exclusively comprised of standardized classical Ayurveda formulations derived from the classical textbooks specified in the First Schedule of the Drugs and Cosmetics Act. These formulations were manufactured following the preparation guidelines outlined in the Ayurvedic Formulary of India (AFI). They included Kashayam (concentrated water decoction), Gulikas (processed Ayurvedic herbo-mineral pills), Arishtas, and Asavas (fermented herbal juices and concentrated water decoctions), Ghritam (clarified butter processed in herbal juices and decoctions), and Choornas (pulverized herbal powder). Notably, no patented, plant-extract-based medicines or proprietary medications were employed in the study. The classical, non-patented Ayurveda formulations underwent rigorous quality control assessments to ascertain their safety and standardization.
Table 1. Details of the medicines used in each group.
*Only the front-line medications as given in the ICMR guidelines have been mentioned above. For a detailed medicine chart, refer to Appendix (Tables 15-18)
| Medicine List | Number of people receiving the medication | |
| Add-on Ayurveda Group (n = 30) | Standard of care Group (n=20) | |
| Ayurvedic formulations | ||
| Indukantham Kashayam | 27 | - |
| Bharangyadi Kashayam | 27 | - |
| Panchathikthakam Kashayam | 22 | - |
| Drakshadi Kashyam | 17 | - |
| Guduchyadi Kashayam | 9 | - |
| Vilwadi Gulika | 2 | - |
| Patolakadurohinyadi Kashayam | 1 | - |
| Pravala Bhasmam | 23 | - |
| Swasanandam Gulika | 24 | - |
| Gorochanadi Gulika | 27 | - |
| Suvarnamukatadi Gulika | 26 | - |
| Brahmarasayana | 11 | - |
| Agasthyarasayanam | 24 | - |
| Dhanwantharam Gritham | 3 | - |
| Vidaryadi Lehyam | 7 | - |
| Baladi Gritham | 20 | - |
| Nayopayam Kashayam | 15 | - |
| Rasonadi Kashayam | 2 | - |
| Allopathic medicines prescribed as per ICMR Guidelines* | ||
| Inj.Remdesivir | 30 | 20 |
| Inj.Clexane | 25 | 19 |
| Inj.Pan | 27 | 19 |
| Inj.Methylprednisolone | 19 | 18 |
| T.Dolo | 26 | 17 |
| T.Zac D | 21 | 17 |
| Syp.Alex | 16 | 11 |
| T.Metformin | 20 | 10 |
| T.Glimepiride | 6 | 1 |
| Inj.Actrapid | 5 | 2 |
| T.Vildagliptin | 5 | 2 |
| T.Glycomet | 4 | 3 |
| Inj.Insulin | 4 | 2 |
| Inj.Mixtard | 4 | 0 |
| T.Janumet | 3 | 0 |
| T.Gluconorm | 2 | 2 |
| T.Gemer | 2 | 1 |
| T.Gliclazide | 2 | 1 |
| T.Teneligliptin | 2 | 1 |
| T.Diamicron | 2 | 0 |
| T.Glycinorm | 2 | 0 |
| T.Voglibose | 1 | 2 |
| Inj.Cantus | 1 | 0 |
| Inj.Lantus | 1 | 0 |
| T.Istamet | 1 | 0 |
| T.Jalra M | 1 | 0 |
| T.Lupisulin | 1 | 0 |
| T.Obimet | 1 | 0 |
| T.Oxra | 1 | 0 |
| T.Pioglitazone | 1 | 0 |
| T.Reclide | 1 | 0 |
| T.Reclimet | 1 | 0 |
| T.Valera | 1 | 0 |
| T.Vilzatom | 1 | 0 |
| T.Vinglyn | 1 | 0 |
| T.Vogs Gm-2 | 1 | 0 |
Statistical analysis
The analysis included available subjects at each follow-up visit, incorporating data from dropouts. The final analysis was performed using SAS 9.4 software, which comprised 30 patients in Group 1 and 20 in Group 2, with an 80% compliance rate (based on active medication days within the study period) and no major protocol deviations. Efficacy between the groups for continuous outcomes was assessed using robust regression, which accounted for outliers and deviations from normality while adjusting for baseline differences. For dichotomous primary symptomatic outcomes, the Cochran-Mantel-Haenszel test was applied, factoring in baseline variations. To address the impact of unequal sample sizes between the groups, weighted analyses were employed. Trend curves, which included all patients at each eligible visit, were used to visualize data changes over time for both the treatment and control groups.
A sensitivity analysis was performed to evaluate the influence of missing data on significant outcomes, utilizing the last observation carried forward (LOCF) method under the missing at random (MAR) assumption. This analysis employed robust regression, ANCOVA, and mixed-effects logistic regression models as appropriate. All statistical tests were conducted with a significance level of 0.05.
Results
Baseline characteristics
Among the 50 enrolled patients (30 in Gp 1; 20 in Gp 2), 44 completed 14 days (25 in Gp 1; 19 in Gp 2), 30 completed 28 days (19 in Gp 1; 11 in Gp 2), and 26 completed 60 days (17 in Gp 1; 9 in Gp 2). Notably, there were no statistically significant differences in demographic characteristics, baseline biomarkers, or presenting symptoms between the two groups. The mean age (Gp 1: 58.9 ± 9.5 yrs vs. Gp 2: 60.15 ± 9.4 yrs, p = NS), gender distribution (Gp 1: 70% males vs. Gp 2: 60% males, p = NS), random blood sugar levels (Gp 1: 259.64 ± 107 mg/dL vs. Gp 2: 254.83 ± 124.58 mg/dL, p = NS), and duration of diabetes (Gp 1: 9.0 ± 6.3 yrs vs. Gp 2: 10.8 ± 6.09 yrs, p = NS) demonstrated no significant differences. Both the add-on Ayurveda group and the Standard of Care group had a median hospitalization duration of eight days. Predominant symptoms, namely cough (Gp 1: 46.7%, vs. Gp 2: 40%, p = NS), fever (Gp 1: 46.7%, vs. Gp 2: 45%, p = NS), and dyspnea (Gp 1: 26.7%, vs. Gp 2: 25%, p = NS), were reported as first symptoms of COVID-19 by a majority of participants in both groups. The time to onset of these symptoms was 5-7 days (median). Baseline assessments, including NLR (Gp 1: 7.88 ± 3.81 vs. Gp 2: 7.19 ± 2.8, p = NS), and HRCT CO-RADS scores (Gp 1: 12.66 ± 4.38 vs. Gp 2: 12.21 ± 4.45, p = NS), were not different between the groups. Furthermore, the proportions of participants receiving Remdesivir and Methylprednisolone were similar (Gp 1: 93.3% vs. Gp 2: 95%, p = NS). Detailed information on baseline demographic characteristics is provided in Table 2.
Table 2. Clinical demography.
Gp 1: Add on Ayurveda Group, Gp 2: Standard of Care Group
A: Values assessed by parametric tests (Independent Samples t-test). B: Values assessed by non-parametric tests (Chi-Square test, Median test, Proportions (Z) test, and Wilcoxon’s Rank-Sum test)
| Gp 1 | Gp 2 | p-value | |
| Total subjects (at baseline) | 30 | 20 | |
| Male | 21 (70%) | 12 (60%) | 0.63b |
| Female | 9 (30%) | 8 (40%) | |
| Age | 58.86 (±9.52) | 60.15 (±9.38) | 0.64a |
| Male | 59.5 (±8.99) | 57.5 (±9.99) | |
| Female | 57.3 (±11.1) | 64.12 (±7.22) | |
| No of subjects with history of co-morbidities | |||
| Diabetes | 30 (100%) | 20 (100%) | |
| Duration (in years) | 9 (±6.3) | 10.8 (±6.09) | 0.19 b |
| Hypertension | 13 (43.3%) | 10 (50%) | |
| Chronic cardiac disease | 3 (10%) | 3 (15%) | |
| Smoking status | 4 (13.3%) | 2 (10%) | |
| Current | 1 | 1 | |
| Past | 3 | 1 | |
| Hypotension | 1 (3.3%) | 0 (0%) | |
| Hypothyroidism | 1 (3.3%) | 0 (0%) | |
| Asthma | 0 (0%) | 1 (5%) | |
| Baseline oxygen saturation | 94 (89 - 96.75) | 94.5 (92.25 - 96.75) | 0.43b |
| Baseline CT scores | 12.66 (±4.38) | 12.21 (±4.45) | 0.72b |
| Baseline neutrophils to lymphocytes ratio | 7.88 (±3.81) | 7.19 (±2.80) | 0.75 b |
| Baseline random blood sugar | 259.64 (±107) | 254.83 (±124.58) | 0.66 b |
| No of subjects at each follow up visit | |||
| 14 days follow up | 25 | 19 | |
| 28 days follow up | 19 | 11 | |
| 60 days follow up | 17 | 9 | |
| Median number of days of hospitalization | 8 (6 - 12) | 8 (6 - 12) | 0.99 b |
| Number of subjects on Remdesivir and Methylprednisolone | 28 (93.3%) | 19 (95%) | 0.99 b |
| Onset of first symptom – median duration | |||
| Cough | 14 (46.7%) - 7 days | 8 (40%) - 7 days | 0.84 b |
| Fever | 14 (46.7%) - 7 days | 9 (45%) - 5 days | 0.09 b |
| Dyspnea | 8 (26.7%) - 5.5 days | 5 (25%) - 6 days | 0.80 b |
| Fatigue | 5 (16.7%) - 7 days | 6 (30%) - 6 days | 0.41 b |
| Tastelessness | 3 (10%) - 5 days | 1 (5%) - 3 days | |
| Anosmia | 2 (6.7%) - 4 days | 3 (15%) - 2.5 days | |
| Nausea | 2 (6.7%) - 9.5 days | 3 (15%) - 4.5 days | |
| Diarrhea | 1 (3.3%) - 7 days | 6 (30%) - 3.5 days | |
| Headache | 1 (3.3%) - 7 days | 2 (10%) - 7 days | |
| Running Nose | 1 (3.3%) - 5 days | 0 (0%) | |
Assessment of markers on day 14
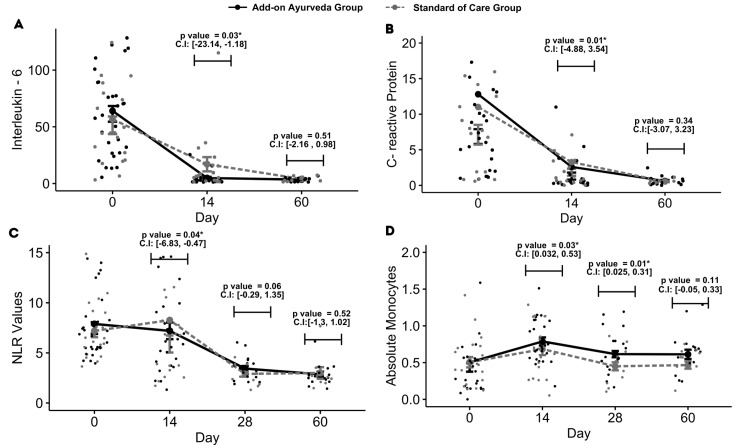
The analysis revealed significant differences between groups across several symptomatic outcomes, namely loss of taste (p = 0.01), cough (p = 0.01), shortness of breath (p = 0.01), and fever (p = 0.04), which exhibited marked improvements within the add-on Ayurveda group. Diarrhea (p = 0.02) exhibited improvements in the standard of care. Other symptoms like anosmia, general weakness, sore throat, irritability, confusion, and nausea displayed consistent patterns across both groups (Tables 3, 4, Figure 1). Robust regression analysis demonstrated a notable decrease in primary biomarkers, namely neutrophil-to-lymphocyte ratio (NLR) (p = 0.04), interleukin-6 (IL-6) levels (p = 0.03), and C-reactive protein (CRP) levels (p = 0.01) within the add-on Ayurveda group (Table 5, Figure 2). Additionally, significant improvements in absolute monocytes (p = 0.03) were observed in the add-on Ayurveda group (Figure 2). Similar results were observed by sensitivity analysis, which accounted for the imputed data.
Table 3. Primary outcomes: Alleviation in sub-acute symptomatic parameters.
Gp 1: Add on Ayurveda Group, Gp 2: Standard of Care Group
*Values at 95% confidence interval where the level of significance, α= 0.05.
A: Values assessed by Cochran-Mantel-Haenszel test after adjusting for baseline difference. B: Values assessed by Chi-square/Fisher’s test over 14 days.
| Parameter | Baseline | Day 14 | Day 28 | Day 60 | |||||||
| Gp 1 (N=17) | Gp 2 (N = 9) | Gp 1 (N=17) | Gp 2 (N = 9) | p value over 14 daysb | Gp 1 (N=17) | Gp 2 (N = 9) | p value over 28 daysa | Gp 1 (N=17) | Gp 2 (N = 9) | p value over 60 daysa | |
| Anosmia | 7 (41.2%) | 2 (22.2%) | 3 (17.6%) | 1 (11.1%) | 0.06 | 2 (11.8%) | 0 (0%) | 0.07 | 1 (5.9%) | 0 (0%) | 0.04* |
| Loss of taste | 12 (70.6%) | 3 (33.3%) | 3 (17.6%) | 1 (11.1%) | 0.01* | 2 (11.8%) | 0 (0%) | 0.03* | 1 (5.9%) | 0 (0%) | 0.02* |
| Cough | 15 (88.2%) | 7 (77.8%) | 7 (41.2%) | 3 (33.3%) | 0.01* | 7 (41.2%) | 1 (11.1%) | 0.59 | 3 (17.6%) | 1 (11.1%) | 0.28 |
| Shortness of breath | 10 (58.8%) | 4 (44.4%) | 4 (23.5%) | 1 (11.1%) | 0.01* | 3 (17.6%) | 1 (11.1%) | 0.03* | 1 (5.9%) | 1 (11.1%) | 0.03* |
| General Weakness | 13 (76.5%) | 6 (66.7%) | 9 (52.9%) | 4 (44.4%) | 0.622 | 6 (35.3%) | 2 (22.2%) | 0.06 | 5 (29.4%) | 3 (33.3%) | 0.02* |
| Headache | 5 (29.4%) | 1 (11.1%) | 1 (5.9%) | 0 (0%) | 0.02* | 5 (29.4%) | 0 (0%) | 0.04* | 1 (5.9%) | 1 (11.1%) | 0.03* |
| Confusion | 2 (11.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.33 | 1 (5.9%) | 0 (0%) | - | 1 (5.9%) | 0 (0%) | - |
Table 4. Primary outcomes: Alleviation in acute symptomatic parameters over 14 days.
Gp 1: Add on Ayurveda Group, Gp 2: Standard of Care Group
*Values at 95% confidence interval where the level of significance, α= 0.05. A: Values assessed by Chi-square/Fisher’s test over 14 days.
| Parameter | Baseline | Day 14 | |||
| Gp 1 (N=24) | Gp 2 (N = 19) | Gp 1 (N=24) | Gp 2 (N = 19) | p-value over 14 days a | |
| Fever | 5 (20.83%) | 2 (10.53%) | 0 (0%) | 0 (0%) | 0.04* |
| Sore throat | 2 (8.33%) | 4 (21.06%) | 5 (20.83%) | 0 (0%) | 0.06 |
| Running Nose | 2 (8.33%) | 0 (0%) | 0 (0%) | 1 (5.26%) | |
| Irritability | 4 (16.67%) | 4 (21.06%) | 1 (4.16%) | 1 (5.26%) | 0.99 |
| Nausea | 5 (20.83%) | 6 (31.58%) | 1 (4.16%) | 0 (0%) | 0.99 |
| Diarrhea | 2 (8.33%) | 8 (42.08%) | 1 (4.16%) | 0 (0%) | 0.02* |
Table 5. Primary outcomes: Interleukin – 6, C-reactive protein, neutrophils to lymphocytes ratio.
Gp 1: Add on Ayurveda Group, Gp 2: Standard of Care Group
* Values at a 95% confidence interval where the level of significance, α= 0.05
The method of Robust regression controlled for baseline differences with treatment group as covariate has been used to testing differences between the treatment groups.
** Parameter not assessed on day 28 as per the protocol; NA: Not applicable
| Parameter | Days of therapy | Gp 1 | Gp 2 | p-value | Estimate | ||||
| N | Mean | SD | N | Mean | SD | ||||
| Interleukin -6 | 0 | 30 | 64.05 | 39.38 | 20 | 56.75 | 40.95 | ||
| 14 | 25 | 4.85 | 5.23 | 18 | 17.01 | 26.61 | 0.03* | -1.79 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 3.43 | 1.52 | 9 | 4.02 | 2.28 | 0.51 | -0.53 | |
| C-Reactive Protein | 0 | 26 | 12.80 | 21.88 | 18 | 11.01 | 14.26 | ||
| 14 | 25 | 2.60 | 6.90 | 17 | 3.27 | 6.18 | 0.01* | -0.5 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 0.62 | 0.56 | 9 | 0.54 | 0.28 | 0.34 | -0.12 | |
| Neutrophils-to-Lymphocytes Ratio | 0 | 30 | 7.88 | 3.81 | 20 | 7.19 | 2.80 | ||
| 14 | 25 | 7.00 | 4.13 | 19 | 10.65 | 6.30 | 0.04* | -3.09 | |
| 28 | 19 | 3.43 | 1.12 | 11 | 2.90 | 0.92 | 0.06 | 0.47 | |
| 60 | 16 | 2.86 | 1.07 | 9 | 3.00 | 1.74 | 0.52 | 0.20 | |
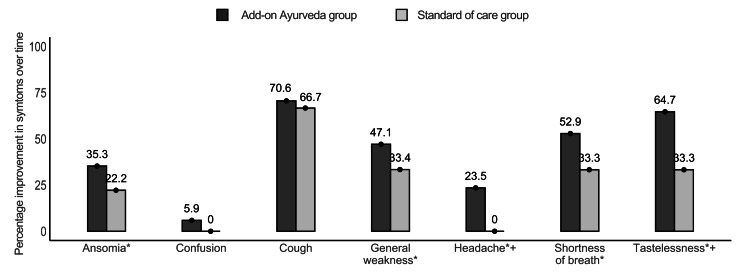
Figure 1. Alleviation in symptomatic parameters over 14 and 60 days (percentage decrease).
* Depicts statistical significance between the groups at 60 days assessed by Cochran-Mantel-Haenszel test after adjusting for baseline values. p-value=0.00-0.04 for all
+ Depicts statistical significance between the groups at 14 days assessed by Chi-square/Fisher’s test. p-value=0.00-0.04 for all
Figure 2. Changes in primary outcomes.
A, B, C, D depicts mean value of patients over time in add-on ayurveda group and standard of care for IL-6, CRP, neutrophils to lymphocytes ratio, and absolute monocytes.
*p-value computed using robust regression controlling for baseline differences between group on the particular visit.
Assessment of markers on day 28
On this day, significant differences from baseline were noted in certain symptoms: loss of taste (p = 0.03), headache (p = 0.04), and shortness of breath (p = 0.03), favoring the add-on Ayurveda group. However, symptoms like anosmia, general weakness, and cough showed similar trends in both groups (Table 3, Figure 1). The neutrophil-to-lymphocyte ratio (NLR) didn't differ significantly between groups, indicating improvement in both. Troponin levels were notably higher in the standard of care (SOC) group (p = 0.01). Additionally, absolute monocytes (p = 0.01) and total monocytes (p = 0.01) significantly improved within the add-on Ayurveda group (Appendix [Table 10]).
Assessment of markers on day 60
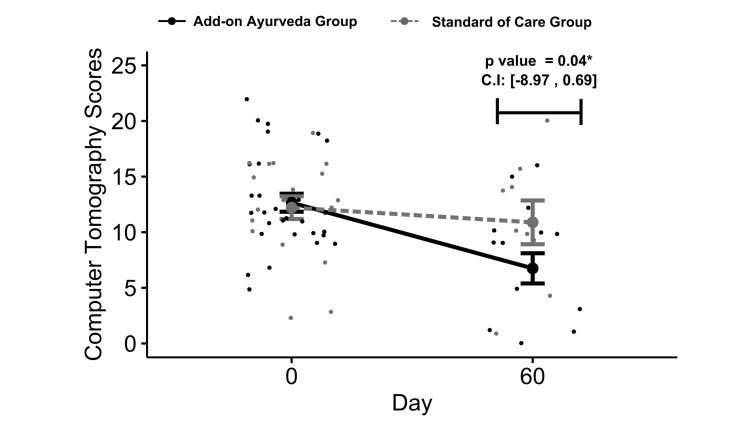
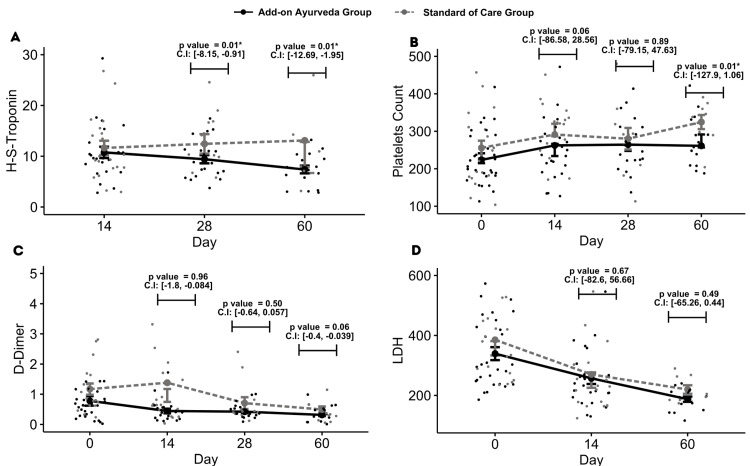
The add-on Ayurveda group exhibited statistically significant improvements in symptoms from baseline, including anosmia (p=0.04), loss of taste (p=0.02), shortness of breath (p=0.03), general weakness (p=0.02), and headache (p=0.03). Cough demonstrated a similar response between the groups (Table 3, Figure 1) Troponin levels and platelet counts were notably higher in the SOC group (p = 0.01 each) (Appendix [Table 10]). Furthermore, HRCT CO-RADS scores displayed a significant decrease in the add-on group compared to SOC (Delta Gp 1 = 47%, Delta in Gp 2 = 10.8%, p = 0.04) (Figure 3).
Figure 3. Changes in CT scores.
*p-value computed using robust regression controlling for baseline differences
Impact on hematological and biochemical markers
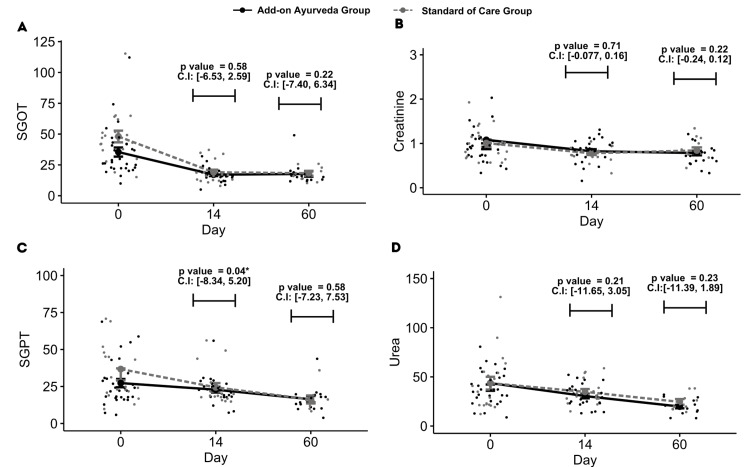
Random blood sugar levels displayed a random pattern with no statistically significant variances observed between the groups (p = NS) over 60 days (Appendix [Table 11]). No consistent or clinically meaningful differences were observed in hemoglobin, WBC, AST, ALT, albumin, total bilirubin, alkaline phosphatase, creatinine, and ferritin levels, or in the vital signs (Appendix [Tables 11-13]). Survival rates did not differ significantly between Gp 1 (96.37%) and Gp 2 (90%) (p = NS) (Appendix [Table 14]). The sensitivity analysis, employing multiple imputations, is in accordance with the results obtained from the primary analyses.
Adverse effects
In Gp 1 (add-on Ayurveda), four out of 30 enrolled patients experienced nine adverse events (AEs). These included two gastrointestinal (blister formation on lips, constipation), one cardiovascular (pedal edema), one ENT (burning sensation in eyes), one skin (itching), one urinary tract (frequent urination), two respiratory (breathing difficulty, traces of blood in sputum), and one general systemic symptom (sweating). Additionally, there were two serious adverse events (SAEs), with one patient dying of disease and another requiring transfer to intensive care due to low oxygen saturation but eventually recovering. In Gp 2 (Standard of Care), one out of 20 patients had a moderate drug-related cardiovascular AE (pedal edema), and there were two SAEs resulting in patient deaths due to the progression of their underlying disease.
The complete adverse event details based on the body system and treatment group can be found in the Appendix (Table 9).
Results of sensitivity analysis
The sensitivity analysis indicated that the outcomes, IL-6, CRP, and NLR, remained consistent with the main analysis over the 60-day period. Secondary outcomes, including H-S troponin and HRCT scores, also demonstrated no significant differences when compared to the main analysis. However, slight variations were observed in symptomatic outcomes.
The analysis showed statistically significant differences between groups at day 14 for fever and anosmia, with improvements noted in the add-on Ayurveda group. By day 28, anosmia showed better improvement in the add-on Ayurveda group. By day 60, both anosmia and dyspnea demonstrated enhanced improvement in the add-on Ayurveda group (p<0.04 for all). Other symptoms showed similar improvements between both groups (Appendix [Tables 6-8]). The sensitivity analysis, despite showing lower statistical significance for symptomatic outcomes, still indicated trends and patterns consistent with the main analysis.
Discussion
In this controlled study, the addition of Ayurvedic therapy to standard treatment (remdesivir/methylprednisolone) for moderately severe COVID-19 patients with type 2 diabetes was found to have a statistically significant impact. The study found statistical improvements in several symptomatic outcomes, inflammatory markers (IL-6, CRP, NLR), and radiological changes (HRCT scores).
Ayurveda's principles, like dosha (pathophysiological attributes) imbalances, agni (gut and cellular metabolism) disruption, and exogenous disease causes, can be applied to COVID-19. The disease primarily presents as Sannipāta jvara (complicated fever) with respiratory involvement and secondary complications. Gastrointestinal and immune factors also contribute. Disease progression shifts from Vata-Kapha predominant Sannipāta jvara to Vata-Pitta predominant Sannipāta jvara (pathological phenotypes) dominance, affecting vital organs (heart, lungs, kidney, brain, etc.). Abnormal immune responses result from Tridosha (pathophysiological attributes), Rakta (blood and related tissues), and Ojus (adaptive and self-regulatory immune system response) afflictions. This host-centric approach offers potential for reducing COVID-19 mortality and improving clinical outcomes, potentially reshaping epidemic management [35].
Ten herbal components, including Ocimum sanctum, Curcuma longa, Tinospora cordifolia, Piper nigrum, Zingiber officinale, Syzygium aromaticum, Elettaria cardamomum, Citrus limon, and Withania somnifera, which have been a part of the polyherbal formulations administered as our study intervention, have been found to exhibit antiviral effects by regulating viral replication and proliferation within host cells in an in-vivo study [36]. Furthermore, in a molecular docking study by Shree et al. bioactive metabolites derived from three ayurvedic herbs namely, Withania somnifera, Tinospora cordifolia, and Ocimum sanctum, have shown promising potential in enhancing immune function against infections [37].
The incorporation of Ayurvedic treatment, including herbal remedies, alongside standard care in the early stages of COVID-19 infection demonstrated notable improvements in symptoms such as fever, diarrhea, loss of taste, cough, shortness of breath, and headache when compared to the standard care-only group. These results are consistent with our earlier study on COVID-19 patients who have Type II diabetes and experience mild disease severity [33]. Despite the limited number of patients available on day 60, it is noteworthy that the Ayurveda add-on group exhibited statistically significant enhancements in symptoms commonly associated with long-term COVID-19, including anosmia, loss of taste, shortness of breath, general weakness, and headache. These findings suggest that Ayurvedic therapy may offer clinical benefits in mitigating or preventing symptoms associated with long-term COVID-19, warranting further investigation.
Biomarkers, such as the Neutrophil Lymphocyte Ratio (NLR), are reliable indicators of disease severity in COVID-19 patients [38,39]. Studies showed that excessive activation of neutrophils and their migration to the lungs can lead to lung injury and acute respiratory distress syndrome (ARDS) [40]. Our trial revealed significant NLR improvement in patients treated with Ayurveda plus standard of care (SOC) compared to SOC alone at 14 days, with a numerical trend continuing through 28 days. This was not seen at 60 days perhaps there were too few patients.
Eleven Ayurvedic herbs, namely Phyllanthus emblica, Withania somnifera, Phyllanthus niruri, Vitis vinifera, Tinospora cordifolia, Curcuma longa, Andrographis paniculata, Solanum xanthocarpum, Allium sativum, Zingiber officinale, and Ocimum tenuiflorum, used during the study, are known to regulate neutrophil function, foster immune balance and reduce thrombosis and coagulation by controlling neutrophil extracellular trap (NET) formation [41].
IL-6 has pro-inflammatory properties in both innate and adaptive immunity, and its levels can correlate with the degree of disease severity [42]. Through increasing oxidative stress, IL-6 can damage proteins, lipids, and DNA and impair the body’s structure and function, and Lim et al. propose that this effect might lead to the rapid progression of COVID-19 in patients with diabetes mellitus [43]. Our study yielded a statistically significant reduction in IL-6 levels in the add-on group when compared to the group receiving standard of care (SOC) alone at 14 days. This observation holds scientific significance as it can contribute to a more effective moderation of cytokine accumulation during the critical initial two weeks post-infection [44]. A recent study shows that nano-curcumin, as an anti-inflammatory herbal-based agent, modulates the increase of inflammatory cytokines, especially serum IL-1β and IL-6 mRNA expression and cytokine secretion in COVID-19 patients, which may reflect an improvement in clinical manifestation and overall recovery [45].
A comparative study revealed that symptomatic COVID-19-positive T2DM patients had significantly higher CRP and absolute neutrophil counts and lower counts of lymphocytes and eosinophils [46]. Another study showed that, compared with conventional treatment, the expression of CRP for patients with severe COVID-19 was significantly inhibited by an herbal Chinese decoction [47]. Ten studies reported the effect of herbal interventions on CRP levels with a net significant decrease after treatment [48]. In our study, the group that received add-on Ayurveda treatment during days 1-14 showed statistically significant improvements in CRP compared to SOC.
It is typically observed that individuals with pre-existing Type 2 diabetes mellitus (T2DM) who contact with COVID-19 experience a reduction in monocyte levels and alterations in their morphology [49-51]. In contrast, Ayurvedic medication in our study administered to patients during the inflammatory phase (days 0-14) and the chronic phase (days 14-28, 28-60) of COVID-19 appeared to result in different patterns. Notably, there was a significant increase in absolute monocyte counts during days 0-14 in the add-on Ayurveda group, probably indicating improved monocyte-macrophage-mediated opsonization compared to the control group [52,53]. The study employed formulations containing Stereospermum suaveolens. (Patala) root extract, which increased monocyte populations [54], and Phyllanthus emblica. (Aamlaki), known to stimulate the reticulo-endothelial system, activate polymorphonuclear and monocyte-macrophage systems, and enhance cell-mediated immunity in in-vivo studies [55].
According to a study by Rajamanickam et al., the progression of COVID-19 is correlated with an increase in the absolute counts of total monocytes and the frequencies of intermediate and non-classical monocytes from days 15-30 to days 61-90, leveling off thereafter [56]. In our study, we observed a significant reduction in monocytes at days 14 and 28 in the Ayurvedic add-on group. Modeling of the predicted trend curve suggests that the polyherbal combination of Ayurvedic medicines effectively managed the post-acute sequelae of COVID-19. However, further blinded, controlled studies are needed to validate these findings.
Contrary to common findings, a study by Junqueira et al. suggests that antibody-mediated uptake of SARS-CoV-2 by monocytes and macrophages triggers inflammatory cell death, leading to systemic inflammation that contributes to COVID-19 pathogenesis [57]. Despite an overall increase in the number of monocytes, the inflammatory markers IL-6 and CRP decreased significantly in our study group. This finding suggests that further investigation into the subsets of monocytes may provide insights into their impact on inflammatory markers and the pathogenesis of COVID-19.
COVID-19 can affect platelet function, increasing clotting risk [58]. In our study, the Ayurvedic add-on group showed stable platelet counts, while the standard care group exhibited an increase, albeit only at 60 days. Platelet count's clinical significance in long-term COVID-19 is unclear, with some studies suggesting lower platelet counts compared to healthy individuals [59,60] while others report higher counts [61-63], requiring further research for comprehension [64].
Elevated troponin levels are frequent in patients with COVID-19 and are significantly associated with fatal outcomes [9,65]. In a recent study involving COVID-19 patients with acute cardiac injury, 55.1% of patients at follow-up had persistently elevated troponin levels for a median of 2.5 months, thus indicating persistent cardiac damage even after the primary infection had resolved [66]. In our study, we found that the group receiving add-on ayurveda intervention showed a significant decrease in high sensitivity troponin values when compared to the standard of care at days 28 and 60, and this may denote a positive effect of add-on ayurveda.
In this study, we examined the use of CO-RADS severity scoring to assess the post-COVID syndrome in recovered patients with lower scores indicating improvement in lung HRCT [67]. After reviewing prior research, we observed that CO-RADS scores improved over time in COVID-19 patients who received antiviral treatment, while they remained stable or worsened in those who did not receive antiviral treatment [68-70]. In our study, despite the small sample size at 60 days, we noted a significant difference in CORADS scores between groups. Lung damage resolved faster in the Ayurveda add-on group compared to standard of care (SOC), suggesting potential benefits in reducing lung inflammation and improving the resolution of ground glass opacities. These findings are promising and merit further investigation.
Both groups displayed comparable trends in random blood sugar levels over the 60-day period as both received standard-of-care (SOC) interventions to manage potential effects on glycemic control.
While the acute COVID-19 pandemic is over, it can be anticipated that new strains of COVID-19 waves will occur, making this study useful for the future. Further, while COVID-19 is a particular virus, the concept of studying ayurvedic medications in well-controlled trials is worth considering. This study exhibits several notable strengths. It is one of the first controlled studies comparing Ayurveda add-on therapy to standard of care in oxygen-dependent, hospitalized, non-vaccinated, diabetic patients with moderate to severe COVID-19 with a 28-day follow-up plus some 60-day observations. The study addresses the safety and compatibility profiles of each intervention. The study design, characterized as an outcome-driven investigation, aligns with the epistemological principles of personalized medicine, particularly well-suited for elucidating the holistic therapeutic approach in Ayurveda. Additionally, the evaluation of a spectrum of innate immunological inflammatory biomarkers and 60-day radiological outcomes is of interest to understanding probable mechanisms of early resolution of lung lesions in the Ayurveda group.
The study also has limitations. While it was controlled, it lacked blinding and randomization as patients volunteered for their groups, introducing selection bias. Additionally, the open nature of the study may have led to evaluation bias. Difficulty in recruiting the planned number of participants was encountered due to challenges in identifying moderate to severe COVID-19 non-vaccinated patients with type 2 diabetes and NLR greater than four as the pandemic waned. Moreover, a high rate of loss to follow-up at 60 days was observed, attributed to concerns about being identified as COVID-19 patients in their communities, personal health issues, family obligations, lack of awareness regarding follow-up, spontaneous recovery during the study period, and reluctance to travel to the center. Consequently, the limited number of 60-day follow-ups impacted the confidence in the analyses at this time point. Fortunately, the 28-day follow-up yielded relatively robust results, and the limited 60-day analyses supported the findings at 28 days. Nevertheless, the presence of a larger effect size allows for the possibility of conducting a more extensive, well-controlled, double-blind study, whose findings could be generalized to a broader population.
Conclusions
This exploratory, controlled, open-label study focused on non-vaccinated COVID-19 patients with pre-existing type II diabetes mellitus. The findings revealed statistically significant improvements in symptomatic outcomes and inflammation markers within a relatively short timeframe of 14 days, as well as sustained benefits over a 28-day period. Additionally, the small subset of subjects followed up at 60 days further supported the initial positive results, with encouraging trends observed in lung health assessments. Other hematological and coagulopathy parameters also showed significantly better responses in the add-on group. Importantly, minimal clinical AEs and no laboratory AEs were observed with the addition of Ayurveda. These promising findings underscore the necessity for a well-controlled, double-blind, randomized study in the future.
Acknowledgments
The authors acknowledge the conceptual guidance and administrative assistance provided by Devidas Varier, Dr Ramkumar Kutty, data collection and therapy execution by Dr. Arun T Namboothiri, Dr. Meera Ramachandran, Dr Nishanth Kumar, Dr Nivethitha, Dr Manikandan N, Dr Saranya T, Dr Dixayarani, Dr Govindrag, Dr Karunamoorthi drug standardization and production oversight by, Dr Aramya A R, Dr Reji Joseph, K Saranya, and V Vani, and contributions to the Ayurvedic Protocol Treatment Group by Dr K G Raveendran, Dr K T Jaikrishnan.
Appendices
Table 6. Sensitivity analyses: Treatment outcomes: Percentage of symptom presence over time (acute and chronic phase) (N = 36)a.
Gp 1: Add on Ayurveda Group, Gp 2: Standard of care group
*Values at 95% Confidence Interval where the level of significance, α= 0.05. a Population with last observation carried forward for 20% of missing values lost to follow-up. b p-values assessed by Chi-square/Fisher’s test over 14 days. c p-values assessed using mixed effects logistic regression with treatment group and time point as covariates, while adjusting for baseline differences with the improvement in response as the outcome.
| Parameters | Baseline | Day 14 | Day 28 | Day 60 | p value between groups over timec | ||||||
| Gp 1 (N = 22) | Gp 2 (N = 14) | Gp 1 (N = 22) | Gp 2 (N = 14) | p value between groups over 14 daysb | Gp 1 (N = 22) | Gp 2 (N = 14) | p value between groups over 28 daysc | Gp 1 (N = 22) | Gp 2 (N = 14) | ||
| Anosmia | 59.10% | 35.70% | 18.20% | 14.30% | 0.029* | 13.60% | 7.10% | 0.036* | 9.10% | 7.10% | 0.045* |
| Tastelessness | 36.40% | 35.70% | 18.20% | 17.10% | 0.609 | 13.60% | 0.00% | 0.048* | 4.50% | 0.00% | 0.427 |
| Cough | 78.60% | 81.80% | 28.60% | 35.90% | 0.633 | 14.30% | 22.70% | 0.648 | 14.30% | 22.70% | 0.176 |
| Shortness of breath | 54.50% | 50.00% | 22.70% | 17.10% | 0.676 | 18.20% | 17.10% | 0.785 | 9.10% | 17.10% | 0.045* |
| General Weakness | 77.30% | 71.40% | 63.60% | 50.00% | 0.809 | 36.40% | 35.70% | 0.899 | 31.80% | 42.90% | 0.859 |
| Headache | 27.30% | 21.40% | 9.10% | 0.00% | 0.693 | 27.30% | 0.00% | 0.788 | 9.10% | 7.10% | 0.998 |
| Confusion | 9.10% | 7.10% | 0.00% | 0.00% | - | 0.00% | 0.00% | - | 4.50% | 0.00% | - |
Table 7. Sensitive analyses: Treatment outcomes: Percentage of symptom presence over time (acute phase) (N = 43)a.
Gp 1: Add on Ayurveda Group, Gp 2: Standard of Care Group
*Values at 95% Confidence Interval where the level of significance, α= 0.05. a Population of cases completing Day 14. b p-values assessed by Chi-square/Fisher’s test over 14 days.
| Parameters | Baseline | Day 14 | p-value between groups over 14 daysᵃ | ||
| Gp 1 (N = 24) | Gp 2 (N = 19) | Gp 1 (N = 24) | Gp 2 (N = 19) | ||
| Fever | 20.83% | 10.53% | 0.00% | 0.00% | 0.04* |
| Sore throat | 8.33% | 21.06% | 20.83% | 0.00% | 0.06 |
| Running nose | 8.33% | 0.00% | 0.00% | 5.26% | - |
| Irritability | 16.67% | 21.06% | 4.16% | 5.26% | 0.99 |
| Nausea | 20.83% | 31.58% | 4.16% | 0.00% | 0.99 |
| Diarrhoea | 8.33% | 42.08% | 4.16% | 0.00% | 0.02* |
Table 8. Sensitivity analyses: Treatment outcomes over time, by group (N = 36)a.
Gp 1: Add on Ayurveda Group, Gp 2: Standard of Care Group
a Population with last observation carried forward for 20% of missing values lost to follow-up. * Values at a 95% confidence interval where the level of significance, α= 0.05. b p-values assessed using the method of robust regression controlled for baseline differences with treatment group as covariate has been used to test differences between the treatment groups unless otherwise noted. c p-values assessed using ANCOVA at 95 CI. ** Parameter not assessed as per the protocol; NA: Not Applicable
| Parameter | Days of therapy | Gp 1 | Gp 2 | p-value | Estimate | ||||
| N | Mean | SD | N | Mean | SD | ||||
| Interleukin-6b | Baseline | 22 | 65.9 | 43.4 | 14 | 56.3 | 43.6 | - | - |
| 14 | 22 | 6.3 | 6.8 | 14 | 18.2 | 29.6 | 0.029* | -14.58 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 22 | 5.3 | 5.6 | 14 | 5.9 | 8.5 | 0.58 | 0.14 | |
| C-reactive proteinb | Baseline | 22 | 7.5 | 5.7 | 14 | 8.9 | 7.0 | - | - |
| 14 | 22 | 2.4 | 4.7 | 14 | 6.5 | 8.9 | 0.037* | -3.65 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 22 | 1.0 | 1.7 | 14 | 1.9 | 4.4 | 0.67 | 0.78 | |
| Neutrophils-Lymphocytes Ratiob | Baseline | 22 | 7.8 | 3.9 | 14 | 7.9 | 3.5 | - | - |
| 14 | 22 | 6.6 | 3.7 | 14 | 8.5 | 4.4 | 0.04* | -1.74 | |
| 28 | 22 | 3.9 | 2.3 | 14 | 5.1 | 3.9 | 0.17 | -1.16 | |
| 60 | 22 | 3.4 | 2.3 | 14 | 4.8 | 3.1 | 0.11 | -1.26 | |
| HS- Troponinb | Baseline | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** |
| 14 | 22 | 10.6 | 5.6 | 14 | 11.7 | 7.2 | - | - | |
| 28 | 22 | 9.1 | 3.9 | 14 | 13.7 | 12.7 | 0.01* | -3.5 | |
| 60 | 22 | 7.7 | 3.8 | 14 | 11.8 | 8.7 | 0.03* | -3.4 | |
| HRCT CORADS scorec | Baseline | 16 | 13.4 | 4.6 | 9 | 13.8 | 2.8 | - | - |
| 14 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 6.3 | 5.0 | 9 | 10.9 | 5.9 | 0.046* | -4.8 | |
Figure 4. Flowchart as per TREND guidelines.
TREND Guidelines as given in the article: Haynes, A. B., Haukoos, J. S., & Dimick, J. B. (2021). TREND Reporting Guidelines for Nonrandomized/Quasi-Experimental Study Designs. JAMA surgery, 156(9), 879–880. https://doi.org/10.1001/jamasurg.2021.0552
Table 9. Details of adverse events.
| Clinical findings of Adverse Events | Total Number of patients with adverse events | |||||||
| Gp 1 (n = 4) | Gp 2 (n = 3) | |||||||
| Day | Freq | Grade | Related to | Day | Freq | Grade | Related to | |
| Burning sensation in eyes (ENT) | 29 | 1 | Mild | Disease | ||||
| Pedal edema (CV) | 27 | 1 | Mild | Drug | 16 | 1 | Moderate | Drug |
| Blister formation on lips (GI) | 19 | 1 | Mild | Disease | ||||
| Breathing Difficulty (RES) | 19 | 1 | Mild | Disease | ||||
| Constipation (GI) | 19 | 1 | Mild | Disease | ||||
| Sweating (GEN) | 19 | 1 | Moderate | Disease | ||||
| Frequent Urination (UT) | 19 | 1 | Mild | Disease | ||||
| Itching (SKIN) | 19 | 1 | Mild | Disease | ||||
| Traces of blood in phlegm (RES) | 1 | 1 | Mild | Disease | ||||
| Serious Adverse Events | ||||||||
| Tachypnoea (RES) | 5 | 1 | Severe | Disease | ||||
| Death | 15 | 1 | Severe | Disease | 18,26 | 2 | Severe | Disease |
Table 10. Secondary outcomes: chronic phase markers.
* Values at a 95% confidence interval where the level of significance, α= 0.05
The method of robust regression controlled for baseline differences with treatment group as a covariate has been used to test differences between the treatment groups.
** Parameter not assessed on day 28 as per the protocol; NA: Not Applicable
| Parameter | Days of therapy | Gp 1 | Gp 2 | p-value | Estimate | ||||
| N | Mean | SD | N | Mean | SD | ||||
| Computed Tomography score | 0 | 29 | 12.66 | 4.38 | 19 | 12.21 | 4.45 | - | - |
| 60 | 16 | 6.75 | 5.43 | 9 | 10.89 | 5.9 | 0.04* | -4.80 | |
| HS Troponin | 0 | 12 | 23 | 29.2 | 7 | 18.44 | 17.24 | - | - |
| 14 | 24 | 10.76 | 5.45 | 20 | 11.63 | 6.34 | 0.71 | -0.97 | |
| 28 | 21 | 9.41 | 3.7 | 12 | 13.94 | 6.56 | 0.01* | -6.59 | |
| 60 | 16 | 7.14 | 3.06 | 9 | 14.46 | 9.69 | 0.35 | -3.36 | |
| Lactate dehydrogenase | 0 | 28 | 339.29 | 114.49 | 19 | 385.47 | 126.05 | - | - |
| 14 | 25 | 257.03 | 94.53 | 19 | 270 | 134.44 | 0.67 | 11.88 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 187.37 | 35.48 | 9 | 219.78 | 42.6 | 0.49 | -15.29 | |
| D Dimer | 0 | 30 | 0.78 | 0.87 | 19 | 1.17 | 0.81 | ||
| 14 | 24 | 0.44 | 0.37 | 19 | 1.38 | 2.04 | 0.96 | -0.03 | |
| 28 | 20 | 0.42 | 0.23 | 13 | 0.71 | 0.71 | 0.5 | 0.04 | |
| 60 | 16 | 0.31 | 0.15 | 9 | 0.53 | 0.29 | 0.06 | -0.11 | |
| Platelets | 0 | 30 | 223.63 | 73.84 | 20 | 255.85 | 116.94 | ||
| 14 | 25 | 262.36 | 98.82 | 19 | 291.37 | 86.47 | 0.06 | -48.29 | |
| 28 | 19 | 264.42 | 72.96 | 11 | 280.18 | 95.4 | 0.89 | -4.65 | |
| 60 | 16 | 261.25 | 83.03 | 9 | 324.67 | 56.25 | 0.01* | -64.28 | |
| Absolute Monocytes | 0 | 30 | 0.5 | 0.48 | 20 | 0.51 | 0.31 | - | - |
| 14 | 25 | 0.94 | 0.45 | 19 | 0.66 | 0.33 | 0.03* | 0.245 | |
| 28 | 19 | 0.62 | 0.19 | 11 | 0.45 | 0.18 | 0.01* | 0.182 | |
| 60 | 16 | 0.61 | 0.25 | 9 | 0.47 | 0.15 | 0.11 | 0.13 | |
| Percentage Monocytes | 0 | 30 | 6.18 | 4.23 | 20 | 7.43 | 2.81 | ||
| 14 | 25 | 6.59 | 2.6 | 19 | 7.11 | 4.27 | 0.72 | 0.28 | |
| 28 | 19 | 7.94 | 1.96 | 11 | 6.97 | 1.73 | 0.01* | 1.38 | |
| 60 | 16 | 7.55 | 2.14 | 9 | 6.37 | 0.9 | 0.20 | 0.73 | |
| Absolute Lymphocytes | 0 | 30 | 0.9 | 0.31 | 20 | 0.85 | 0.28 | - | - |
| 14 | 25 | 1.5 | 0.65 | 19 | 1.68 | 1.18 | 0.54 | -0.12 | |
| 28 | 19 | 1.65 | 0.51 | 11 | 1.77 | 0.73 | 0.94 | -0.02 | |
| 60 | 16 | 1.92 | 0.60 | 9 | 1.93 | 0.52 | 0.70 | -0.08 | |
| Percentage Lymphocytes | 0 | 30 | 12.03 | 3.66 | 20 | 12.48 | 3.78 | - | - |
| 14 | 25 | 14.45 | 7.96 | 19 | 14.92 | 8.65 | 0.58 | -1.626 | |
| 28 | 19 | 20.91 | 5.2 | 11 | 24.37 | 6.25 | 0.18 | -3.05 | |
| 60 | 16 | 24.30 | 5.79 | 9 | 24.59 | 6.32 | 0.61 | -1.32 | |
| Absolute Neutrophils | 0 | 30 | 6.61 | 2.89 | 20 | 5.83 | 2.23 | - | - |
| 14 | 25 | 8.99 | 3.18 | 19 | 10.01 | 4.59 | 0.62 | -0.03 | |
| 28 | 19 | 5.33 | 1.44 | 11 | 4.67 | 0.89 | 0.53 | 0.03 | |
| 60 | 16 | 5.14 | 1.29 | 9 | 5.4 | 1.49 | 0.92 | -0.06 | |
| Percentage Neutrophils | 0 | 30 | 81.33 | 6.07 | 20 | 80.19 | 5.42 | - | - |
| 14 | 25 | 77.37 | 9.72 | 19 | 77.06 | 10 | 0.88 | -0.003 | |
| 28 | 19 | 67.06 | 5.56 | 11 | 65.45 | 5.78 | 0.11 | 0.0248 | |
| 60 | 16 | 64.53 | 6.47 | 9 | 64.87 | 8.3 | 0.92 | -0.17 | |
| Ferritin | 0 | 30 | 589.4 | 477.3 | 20 | 805.7 | 523 | - | |
| 14 | 25 | 369.9 | 364.2 | 19 | 456.8 | 341.8 | 0.38 | -39.36 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 167.23 | 83.03 | 9 | 172.9 | 151.6 | 0.44 | 32.31 | |
Figure 5. Changes in secondary outcomes.
A, B, C, D Depicts mean value of patients over time in the add-on ayurveda group and standard of care for H-S troponin, platelets count, D-dimer and LDH.
*p-value computed using robust regression controlling for baseline differences between groups on the particular visit.
Table 11. Hematological parameters.
* Values at a 95% confidence interval where the level of significance, α= 0.05
The method of robust regression controlled for baseline differences with treatment group as a covariate has been used to test differences between the treatment groups.
| Parameter | Days of Therapy | Gp 1 | Gp 2 | p-value | Estimate | ||||
| N | Mean | SD | N | Mean | SD | ||||
| White Blood Cells | 0 | 30 | 8.03 | 3.16 | 20 | 7.37 | 2.74 | - | - |
| 14 | 25 | 11.36 | 3.29 | 19 | 12.61 | 4.68 | 0.83 | -0.22 | |
| 28 | 19 | 7.93 | 1.82 | 11 | 7.13 | 1.45 | 0.34 | 0.61 | |
| 60 | 16 | 7.94 | 1.80 | 9 | 8.21 | 1.93 | 0.98 | -0.01 | |
| Red Blood Cells | 0 | 30 | 4.51 | 0.62 | 20 | 4.26 | 0.6 | - | - |
| 14 | 25 | 4.69 | 0.54 | 19 | 4.31 | 0.73 | 0.05* | 0.21 | |
| 28 | 19 | 4.47 | 0.36 | 11 | 4.04 | 0.68 | 0.24 | 0.16 | |
| 60 | 16 | 4.54 | 0.41 | 9 | 4.37 | 0.62 | 0.50 | 0.09 | |
| Random Blood Sugar | 0 | 25 | 259.64 | 107 | 18 | 254.83 | 124.58 | - | - |
| 14 | 24 | 311.17 | 91.16 | 18 | 355.37 | 152.19 | 0.18 | -56.03 | |
| 28 | 18 | 242.61 | 76.88 | 12 | 254.08 | 91.42 | 0.17 | -43.47 | |
| 60 | 16 | 225.12 | 64.57 | 9 | 244.22 | 125.11 | 0.62 | -20.83 | |
| Hemoglobin | 0 | 30 | 12.82 | 1.66 | 20 | 12.3 | 1.84 | - | - |
| 14 | 25 | 13.26 | 1.77 | 19 | 12.34 | 1.8 | 0.09 | 0.52 | |
| 28 | 19 | 12.86 | 1.23 | 11 | 11.87 | 2.22 | 0.4 | 0.35 | |
| 60 | 16 | 13.12 | 1.05 | 9 | 12.88 | 1.95 | 0.57 | 0.20 | |
Figure 6. Changes in secondary outcomes: Safety markers.
A, B, C, D depicts mean value of patients over time in add-on ayurveda group and standard of care for SGOT, creatinine, SGPT and urea.
*p-value computed using robust regression controlling for baseline differences between group on the particular visit.
Table 12. Liver and renal markers.
* Values at a 95% confidence interval where the level of significance, α= 0.05
The method of robust regression controlled for baseline differences with treatment group as a covariate has been used to testing differences between the treatment groups.
** Parameter not assessed on Day 28 as per the protocol; NA – Not Applicable
| Parameter | Days of therapy | Gp 1 | Gp 2 | p-value | Estimate | ||||
| N | Mean | SD | N | Mean | SD | ||||
| Aspartate transaminase | 0 | 30 | 35.42 | 20.2 | 20 | 48.05 | 21.17 | - | - |
| 14 | 24 | 17.09 | 5.7 | 18 | 19.06 | 8.9 | 0.58 | 1.12 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 17.69 | 8.84 | 9 | 18.22 | 6.02 | 0.22 | -2.46 | |
| Alanine transaminase | 0 | 30 | 27.27 | 15.3 | 20 | 36.75 | 23.65 | - | - |
| 14 | 24 | 22.85 | 9.5 | 18 | 24.42 | 12.21 | 0.04* | -2.54 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 16.37 | 8.78 | 9 | 16.22 | 8.14 | 0.58 | 1.28 | |
| Alkaline phosphatase | 0 | 29 | 69.21 | 28.1 | 20 | 91.15 | 30.15 | - | - |
| 14 | 24 | 84.53 | 21.5 | 18 | 104.51 | 23.89 | 0.07 | -11.51 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 83.06 | 26.60 | 9 | 84.44 | 26.02 | 0.94 | -0.75 | |
| Direct Bilirubin | 0 | 30 | 0.21 | 0.1 | 20 | 0.23 | 0.11 | - | - |
| 14 | 24 | 0.22 | 0.1 | 18 | 0.21 | 0.1 | 0.79 | 0.01 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 0.18 | 0.06 | 9 | 0.19 | 0.14 | 0.09 | 0.04 | |
| Total Bilirubin | 0 | 30 | 0.52 | 0.2 | 20 | 0.49 | 0.18 | - | - |
| 14 | 24 | 0.53 | 0.3 | 18 | 0.52 | 0.18 | 0.52 | -0.04 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 0.42 | 0.18 | 9 | 0.47 | 0.31 | 0.24 | 0.07 | |
| Urea | 0 | 30 | 43.62 | 30.1 | 20 | 43.6 | 28.98 | - | - |
| 14 | 25 | 30.48 | 11.5 | 18 | 34.78 | 12.15 | 0.21 | -3.87 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 19.81 | 7.11 | 9 | 24.56 | 8.71 | 0.23 | -4.24 | |
| Albumin | 0 | 29 | 3.46 | 0.4 | 20 | 3.45 | 0.44 | - | - |
| 14 | 24 | 3.88 | 0.3 | 18 | 3.61 | 0.44 | 0.02* | 0.28 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 4.33 | 0.23 | 9 | 4.34 | 0.27 | 0.76 | -0.03 | |
| Total Protein | 0 | 29 | 6.9 | 0.6 | 20 | 7.19 | 0.6 | - | - |
| 14 | 24 | 6.65 | 0.5 | 18 | 6.73 | 0.7 | 0.84 | -0.04 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 6.86 | 0.40 | 9 | 7.28 | 0.32 | 0.01* | -0.46 | |
| Creatinine | 0 | 30 | 1.08 | 0.9 | 20 | 1.01 | 0.37 | - | - |
| 14 | 25 | 0.82 | 0.2 | 18 | 0.78 | 0.17 | 0.71 | 0.02 | |
| 28 | NA** | NA** | NA** | NA** | NA** | NA** | NA** | NA** | |
| 60 | 16 | 0.78 | 0.21 | 9 | 0.84 | 0.2 | 0.22 | -0.08 | |
Table 13. Vitals.
* Values at a 95% confidence interval where the level of significance, α= 0.05
The method of robust regression controlled for baseline differences with treatment group as a covariate has been used to test differences between the treatment groups.
| Parameter | Day of therapy | Gp 1 | Gp 2 | p-value | Estimate | ||||||
| N | Q1 | Median | Q3 | N | Q1 | Median | Q3 | ||||
| Temperature | 0 | 29 | 36.1 | 36.5 | 36.9 | 18 | 35.575 | 36.3 | 36.625 | - | - |
| 14 | 23 | 35.8 | 36.5 | 36.6 | 15 | 35.6 | 36 | 36.5 | 0.06 | 0.44 | |
| 28 | 21 | 36.2 | 36.4 | 36.6 | 10 | 35.2 | 36.25 | 36.45 | 0.84 | 0.03 | |
| 60 | 17 | 34.3 | 36 | 36.45 | 9 | 36.035 | 36.3 | 36.6 | 0.90 | 0.02 | |
| Pulse rate | 0 | 30 | 74.7 | 86.5 | 95.25 | 17 | 75.5 | 85 | 97 | - | - |
| 14 | 23 | 85 | 94 | 102 | 16 | 83 | 101.5 | 123.75 | 0.02* | -11.77 | |
| 28 | 21 | 84 | 97 | 104 | 10 | 88 | 94 | 102.75 | 0.84 | -0.87 | |
| 60 | 17 | 86 | 91 | 96 | 9 | 72 | 90 | 97 | 0.81 | -0.85 | |
| Respiratory rate | 0 | 25 | 22.5 | 25 | 29 | 18 | 20 | 22 | 26.75 | - | - |
| 14 | 22 | 19.5 | 22 | 25.25 | 13 | 22 | 24 | 25 | 0.25 | -1.55 | |
| 28 | 16 | 18 | 22.5 | 26 | 8 | 20.5 | 22 | 24 | 0.45 | -1.44 | |
| 60 | 15 | 22 | 24 | 27 | 8 | 22.5 | 24 | 25.5 | 0.99 | -0.02 | |
| Systolic BP | 0 | 29 | 118 | 128 | 140 | 19 | 130 | 135 | 147 | - | - |
| 14 | 22 | 109. | 125.5 | 136.7 | 15 | 120 | 128 | 149 | 0.84 | -1.41 | |
| 28 | 21 | 119.5 | 127 | 142 | 11 | 109 | 132 | 150 | 0.65 | 3.59 | |
| 60 | 17 | 126.5 | 132 | 137 | 9 | 114 | 128 | 130.5 | 0.04* | 9.27 | |
| Diastolic BP | 0 | 29 | 69 | 80 | 84.5 | 19 | 70 | 81 | 90 | - | - |
| 14 | 22 | 72.75 | 81 | 84.2 | 15 | 75 | 83 | 91 | 0.18 | -5.35 | |
| 28 | 21 | 74 | 80 | 89 | 11 | 70 | 77 | 91 | 0.58 | 2.41 | |
| 60 | 17 | 74 | 80 | 88.5 | 9 | 73 | 79 | 84 | 0.19 | -4.94 | |
| Oxygen Saturation | 0 | 28 | 89 | 94 | 96.7 | 16 | 92.2 | 94.5 | 96.7 | - | - |
| 14 | 23 | 94 | 97 | 98 | 16 | 95 | 96 | 96.7 | 0.18 | 1.06 | |
| 28 | 21 | 95 | 96 | 97 | 11 | 95 | 96 | 98 | 0.61 | -0.35 | |
| 60 | 17 | 95 | 96 | 97 | 9 | 96 | 97 | 99 | 0.63 | 2.03 | |
Table 14. Survival analysis.
p-value computed using the Log-rank test at a 95% confidence interval where the level of significance, α = 0.05
| Gp 1 | Gp 2 | |
| N=50 | 30 | 20 |
| Number of ICU Admissions | 2 | 2 |
| Number of deaths | 1 | 2 |
| Rate of Survival (in %) | 96.7 | 90 |
| Rate of ICU Admissions (in %) | 6.67 | 10 |
| Death Rate (in %) | 3.33 | 10 |
| P value | 0.38 | |
Table 15. Rationality behind the formulation used in COVID-19 during Baseline-14 days (Ama or Pachyamana Jwara phase) in add-on Ayurveda Group (Group 1) – Ayurvedic Medicine Track.
*n represents the count of patients who received the specified medication within the given timeframe
+Dosage and frequency were decided based on the Ayurveda physician’s clinical judgement
| Medicine Name | Rationale | Dosage+ | n (%)* | Frequency of intake+ |
| Kashyam | ||||
| Bharangyadi Kashayam | Sannipataja jwara(complicated fevers involving all three doshas), vishamajwara(intermittent fever), jeerna jwara(chronic fever), shirograha(headache), parshwashoola (pain in the flanks of the chest), hrith shola(chest pain around the heart), kasa(cough), shwasa(dyspnea) , tandra(semi-comatose condition) and agnimandhya(low metabolism). | 7.5 - 15 ml | 21 (100%) | Once/Twice |
| Indukantham Kashayam | Vata predominant type of fever associated with cough, breathing difficulty and fluid accumulation. | 7.5 - 15 ml | 20 (95.2%) | Once/Twice |
| Panchathikthakam Kashayam | All eight type of fever as classified in Ayurveda associated with Kasa swasa. | 7.5 ml | 15 (71.4%) | Once/Twice |
| Nayopayam Kashayam | Given during acute phase of COVID-19 with acute dyspnea, bloatedness, reverse peristalsis, belching associated with chest discomfort. | 7.5 ml | 12 (57.1%) | Once/Twice |
| Drakshadi Kashyam | In Chronic fever and Vomiting | 7.5 - 15 ml | 8 (38.1%) | Once/Twice |
| Guduchyadi Kashayam | In fever, vomiting, renal disorders, migraine, and diabetes. | 7.5 - 20 ml | 7 (33.3%) | Once/Twice |
| Gulika/Pills | ||||
| Gorochanadi Gulika | Fever associated with giddiness, nausea, breathlessness, General weakness, headache, Asthma, hiccups, cough, Hemiplegia and Ansomia. | 100 mg | 21 (100%) | Once/Twice/four |
| Suvarnamukatadi | Fever, vertigo, fatigue, loss of smell, and semi-comatose conditions. | 27 - 54 mg | 17 (81%) | Once/Twice/four |
| Swasanandam | Acute dyspnea. | 50 - 200 mg | 16 (76.2%) | Once/Twice |
| Vilwadi Gulika | In Vata Kapha fever associated with indigestion, loose motion, vomiting and symptoms of toxicity. | 325 - 650 mg | 3 (14.3%) | Once/Twice |
| Bhasmam | ||||
| Pravala Bhasmam | Acute dyspnea associated with hyperacidity and to iniate innate immune response. | 125 - 250 mg | 16 (76.2%) | Every 3 hours/Twice/once |
| Lehyam | ||||
| Agasthyarasayanam | Given both in acute and chronic condition of Cough, Dyspnoea, Rhinitis, Hiccup, Fever, Lack of appetite in COVID patients. | 5 - 6 g | 16 (76.2%) | Every 3 hours/Twice/once |
| Vidaryadi Lehyam | Given during both acute and chronic COVID-19 patients showing symptoms of fatigue, weight loss, myalgia, shortness of breath having moderate or good digestive activity. | 5 - 6 g | 3 (14.3%) | Every 3 hours/Twice |
| Brahmarasayana | Given both in acute and chronic condition of Cough, Dyspnoea, Rhinitis, Hiccup, Fever, Lack of appetite in COVID patients. | 5 - 6 g | 1 (4.8%) | Every 3 hours/Twice/once |
| Gritham | ||||
| Baladi Gritham | Given during both acute and chronic COVID-19 patients showing symptoms of fatigue, shortness of breath, persistent reoccurring fever, and lung consolidation. | 1/2 - 5 - 6 g | 3 (14.3%) | Once/Twice |
Table 16. Rationality behind the used formulation in COVID-19 during Baseline-14 days (Ama or Pachyamana Jwara phase) in Standard of care Group (Group 2) – Allopathic Medicine Track.
*n represents the count of patients who received the specified medication within the given timeframe
+Dosage and frequency were decided based on the Allopathic physician’s clinical judgement
| Medicine Name | Rationale | Dosage+ | n (%)* | Frequency of intake+ |
| Enoxaparin | An anticoagulant used to prevent harmful blood clots | 0-4-0.6 cc | 9 (100%) | Once-Twice |
| Remdesivir | This is a broad-spectrum antiviral medicine. It is used for the treatment of suspected or laboratory-confirmed coronavirus disease 2019 (COVID-19) in adults and children hospitalized with severe disease. | 200 mg via infusion loading dose followed by 100 mg per day for 5-10 days | 9 (100%) | Once |
| Metformin | Anti-diabetic drugs | 500mg | 7 (77.7%) | Once-Twice |
| Zac D | Vitamin supplements | - | 7 (77.8%) | Once-Twice |
| Methylprednisolone | Steroids. It is used to treat different conditions including rheumatic disorder, asthma, severe allergic reactions and conditions that affect your eyes and skin. | 1-2mg /kg /day <=7 days | 5 (55.6%) | Once-Twice |
| Pantoprazole | Antacids | 40mg | 5 (55.6%) | Once |
| Chlorpheniramine Maleate | Cough remedial Medication | 10-15 ml | 4 (44.4%) | Once-Twice |
| Pantoprazole | Antacids | 40mg | 4 (44.4%) | Once-Twice |
| Paracetamol | Helps relieve pain and fever by blocking the release of certain chemical messengers responsible for fever and pain. | 650mg | 3 (33.3%) | Once |
| Paracetamol | Helps relieve pain and fever by blocking the release of certain chemical messengers responsible for fever and pain. | 650mg | 3 (33.3%) | Thrice |
| Ondansetron | Used to control nausea and vomiting | 4-11mg | 2 (22.2%) | Once |
| Methylprednisolone | Steroids. It is used to treat different conditions including rheumatic disorder, asthma, severe allergic reactions and conditions that affect your eyes and skin. | 1-2mg /kg /day <=7 days | 2 (22.2%) | Twice |
| Cefoperazone and Sulbactam | It is prescribed to treat various types of bacterial infections. It fights against the microorganisms to prevent their growth and further spread of the infection. | 1.5 -3 g/dose | 2 (22.2%) | Once-Twice |
| Chlorpheniramine Maleate | Cough remedial Medication | 10-15 ml | 2 (22.2%) | Twice |
| Glimepiride | Anti-diabetic drugs | 1-2mg/dose | 2 (22.2%) | Once-Twice |
| Methylprednisolone | Steroids. It is used to treat different conditions including rheumatic disorder, asthma, severe allergic reactions and conditions that affect your eyes and skin. | 1-2mg /kg /day <=7 days | 2 (22.2%) | Once |
| Ursodeoxycholic acid | Dissolves gallstones and prevents them from forming. | 150-300mg | 2 (22.2%) | Twice |
Table 17. Rationality behind the used formulation in COVID-19 during 14 days-28 days (Jirna Jwara phase) in Add-on Ayurveda Group (Group 1) – Ayurvedic Medicine Track.
*n represents the count of patients who received the specified medication within the given timeframe
+Dosage and frequency were decided based on the Ayurveda physician’s clinical judgement
| Medicine Name | Rationale | Dosage+ | n (%)* | Frequency of intake+ |
| Kashyam | ||||
| Bharangyadi Kashayam | Sannipataja jwara(complicated fevers involving all three doshas), vishamajwara(intermittent fever), jeerna jwara(chronic fever), shirograha(headache), parshwashoola (pain in the flanks of the chest), hrith shola(chest pain around the heart), kasa(cough), shwasa(dyspnea) , tandra(semi-comatose condition) and agnimandhya(low metabolism). | 7.5 - 15 ml | 20 (95.2%) | Once/Twice |
| Indukantham Kashayam | Vata predominant type of fever associated with cough, breathing difficulty and fluid accumulation. | 7.5 - 15 ml | 18 (85.7%) | Once/Twice |
| Nayopayam Kashayam | Given during acute phase of COVID-19 with acute dyspnea, bloatedness, reverse peristalsis, belching associated with chest discomfort. | 7.5 ml | 11 (52.4%) | Once/Twice |
| Panchathikthakam Kashayam | All eight type of fever as classified in Ayurveda associated with Kasa swasa. | 7.5 ml | 6 (28.6%) | Once/Twice |
| Drakshadi Kashyam | In Chronic fever and Vomiting | 7.5 - 15 ml | 5 (23.8%) | Once/Twice |
| Guduchyadi Kashayam | In fever, vomiting, renal disorders, migraine, and diabetes. | 7.5 ml | 4 (19%) | Once/Twice |
| Rasonadi kashayam | Given in both acute and chronic COVID-19 cases where cardiovascular stress, and coagulopathy symptoms are high. | 7.5 ml | 2 (9.5%) | Twice |
| Gulika/Pills | ||||
| Gorochanadi Gulika | Fever associated with giddiness, nausea, breathlessness, General weakness, headache, Asthma, hiccups, cough, Hemiplegia and Ansomia. | 100 mg | 16 (76.2%) | Once/Twice/four |
| Suvarnamukatadi | Fever, vertigo, fatigue, loss of smell, and semi-comatose conditions. | 27 - 54 mg | 15 (71.4%) | Once/Twice/four |
| Swasanandam | Acute dyspnea. | 50 - 200 mg | 9 (42.9%) | Once/Twice |
| Bhasmam | ||||
| Pravala Bhasmam | Acute dyspnea associated with hyperacidity and to iniate innate immune response. | 125 - 250 mg | 16 (76.2%) | Every 3 hours/Twice/once |
| Lehyam | ||||
| Agasthyarasayanam | Given both in acute and chronic condition of Cough, Dyspnoea, Rhinitis, Hiccup, Fever, Lack of appetite in COVID patients. | 5 - 6 g | 17 (81%) | Every 3 hours/Twice/once |
| Brahmarasayana | Given both in acute and chronic condition of Cough, Dyspnoea, Rhinitis, Hiccup, Fever, Lack of appetite in COVID patients. | 5 - 6 g | 6 (28.6%) | Every 3 hours/Twice/once |
| Vidaryadi Lehyam | Given during both acute and chronic COVID-19 patients showing symptoms of fatigue, weight loss, myalgia, shortness of breath having moderate or good digestive activity. | 5 - 6 g | 4 (19%) | Every 3 hours/Twice |
| Gritham | ||||
| Baladi Gritham | Given during both acute and chronic COVID-19 patients showing symptoms of fatigue, shortness of breath, persistent reoccurring fever, and lung consolidation. | 1/2 - 5 - 6 g | 14 (66.7%) | Once/Twice |
| Dhanwantharam Gritham | Given in COVID-19 patients with diabetes after acute inflammatory phase with persistent symptoms of shortness of breath, dyspnea, lung consolidation, and brain fog. | 5 - 6 g | 1 (4.8%) | Once |
Table 18. Rationality behind the used formulation in COVID-19 during 14 days-28 days (Jirna Jwara phase) in Standard of care Group (Group 2) – Allopathic Medicine Track.
*n represents the count of patients who received the specified medication within the given timeframe
+Dosage and frequency were decided based on the Allopathic physician’s clinical judgement
| Medicine Name | Rationale | Dosage+ | n (%)* | Frequency of intake+ |
| Metformin | Anti-diabetic drugs | 500mg | 7 (77.7%) | Once-Twice |
| Apixaban | Antihypertensive drugs | 2.5mg | 2 (22.2%) | Twice |
| Paracetamol | Helps relieve pain and fever by blocking the release of certain chemical messengers responsible for fever and pain. | 650mg | 2 (22.2%) | SOS |
| Aspirin | An antiplatelet medicine used to treat and prevent heart attacks, strokes, and heart-related chest pain (angina). | 75mg | 2 (22.2%) | Once |
| Glimepiride | Anti-diabetic drugs | 1-2mg/dose | 2 (22.2%) | Once-Twice |
Table 19. Rationality behind the used formulation in COVID-19 during 28 days-60 days (Punaravartaka Jwara phase) in Add-on Ayurveda Group (Group 1) – Ayurvedic Medicine Track.
*n represents the count of patients who received the specified medication within the given timeframe
+Dosage and frequency were decided based on the Ayurveda physician’s clinical judgement
| Medicine Name | Rationale | Dosage+ | n (%)* | Frequency of intake+ |
| Kashyam | ||||
| Bharangyadi Kashayam | Sannipataja jwara(complicated fevers involving all three doshas), vishamajwara(intermittent fever), jeerna jwara(chronic fever), shirograha(headache), parshwashoola (pain in the flanks of the chest), hrith shola(chest pain around the heart), kasa(cough), shwasa(dyspnea) , tandra(semi-comatose condition) and agnimandhya(low metabolism). | 7.5 - 15 ml | 19 (90.5%) | Once/Twice |
| Indukantham Kashayam | Vata predominant type of fever associated with cough, breathing difficulty and fluid accumulation. | 7.5 - 15 ml | 15 (71.4%) | Once/Twice |
| Nayopayam Kashayam | Given during acute phase of COVID-19 with acute dyspnea, bloatedness, reverse peristalsis, belching associated with chest discomfort. | 7.5 ml | 8 (38.1%) | Once/Twice |
| Drakshadi Kashyam | In Chronic fever and Vomiting | 7.5 - 15 ml | 6 (28.6%) | Once/Twice |
| Panchathikthakam Kashayam | All eight type of fever as classified in Ayurveda associated with Kasa swasa. | 7.5 ml | 3 (14.3%) | Once/Twice |
| Guduchyadi Kashayam | In fever, vomiting, renal disorders, migraine, and diabetes. | 7.5 ml | 2 (9.5%) | Once/Twice |
| Rasonadi kashayam | Given in both acute and chronic COVID-19 cases where cardiovascular stress, and coagulopathy symptoms are high. | 7.5 ml | 2 (9.5%) | Twice |
| Patolakadurohinyadi Kashayam | In Kapha Pitta type of fever associated with Vomiting, Loss of appetite, skin rashes and symptoms of toxicity. | 7.5 ml | 1 (4.8%) | Twice |
| Gulika/Pills | ||||
| Suvarnamukatadi | Fever, vertigo, fatigue, loss of smell, and semi-comatose conditions. | 27 - 54 mg | 14 (66.7%) | Once/Twice/four |
| Gorochanadi Gulika | Fever associated with giddiness, nausea, breathlessness, General weakness, headache, Asthma, hiccups, cough, Hemiplegia and Ansomia. | 100 mg | 12 (57.1%) | Once/Twice/four |
| Swasanandam | Acute dyspnea. | 50mg | 3 (14.3%) | Once/Twice |
| Bhasmam | ||||
| Pravala Bhasmam | Acute dyspnea associated with hyperacidity and to iniate innate immune response. | 125 - 250 mg | 12 (57.1%) | Every 3 hours/Twice/once |
| Lehyam | ||||
| Agasthyarasayanam | Given both in acute and chronic condition of Cough, Dyspnoea, Rhinitis, Hiccup, Fever, Lack of appetite in COVID patients. | 5 - 6 g | 12 (57.1%) | Every 3 hours/Twice/once |
| Vidaryadi Lehyam | Given during both acute and chronic COVID-19 patients showing symptoms of fatigue, weight loss, myalgia, shortness of breath having moderate or good digestive activity. | 5 - 6 g | 6 (28.6%) | Every 3 hours/Twice |
| Brahmarasayana | Given both in acute and chronic condition of Cough, Dyspnoea, Rhinitis, Hiccup, Fever, Lack of appetite in COVID patients. | 5 - 6 g | 4 (19%) | Every 3 hours/Twice/once |
| Gritham | ||||
| Baladi Gritham | Given during both acute and chronic COVID-19 patients showing symptoms of fatigue, shortness of breath, persistent reoccurring fever, and lung consolidation. | 1/2 - 5 - 6 g | 18 (85.7%) | Once/Twice |
Funding Statement
The clinical study was done jointly funded by AVP Research Foundation, PSG Institute of Medical Sciences and Research, The Arya Vaidya Pharmacy (Coimbatore) Ltd., Kama Ayurveda, Madhuram Narayanan Charitable trust, Vivek Sahni, and OPG Power Generation Private Limited.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. PSG Institute's Institutional Human Ethics Committee issued approval PSG/IHEC/2020/Appr/FB/034. The study is approved in its present form.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: The clinical study was done jointly funded by AVP Research Foundation, PSG Institute of Medical Sciences and Research, The Arya Vaidya Pharmacy (Coimbatore) Ltd., Kama Ayurveda, Madhuram Narayanan Charitable trust, Vivek Sahni, and OPG Power Generation Private Limited. .
Financial relationships: Somit Kumar declare(s) employment and Supplied the trial medications from The Arya Vaidya Pharmacy (Cbe) Ltd. Somit Kumar, Sujith S Eranezhath, Mitravinda S Kakarla declare(s) a grant, employment and stock/stock options from AVP Research Foundation.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Somit Kumar, Karthikeyan Ramaraju, Sujith S. Eranezhath, Chaithanya Chenthamarakshan, Murali Alagesan, Balagopal Satheesan, Indulal Unniappan, Valdis Pīrāgs
Acquisition, analysis, or interpretation of data: Somit Kumar, Karthikeyan Ramaraju, Mitravinda S. Kakarla, Chaithanya Chenthamarakshan, Balagopal Satheesan, Holly Wilhalme, Daniel E. Furst
Drafting of the manuscript: Somit Kumar, Mitravinda S. Kakarla, Sujith S. Eranezhath, Chaithanya Chenthamarakshan, Indulal Unniappan, Valdis Pīrāgs, Daniel E. Furst
Critical review of the manuscript for important intellectual content: Somit Kumar, Karthikeyan Ramaraju, Mitravinda S. Kakarla, Sujith S. Eranezhath, Murali Alagesan, Balagopal Satheesan, Indulal Unniappan, Holly Wilhalme, Valdis Pīrāgs, Daniel E. Furst
Supervision: Somit Kumar, Karthikeyan Ramaraju, Sujith S. Eranezhath, Chaithanya Chenthamarakshan
References
- 1.COVID-19 and diabetes mellitus: An unholy interaction of two pandemics. Pal R, Bhadada SK. Diabetes Metab Syndr. 2020;14:513–517. doi: 10.1016/j.dsx.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. Yan Y, Yang Y, Wang F, et al. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Maddaloni E, Buzzetti R. Diabetes Metab Res Rev. 2020;36:0. doi: 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19: consider cytokine storm syndromes and immunosuppression. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silent hypoxia in COVID-19: pathomechanism and possible management strategy. Rahman A, Tabassum T, Araf Y, Al Nahid A, Ullah MA, Hosen MJ. Mol Biol Rep. 2021;48:3863–3869. doi: 10.1007/s11033-021-06358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biomarkers associated with COVID-19 disease progression. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Crit Rev Clin Lab Sci. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Proal AD, VanElzakker MB. Front Microbiol. 2021;12:698169. doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longitudinal assessment of chest CT findings and pulmonary function after COVID-19 infection. Han X, Chen L, Fan Y, et al. Radiology. 2023;307:0. doi: 10.1148/radiol.222888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. J Card Fail. 2020;26:470–475. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The assessment of high sensitivity cardiac troponin in patients with COVID-19: A multicenter study. Perrone MA, Spolaore F, Ammirabile M, Romeo F, Caciagli P, Ceriotti F, Bernardini S. Int J Cardiol Heart Vasc. 2021;32:100715. doi: 10.1016/j.ijcha.2021.100715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remdesivir for the treatment of Covid-19 - final report. Beigel JH, Tomashek KM, Dodd LE, et al. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Götte M. J Biol Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remdesivir inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. Pruijssers AJ, George AS, Schäfer A, et al. Cell Rep. 2020;32:107940. doi: 10.1016/j.celrep.2020.107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rapid review of suspected adverse drug events due to remdesivir in the WHO database; findings and implications. Charan J, Kaur RJ, Bhardwaj P, Haque M, Sharma P, Misra S, Godman B. Expert Rev Clin Pharmacol. 2021;14:95–103. doi: 10.1080/17512433.2021.1856655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dexamethasone in hospitalized patients with Covid-19. Horby P, Lim WS, Emberson JR, et al. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The roles of methylprednisolone treatment in patients with COVID-19: A systematic review and meta-analysis. Hong S, Wang H, Zhang Z, Qiao L. Steroids. 2022;183:109022. doi: 10.1016/j.steroids.2022.109022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2023;401:1499–1507. doi: 10.1016/S0140-6736(23)00510-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study. Liu J, Pan X, Zhang S, et al. Lancet Reg Health West Pac. 2023;33:100694. doi: 10.1016/j.lanwpc.2023.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Effective treatment of severe COVID-19 patients with tocilizumab. Xu X, Han M, Li T, et al. Proc Natl Acad Sci U S A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Differential efficacy and safety of anti-SARS-CoV-2 antibody therapies for the management of COVID-19: a systematic review and network meta-analysis. Deng J, Heybati K, Ramaraju HB, Zhou F, Rayner D, Heybati S. Infection. 2023;51:21–35. doi: 10.1007/s15010-022-01825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Co-administration of AYUSH 64 as an adjunct to standard of care in mild and moderate COVID-19: A randomized, controlled, multicentric clinical trial. Chopra A, Tillu G, Chuadhary K, et al. PLoS One. 2023;18:0. doi: 10.1371/journal.pone.0282688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayurvedic clinical profile of COVID-19 - A preliminary report. Puthiyedath R, Kataria S, Payyappallimana U, et al. J Ayurveda Integr Med. 2022;13:100326. doi: 10.1016/j.jaim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A randomized, controlled, blinded, parallel group, clinical trial to study the role of Ayurcov (AyurCoro3), one day regimen as an adjuvant therapy for COVID-19 disease management, at dedicated Covid Hospital (DCH) in India. Sankhe AP, Memane NS, Gawali VP, et al. Complement Ther Med. 2022;67:102824. doi: 10.1016/j.ctim.2022.102824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A prospective clinical study of an Ayurveda regimen in COVID 19 patients. Wanjarkhedkar P, Sarade G, Purandare B, Kelkar D. J Ayurveda Integr Med. 2022;13:100365. doi: 10.1016/j.jaim.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayurvedic management through telemedicine of covid hypoxia non-dependent of oxygen support in a patient with chest CT score of 18/25- A case report. A S, G M. J Ayurveda Integr Med. 2022;13:100660. doi: 10.1016/j.jaim.2022.100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Standalone Ayurvedic treatment of high-risk COVID-19 patients with multiple co-morbidities: A case series. Girija PL, Sivan N, Naik P, Murugavel YA, M Ravindranath T, Cv K. J Ayurveda Integr Med. 2022;13:100466. doi: 10.1016/j.jaim.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayurvedic management of persistent hypoxia in a diabetic and hypertensive COVID-19 patient in the post-hospitalization period-A case report. Joshi J, Naranappa Salethoor S, Kulangara S, Edamala Narayanan PN, Puthiyedath R. J Ayurveda Integr Med. 2022;13:100509. doi: 10.1016/j.jaim.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The use of integrative therapy based on Yoga and Ayurveda in the treatment of a high-risk case of COVID-19/SARS-CoV-2 with multiple comorbidities: a case report. Mishra A, Bentur SA, Thakral S, Garg R, Duggal B. J Med Case Rep. 2021;15:95. doi: 10.1186/s13256-020-02624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Multimodal ayurvedic approach in the management of moderate SARS-COV2 infection with co-morbidities - A case report. Mythri HS, Mahto RR. J Family Med Prim Care. 2022;11:344–349. doi: 10.4103/jfmpc.jfmpc_495_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayurvedic management of COVID-19/SARS-CoV-2 along with chronic diabetes mellitus: A case study. Nesari T, Galib R, Dharmarajan P, et al. J Ayurveda. 2021;15:311. [Google Scholar]
- 32.A case series sharing novel experience of treating viral pandemic cases of morbid, mid aged, mild, moderate & severe grade with only ayurvedic medicines. Patil S. J Ayurveda Integr Med. 2022;13:100420. doi: 10.1016/j.jaim.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.A prospective, controlled, pilot study of personalised add-on ayurveda treatment in high-risk type II diabetes COVID-19 patients. Kumar S, Eranezhath SS, Vishwanathan VK, et al. Jr Her Med. 2024;43:100836. [Google Scholar]
- 34.ICMR: Clinical guidance for management of adult covid-19 patients. 2021. https://www.icmr.gov.in/pdf/covid/techdoc/COVID_Clinical_Management_19032023.pdf https://www.icmr.gov.in/pdf/covid/techdoc/COVID_Clinical_Management_19032023.pdf
- 35.Pathophysiology of Covid-19 and host centric approaches in Ayurveda. Pandkar PD, Sachdeva V. J Ayurveda Integr Med. 2022;13:100380. doi: 10.1016/j.jaim.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evaluation of traditional ayurvedic Kadha for prevention and management of the novel Coronavirus (SARS-CoV-2) using in silico approach. Maurya DK, Sharma D. J Biomol Struct Dyn. 2022;40:3949–3964. doi: 10.1080/07391102.2020.1852119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants - Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi) - a molecular docking study. Shree P, Mishra P, Selvaraj C, Singh SK, Chaube R, Garg N, Tripathi YB. J Biomol Struct Dyn. 2022;40:190–203. doi: 10.1080/07391102.2020.1810778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neutrophils and COVID-19: Active participants and rational therapeutic targets. Hazeldine J, Lord JM. Front Immunol. 2021;12:680134. doi: 10.3389/fimmu.2021.680134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neutrophils in COVID-19. Reusch N, De Domenico E, Bonaguro L, Schulte-Schrepping J, Baßler K, Schultze JL, Aschenbrenner AC. Front Immunol. 2021;12:652470. doi: 10.3389/fimmu.2021.652470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Understanding the role of neutrophils in acute respiratory distress syndrome. Yang SC, Tsai YF, Pan YL, Hwang TL. Biomed J. 2021;44:439–446. doi: 10.1016/j.bj.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modulation of neutrophil (dys)function by Ayurvedic herbs and its potential influence on SARS-CoV-2 infection. Joshi MB, Kamath A, Nair AS, Yedehali Thimmappa P, Sriranjini SJ, Gangadharan GG, Satyamoorthy K. J Ayurveda Integr Med. 2022;13:100424. doi: 10.1016/j.jaim.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inflammation and thrombosis: the clot thickens. Libby P, Simon DI. Circulation. 2001;103:1718–1720. doi: 10.1161/01.cir.103.13.1718. [DOI] [PubMed] [Google Scholar]
- 43.COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Lim S, Bae JH, Kwon HS, Nauck MA. Nat Rev Endocrinol. 2021;17:11–30. doi: 10.1038/s41574-020-00435-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Huang C, Wang Y, Li X, et al. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Valizadeh H, Abdolmohammadi-Vahid S, Danshina S, et al. Int Immunopharmacol. 2020;89:107088. doi: 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prevalence, clinical manifestations, and biochemical data of type 2 diabetes mellitus versus nondiabetic symptomatic patients with COVID-19: A comparative study. Soliman AT, Prabhakaran Nair A, Al Masalamani MS, et al. Acta Biomed. 2020;91:0. doi: 10.23750/abm.v91i3.10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19:A pilot randomized clinical trial. Xiong WZ, Wang G, Du J, Ai W. Integr Med Res. 2020;9:100489. doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Therapeutic effects of herbal-medicine combined therapy for COVID-19: A systematic review and meta-analysis of randomized controlled trials. Chien TJ, Liu CY, Chang YI, Fang CJ, Pai JH, Wu YX, Chen SW. Front Pharmacol. 2022;13:950012. doi: 10.3389/fphar.2022.950012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monocytopenia, monocyte morphological anomalies and hyperinflammation characterise severe COVID-19 in type 2 diabetes. Alzaid F, Julla JB, Diedisheim M, et al. EMBO Mol Med. 2020;12:0. doi: 10.15252/emmm.202013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neutrophil-to-lymphocyte, lymphocyte-to-monocyte, and platelet-to-lymphocyte ratios: prognostic significance in COVID-19. Bg S, Gosavi S, Ananda Rao A, et al. Cureus. 2021;13:0. doi: 10.7759/cureus.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Identification of macrophage activation-related biomarkers in obese type 2 diabetes that may be indicative of enhanced respiratory risk in COVID-19. Moin AS, Sathyapalan T, Diboun I, Atkin SL, Butler AE. Sci Rep. 2021;11:6428. doi: 10.1038/s41598-021-85760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Decrease of non-classical and intermediate monocyte subsets in severe acute SARS-CoV-2 infection. Gatti A, Radrizzani D, Viganò P, Mazzone A, Brando B. Cytometry A. 2020;97:887–890. doi: 10.1002/cyto.a.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Can monocytopenia be a new indicator in determining survival in covid-19 disease? Pehlivan O, Serin SO, Yesil EE, Demirdag F. Clin Lab. 2021;67 doi: 10.7754/Clin.Lab.2021.210215. [DOI] [PubMed] [Google Scholar]
- 54.In-vivo immunomodulatory activity of standardized Stereospermum suaveolens (Roxb.) DC. root extract. Maji AK, Samanta SK, Mahapatra S, Banerji P, Banerjee D. Orient Pharm Exp Med. 2014;14:47–54. [Google Scholar]
- 55.Ayurveda Rasayana as antivirals and immunomodulators: potential applications in COVID-19. Singh R, Goel S, Bourgeade P, Aleya L, Tewari D. Environ Sci Pollut Res Int. 2021;28:55925–55951. doi: 10.1007/s11356-021-16280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dynamic alterations in monocyte numbers, subset frequencies and activation markers in acute and convalescent COVID-19 individuals. Rajamanickam A, Kumar NP, Pandiarajan AN, et al. Sci Rep. 2021;11:20254. doi: 10.1038/s41598-021-99705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Junqueira C, Crespo Â, Ranjbar S, et al. Nature. 2022;606:576–584. doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Platelets and COVID-19. Rohlfing AK, Rath D, Geisler T, Gawaz M. Hamostaseologie. 2021;41:379–385. doi: 10.1055/a-1581-4355. [DOI] [PubMed] [Google Scholar]
- 59.The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants. Jiang SQ, Huang QF, Xie WM, Lv C, Quan XQ. Br J Haematol. 2020;190:0–33. doi: 10.1111/bjh.16817. [DOI] [PubMed] [Google Scholar]
- 60.Mechanism of thrombocytopenia in COVID-19 patients. Xu P, Zhou Q, Xu J. Ann Hematol. 2020;99:1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The COVID complex: a review of platelet activation and immune complexes in COVID-19. Jevtic SD, Nazy I. Front Immunol. 2022;13:807934. doi: 10.3389/fimmu.2022.807934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. Qu R, Ling Y, Zhang YH, et al. J Med Virol. 2020;92:1533–1541. doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.The impact of COVID-19 disease on platelets and coagulation. Wool GD, Miller JL. Pathobiology. 2021;88:15–27. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hemostatic laboratory derangements in COVID-19 with a focus on platelet count. Amgalan A, Othman M. Platelets. 2020;31:740–745. doi: 10.1080/09537104.2020.1768523. [DOI] [PubMed] [Google Scholar]
- 65.Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Banerjee A, Pasea L, Harris S, et al. Lancet. 2020;395:1715–1725. doi: 10.1016/S0140-6736(20)30854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Persistent cardiac injury - An important component of long COVID-19 syndrome. Chidambaram V, Kumar A, Calcaterra G, Mehta JL. EBioMedicine. 2022;77:103892. doi: 10.1016/j.ebiom.2022.103892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.CO-RADS: A categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Prokop M, van Everdingen W, van Rees Vellinga T, et al. Radiology. 2020;296:0. doi: 10.1148/radiol.2020201473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Han X, Fan Y, Alwalid O, et al. Radiology. 2021;299:0–86. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Pan F, Ye T, Sun P, et al. Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chest CT patterns from diagnosis to 1 year of follow-up in patients with COVID-19. Pan F, Yang L, Liang B, et al. Radiology. 2022;302:709–719. doi: 10.1148/radiol.2021211199. [DOI] [PMC free article] [PubMed] [Google Scholar]