Abstract
Objectives
This study aimed to provide patients insights on the management of exocrine pancreatic insufficiency (EPI) with pancreatic enzyme replacement therapy (PERT).
Materials and Methods
A survey of 75 members of Inspire's Pancreatitis or Pancreatic Cancer Support communities was conducted. Eligibility included having EPI secondary to chronic pancreatitis, pancreatic cancer, pancreatic surgery, or acute pancreatitis, and current/past PERT experience.
Results
Patients were 73% female, 57% aged 50 to 69 years, and 85% White, with PERT prescribed by a gastroenterologist/pancreatologist for 64%. Only approximately half of respondents agreed that their healthcare provider provided detailed information about EPI (54%) or how PERT works to treat EPI (56%). Most respondents (83%) reported searching for information about EPI, 56% were taking PERT solely before or after eating, 36% reported taking suboptimal PERT doses, and 39% reported no follow-up. In addition, 24% decreased their PERT dosage without consulting their physician, and 21% reported purposely skipping PERT.
Conclusions
This study reveals potential barriers to effective treatment of EPI with PERT, including lack of patient education, mainly how and when to take PERT, gaps in appropriate dosing, and lack of patient follow-up. Continued focus on patient and provider education is essential to address these gaps and optimize the treatment of EPI.
Key Words: exocrine pancreatic insufficiency, pancreatic enzyme replacement therapy, education, patient understanding, adherence, dosing
Exocrine pancreatic insufficiency (EPI) is a common complication of chronic pancreatitis (CP), pancreatic cancer (PC), and pancreatic surgery and may occur in other underlying conditions, such as acute pancreatitis (AP).1 Exocrine pancreatic insufficiency causes maldigestion of food and nutrients, leading to symptoms of maldigestion, such as steatorrhea, diarrhea, bloating, and flatulence; nutritional deficiencies; unintentional weight loss; increased risk of malnutrition-related complications; and decreased quality of life.2–9 Regardless of its etiology, the current standard of care for EPI treatment is pancreatic enzyme replacement therapy (PERT). The goal of PERT is to correct maldigestion of dietary nutrients, thereby reducing associated maldigestive symptoms, nutritional deficiencies, and risk of complications of malnutrition.2–9 Effective treatment with PERT is dependent on PERT being dosed appropriately and taken correctly, patient persistency, and timely follow-up of patients by healthcare providers (HCPs) to assess the need for dose adjustment.1,7,10 International and national guidelines have established PERT recommendations regarding the minimum initial dose, timing of ingestion, and need for patient follow-up for dose adjustment.1,7,10,11 Initial dosing is recommended to be a minimum of 40,000 to 50,000 lipase units (LU) per meal for conditions like CP1,7,10 and 72,000 to 75,000 in PC or after pancreatic surgery.12,13 Recommendations on administration include PERT to be taken with meals and snacks and to be taken intermittently throughout the meal rather than all at once at the start or end of meals, before meals, or after meals, to better simulate the natural secretion of pancreatic enzymes during the digestive process.1,7,14 Patient follow-up is critical to assess the response to therapy and the need to adjust dosing, with successful response being evaluated by the relief of maldigestion-related symptoms and the normalization of the patient's nutritional status.7,14–16 Thus, management of patients with EPI requires understanding the goals of PERT and close communication between the treating clinician and EPI patient.
Gaps in the diagnosis and treatment of EPI continue to exist, with data pointing to infrequent and inconsistent screening for EPI in patients at high risk for EPI, low PERT prescription fill rates, low rates of adequate dosing of PERT or appropriate administration,17–19 and disparity of care affecting minority patients.20,21 Patient-level data on their understanding of EPI and its management and their experience with PERT do not exist. Therefore, the objective of the current study was to provide insights on the real-world experience of patients with EPI (and their caregivers) and PERT use.
METHODS AND MATERIALS
Online Survey
An online survey was conducted with 75 eligible members of the Inspire Pancreatitis or Pancreatic Cancer Support communities22 from December 3 through December 9, 2020. Participants were eligible if they met all of the following criteria: aged ≥18 years; residing in the United States; reported having received a physician's diagnosis of CP, PC, or AP, or having undergone pancreatic surgery; reported having received a physician's diagnosis of EPI; and reporting current or past use of PERT.
The survey was composed of 64 questions assessing the respondent's eligibility, demographic characteristics, clinical characteristics relating to their underlying conditions, diagnosis of EPI, specialty of EPI-treating clinician, and prescribed PERT dosage. The main part of the survey questions centered around patients' understanding of EPI and PERT, patients' behaviors related to taking PERT, and patient-reported clinician guidance and information provided to them on EPI and PERT. Most questions were multiple choice, whereas others were scale based (ie, extent of agreement or disagreement with certain statements) or open ended where appropriate (eg, text fields for additional details). The survey was approved by the WCG Institutional Review Board (https://www.wcgirb.com).
Descriptive statistics were used to analyze the responses. Data were expressed as absolute number of respondents and percentage of total.
RESULTS
Survey Respondents' Demographic and Clinical Characteristics
Two hundred fifty-two individuals indicated their interest in the survey of whom 75 patients qualified and completed the survey. The vast majority of the 75 respondents identified themselves as being patients (93%), and the remainder identified themselves as being caregivers (7%). Patient characteristics (as reported by the patients or on their behalf by their caregiver) are summarized in Table 1. Overall, 73% of patients were women, 57% were aged 50 to 69 years, 85% were White, and 56% lived in a large city or a suburb of a large city. Patients who reported more than 1 condition (eg, AP and CP) were classified under the more chronic condition (eg, CP) for the purpose of understanding the underlying condition associated with EPI. As such, 67% had CP, 19% had PC, and 73% indicated receiving a diagnosis of EPI more than 1 year ago. Eighty percent (n = 60) were taking PERT, and 20% (n = 15) had previously taken PERT.
TABLE 1.
Survey Data From Respondents
| Demographic Characteristic | Respondents (N = 75) |
|---|---|
| Respondent type, n (%) | |
| Patient | 70 (93) |
| Caregiver* | 5 (7) |
| Patient age cohort, n (%) | |
| 18–29 y | 1 (1) |
| 30–49 y | 19 (25) |
| 50–69 y | 43 (57) |
| ≥70 y | 12 (16) |
| Female sex (patients), n (%) | 55 (73) |
| Race or ethnicity†, n (%) | |
| White | 64 (85) |
| Black | 4 (5) |
| Asian | 3 (4) |
| American Indian/Alaskan Native | 3 (4) |
| Hispanic or Latino | 3 (4) |
| Native Hawaiian/Pacific Islander | 1 (1) |
| Employment status †, n (%) | |
| Unemployed | 26 (35) |
| Retired | 23 (31) |
| Employed | 21 (28) |
| Other | 8 (11) |
| Medical leave | 2 (3) |
| Type of insurance†, n (%) | |
| Commercial | 45 (60) |
| Medicare | 25 (33) |
| Military, DoD, VA | 7 (9) |
| Other | 6 (8) |
| Medicaid | 5 (7) |
| Preferred not to answer | 1 (1) |
| Type of location, n (%) | |
| Suburb of a large city | 34 (45) |
| Small town/city | 26 (35) |
| Large city | 8 (11) |
| Rural area | 7 (9) |
*Caregiver characteristics: 4 women, 1 man; 3 spouse/partner, 1 friend, and 1 child.
†Respondents could select more than 1 option.
DoD indicates Department of Defense; VA, Veteran's Administration.
Patients' Reports on Education Provided by Their HCP and Patient Follow-up
Pancreatic enzyme replacement therapy was prescribed by a gastroenterologist for 57% of respondents. Other prescribers included oncologists (17%), surgeons (11%), pancreatologists (7%), primary care physicians (5%), nurse practitioners (1%), and other (2%) (Table 2).
TABLE 2.
Survey Data for Patients With EPI
| Clinical Characteristic | Respondents (N = 75) |
|---|---|
| Underlying conditions, n (%) | |
| Chronic pancreatitis (CP) | 50 (67) |
| Pancreatic cancer | 14 (19) |
| AP (no concomitant CP) | 7 (9) |
| Pancreatic surgery (non-CP/PC) | 4 (5) |
| Time since diagnosis, n (%) | |
| <6 mo | 5 (6.66) |
| 6–11 mo | 15 (20) |
| 1–2 y | 14 (18.67) |
| 3–4 y | 18 (24) |
| 5–6 y | 6 (8) |
| >6 y | 17 (22.67) |
| Currently taking PERT, (%) | 60 (80) |
| PERT prescriber, n (%) | |
| Gastroenterologist | 43 (57) |
| Oncologist | 13 (17) |
| Surgeon | 8 (11) |
| Pancreatologist | 5 (7) |
| PCP | 4 (5) |
| NP | 1 (1) |
| Other | 1 (2) |
| Frequency of PERT, times/day, n (%) | (n = 72) |
| 1 | 2 (3) |
| 2–3 | 43 (60) |
| 4–5 | 19 (26) |
| 6–8 | 7 (10) |
| Not sure | 1 (1) |
| Frequency of PERT, days/week, n (%) | (n = 72) |
| 3–4 | 3 (4) |
| 5–6 | 6 (8) |
| 7 | 63 (88) |
NP indicates nurse practitioner; PCP, primary care provider.
Fifty-four percent of respondents agreed that their HCPs provided detailed information about EPI, 56% agreed on receiving information from their HCPs on how PERT works to treat EPI, and 71% of respondents agreed that their HCPs provided information on why to take PERT to treat EPI. The remaining respondents were neutral or disagreed (Fig. 1).
FIGURE 1.
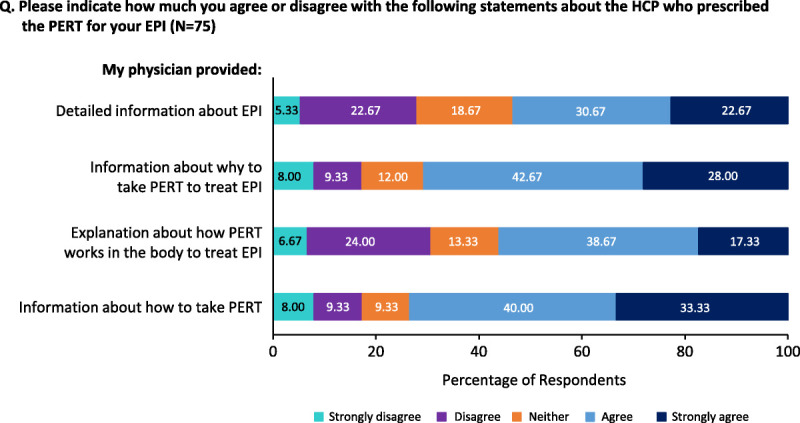
Respondents' assessment of information their HCPs provided on EPI, the role of PERT in EPI, and how to take PERT.
Seventy-three percent of patients agreed that their HCP provided information on how to take PERT. However, 11% reported receiving no instructions on how or when to take PERT. Seventy-nine percent of respondents were instructed to take PERT with meals and snacks and notably 21% reported not being instructed to take PERT with snacks.
With regard to the timing of PERT ingestion, respondents were instructed to take PERT as follows: before eating (56%), while eating food (24%), before and while eating food (17%), and after eating food (1.5%); 1 respondent chose none of the above options (1.5%). For the open-ended items relating to HCP instructions received by patients on when to take PERT, text entered regarding administration timing instructions included: “30 minutes before meals,” “20 minutes before a meal,” and “15 minutes before or after meals.”
Fifty-nine percent of respondents reported that their HCP recommended taking PERT for the rest of their lives, while 33% reported that their HCP did not specify how long they should take PERT. A small fraction was advised to take PERT for a few months (3%) to a few years (5%).
Thirty-nine percent of respondents reported an absence of HCP follow-up after starting PERT. Patients who had an HCP follow-up reported being asked and/or discussing PERT effectiveness/EPI symptom status (56%), compliance (22%), perceived adverse effects (eg, constipation) (18%), and dose adjustment (4%).
Patients' Reports on Their Understanding of EPI and PERT
The vast majority of respondents (83%) reported actively searching for information about EPI. The largest percentage of respondents used an online search engine (69%), followed by patient organizations (60%), medical or academic research organizations (58%), and specialist physicians (58%).
Respondents reported having a variable understanding of EPI, with notably more agreement on understanding that EPI was a complication of their underlying condition (93%) and less agreement on how EPI affects their body (68%) (Fig. 2A). Most agreed on understanding that PERT should be part of their daily routine and knowing how many pills to take with their meals and snacks. However, approximately one quarter of respondents were neutral or disagreed about their understanding of how to take PERT (23%) and how many pills to take, especially with snacks (28%) (Fig. 2B).
FIGURE 2.
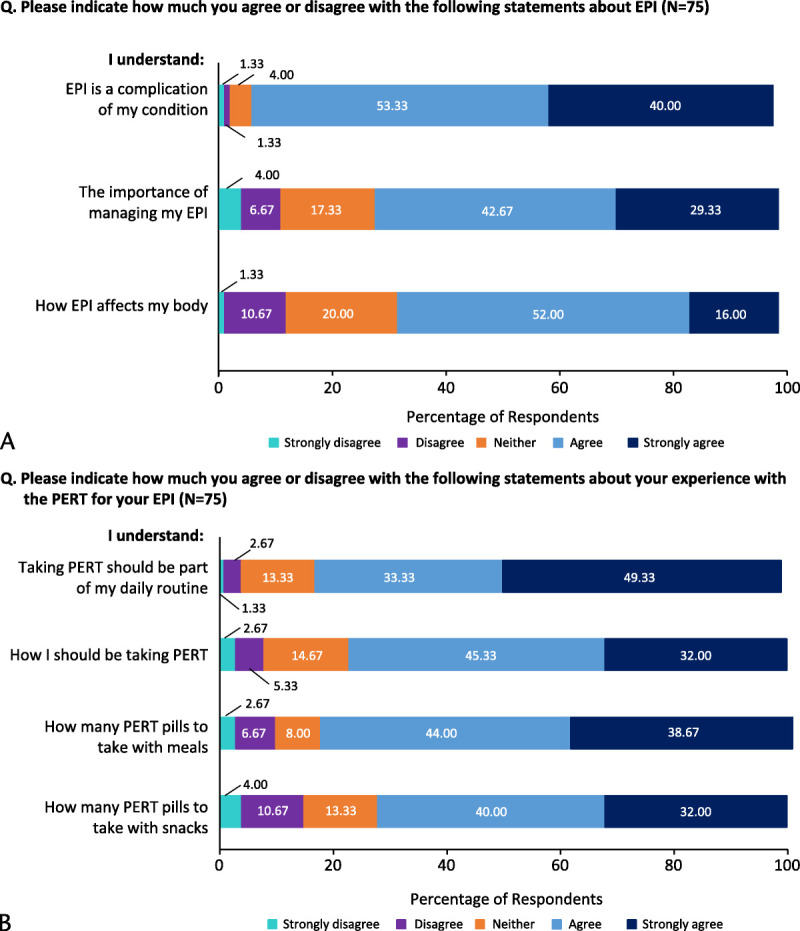
Respondents' understanding of (A) EPI and (B) experience with PERT for EPI.
Patients' Reports on PERT Use
Most respondents indicated taking PERT on average 2 to 5 times per day (86%) and on average 7 days per week (88%) (Table 2).
Among respondents reporting PERT capsule strength information and number of pills taken with meals, 36% were taking PERT doses that were less than 40,000 LU/meal (Table 3). Of these patients, 62.5% had CP. Moreover, 24% reported having received PERT prescriptions for low-strength capsules (ie, 3,000–15,000 LU/capsule) (Table 4).
TABLE 3.
Estimated PERT Dose Taken at Meals
| PERT Dose/Meal, LU | Patients (n = 66) |
|---|---|
| <40,000 | 36.4% |
| 40,000–50,000 | 15.1% |
| 50,000–175,000 | 47% |
| 252,000–280,000 | 1.5% |
TABLE 4.
Patient-Reported Prescribed PERT Capsule Strength and Number of Capsules Taken at Meals
| PERT Capsule Strengths, LU | Capsules/Meal, Median | Capsules/Meal, Min–Max | Patients (n = 71) |
|---|---|---|---|
| 3000–5000 | 1.5 | 1–3 | 6% |
| 10,000–15,000 | 2 | 1–4 | 18% |
| 16,000–20,000 | 2 | 2–2 | 6% |
| 21,000–25,000 | 3 | 1–7 | 28% |
| 36,000–40,000 | 2 | 1–7 | 42% |
Among evaluable respondents (n = 71) who reported how they take PERT in relation to meals and snacks, 34% reported taking PERT with all meals and all snacks, 32% with all meals and some snacks, 13% with meals only, variable (20%), and unknown (1%). In summary, 79% reported taking PERT with all meals, and 21% reported not taking PERT with any snacks.
With regard to the timing of PERT ingestion, respondents reported taking PERT as follows: 55% before eating food, 28% before and while eating food, 15.5% while eating food, and 1.5% after eating. For the open-ended items corresponding to HCP instructions of taking PERT before eating, text entered by patients included comments such as, “After doing more research, I now take one cap with my first bite and my second cap halfway through the meal.”
Sixty-eight percent of respondents reported that they occasionally forget to take PERT (Fig. 3), mainly because it is difficult to remember taking PERT with every meal and/or snack or that other medications were more important to remember (Figure, Supplemental Digital Content 1, http://links.lww.com/MPA/B42). Twenty-one percent reported purposely skipping a dose of PERT (Fig. 3), mainly because they felt the medication was not needed with every meal and/or snack or there would be no health consequences for doing so (Figure, Supplemental Digital Content 2, http://links.lww.com/MPA/B43). Twenty-four percent of respondents reported decreasing their PERT dose without consulting their HCP (Fig. 3), mainly because they felt there would be no health consequences for doing so or because of concerns about potential adverse effects (Figure, Supplemental Digital Content 3, http://links.lww.com/MPA/B44).
FIGURE 3.
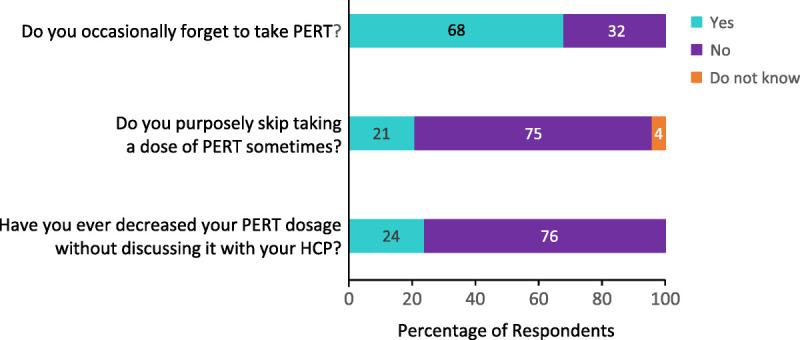
Respondent's PERT-taking behavior.
Of the 15 respondents (20%) who had stopped taking PERT (9 had CP, 4 had PC, 1 had AP, and 1 had undergone pancreatic surgery), 67% reported making the decision on their own, mainly due to high pill burden, overwhelming administration schedule, or changes in their diet to replace PERT (Figure, Supplemental Digital Content 4, http://links.lww.com/MPA/B45).
DISCUSSION
In recent years, data have emerged indicating that EPI is underdiagnosed and undertreated. Forsmark et al18 found in a large insured population-based study in the United States that only 30% of patients with CP and 22% of patients with PC were treated with PERT, and of those patients treated with PERT, 69% of CP and 75% of PC patients received subtherapeutic PERT doses. Chittajallu et al21 reported racial and ethnic disparities as well as age-related disparities in PERT prescribing that disproportionately negatively affected African American and Hispanic patients with CP20 and older and African American patients with PC. Although PERT is a lifelong therapy for most patients with EPI, the rate of PERT discontinuation is high, with a median duration of therapy of only 8 months.18
Few studies have evaluated real-world, patient-reported outcomes in patients with EPI and the effects of PERT treatment, and data on patient experiences and understanding of PERT treatment through surveys are even more limited.17 To date, no US-based research has evaluated patients' understanding of EPI and PERT, their daily use of PERT, and their assessment of their experience with their HCP managing their EPI. Johansson et al23 have reported that in recent years, the use of online support communities has increased as patients seek information about their health maladies, guidance for treatment, and support from peers. In an effort to advance our understanding of patients' real-world experiences with EPI and PERT, we conducted a survey with 75 EPI patients (or their caregivers) who are members of the online support platform, Inspire and its support communities for pancreatitis or pancreatic cancer.19
Patient-level data in this study indicate patient needs for additional information and education on EPI and PERT because only about half of respondents agreed that their HCP provided detailed information about EPI (54%) or how PERT works to treat EPI (56%), and most respondents (83%) reported actively searching for information about their condition, mainly online. Respondents reported having a variable understanding of EPI, mainly less understanding of how EPI affects their body, how PERT works to treat EPI, and how and when to take PERT. Counseling on the need for chronic PERT use was inconsistent, and only 59% of patients were informed about the chronicity of their condition and the need for lifelong therapy.
Information reported on administration of PERT indicates gaps in patient onboarding to therapy. Inconsistencies in instructions given to patients exist, ranging from patients not receiving any instructions at all on how and/or when to take PERT, to incomplete instructions, such as omitting instructions on taking PERT with snacks, and inaccurate guidance on timing of ingestion, such as before or after eating. Such inconsistencies are mirrored by patients' behavior, with patients reporting not taking PERT with any snacks, and more than half reporting taking PERT incorrectly as it related to timing of ingestion before or after eating.
Appropriate dosing of PERT is the foundation of its physiologic actions to improve maldigestion. The American College of Gastroenterology recommends a minimum starting dose for adults with CP and EPI of 40,000 to 50,000 LU/meal,1,7,10,12 with other international-based guidelines having similar or higher benchmarks.1,7,10,12,13 In our study, 36% of all patients were taking PERT doses that were less than 40,000 LU/meal at the time of the survey. Intake of an increased number of pills, the so-called pill burden, negatively affects patient compliance.24 In the United States, PERT capsule strengths range from infant dosing strengths to full adult dosing strengths, with the highest range of 36,000 to 40,000 LU. Only 42% of the adult patients in our study received PERT individual capsule strengths in the range of 36,000 to 40,000 LU, with a number of patients receiving lower LU capsule strengths, requiring multiple extra pills to achieve appropriate minimum dosing levels, thereby increasing pill burden and potentially decreasing compliance. The prescribing HCPs were primarily specialists—for example, gastroenterologists (57%), pancreatologists (7%), oncologists (17%), and surgeons (11%)—with 8% as other HCP types. Thus, further education regarding PERT dosing should be focused to HCPs as well.
Patient follow-up after starting PERT is important to evaluate the need for dose adjustment, assess patient adherence to instructions for dosing and administration (appropriate timing of PERT ingestion in relation to food, number of pills taken), address any gaps in the provision of these instructions, and inquire for any concerns (eg, management of any perceived side effects). However, 39% of patients reported an absence of follow-up. This results in missed opportunities to assess patient response to therapy and to clarify and reinforce adherence to instructions. Patient behaviors of occasionally forgetting to take their medication are consistent with behaviors associated with medications for other chronic conditions.25 However, many patients indicated purposely skipping, decreasing their dose, or stopping their PERT without consulting their HCP. This may be due to many factors including lack of information, pill burden, lack of response, and cost of medication, among others. This emphasizes the need for patient information and education and close patient follow-up.
Patients expressed that their HCP could help them be adherent with their PERT by improvements in several key areas: (1) education on why PERT is needed (eg, “explain why PERT is important and what it actually is doing,” “make the reason I need it clearer and more convincing, rather than it can't hurt”), (2) clarity on PERT dosage and dose administration, (3) understanding potential side effects of PERT (eg, constipation), and (4) close HCP follow-up regarding treatment response and decreasing pill burden.
The results from this survey suggest that further educational resources and tools for patients and HCPs as well as strategies for widespread dissemination of this information may improve patients' experience with PERT, thereby potentially improving adherence to therapy to maximize patient benefit of PERT. For HCPs, educational tools could focus on material to help with patient education, such as an onboarding checklist, education on pill burden, and adequate PERT dosing. This may include advice on prescribing higher lipase strength dosage units and sufficient PERT doses per meal and per snack according to published treatment guidelines.
The study findings should be interpreted considering some limitations. First, the sample size was small. Exocrine pancreatic insufficiency diagnosis was represented by patient/caregiver answer “Yes” only and not verified by EPI diagnostic test results and/or clinician attestation/verification, as is reasonable for a patient survey. In addition, as Inspire network members, the respondents likely reflect those who are more engaged in online patient forums and information sharing and those more engaged in understanding EPI and PERT. As such, the respondents may not be representative of the general EPI patient population, and potential gaps in therapy may actually be more pronounced in a potentially less engaged general population with EPI than those reported in the study population here.
In summary, this study identified barriers to effective treatment of EPI with PERT, including (1) lack of patient education on EPI and PERT, mainly how and when to take PERT, (2) gaps in appropriate dosing, leading to suboptimal patient response, patient perception of therapy being ineffective, and increased pill burden, and (3) lack of patient follow-up to assess the need for dose adjustment, management of any perceived side effects, assessment of patient adherence to instructions, and addressing any gaps in the provision of these instructions. Continued focus on patient and provider education is essential to address these gaps in care, optimize the treatment of EPI, and prevent its associated sequelae.
ACKNOWLEDGMENTS
The authors and AbbVie thank the patients and caregivers who participated in this survey as well as the Inspire research team, including Marina Ness, Hannah Eccard, Jessica Wood, and Jennifer Hlawacz, for their pivotal role in this research.
Footnotes
AbbVie funded this survey and participated in the survey design, research, analysis, data collection, interpretation of data, and the review and approval of the presentation. All authors had access to relevant data and participated in the drafting, review, and approval of this presentation. No honoraria or payments were made for authorship. Medical writing support was provided by Linda Goldstein, PhD, and Janet Matsuura, PhD, of ICON (Blue Bell, PA) and was funded by AbbVie. J.A.B. has served as a scientific advisor for AbbVie. D.H. and J.K. are full-time employees of AbbVie and may hold AbbVie stock. J.S.B. has served as a scientific advisor for AbbVie.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
Contributor Information
Diala Harb, Email: Diala.Harb@abbvie.com.
Jens Kort, Email: jens.kort@abbvie.com.
Jamie S. Barkin, Email: jsbarkin@med.miami.edu.
REFERENCES
- 1.Phillips ME Hopper AD Leeds JS, et al. Consensus for the management of pancreatic exocrine insufficiency: UK practical guidelines. BMJ Open Gastroenterol. 2021;8:e000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Haese JG Ceyhan GO Demir IE, et al. Pancreatic enzyme replacement therapy in patients with exocrine pancreatic insufficiency due to chronic pancreatitis: a 1-year disease management study on symptom control and quality of life. Pancreas. 2014;43:834–841. [DOI] [PubMed] [Google Scholar]
- 3.de la Iglesia-Garcia D Huang W Szatmary P, et al. Efficacy of pancreatic enzyme replacement therapy in chronic pancreatitis: systematic review and meta-analysis. Gut. 2017;66:1354–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez-Munoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep. 2007;9:116–122. [DOI] [PubMed] [Google Scholar]
- 5.Dominguez-Munoz JE Nieto-Garcia L Lopez-Diaz J, et al. Impact of the treatment of pancreatic exocrine insufficiency on survival of patients with unresectable pancreatic cancer: a retrospective analysis. BMC Cancer. 2018;18:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durie P Baillargeon JD Bouchard S, et al. Diagnosis and management of pancreatic exocrine insufficiency (PEI) in primary care: consensus guidance of a Canadian expert panel. Curr Med Res Opin. 2018;34:25–33. [DOI] [PubMed] [Google Scholar]
- 7.Löhr JM Domínguez-Muñoz E Rosendahl J, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5:153–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramesh H Reddy N Bhatia S, et al. A 51-week, open-label clinical trial in India to assess the efficacy and safety of pancreatin 40000 enteric-coated minimicrospheres in patients with pancreatic exocrine insufficiency due to chronic pancreatitis. Pancreatology. 2013;13:133–139. [DOI] [PubMed] [Google Scholar]
- 9.Roberts KJ, Bannister CA, Schrem H. Enzyme replacement improves survival among patients with pancreatic cancer: results of a population based study. Pancreatology. 2019;19:114–121. [DOI] [PubMed] [Google Scholar]
- 10.Gardner TB Adler DG Forsmark CE, et al. ACG clinical guideline: chronic pancreatitis. Am J Gastroenterol. 2020;115:322–339. [DOI] [PubMed] [Google Scholar]
- 11.Hendifar AE Petzel MQB Zimmers TA, et al. Pancreas cancer-associated weight loss. Oncologist. 2019;24:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Perez E Dominguez-Munoz JE Botella-Romero F, et al. Multidisciplinary consensus statement on the clinical management of patients with pancreatic cancer. Clin Transl Oncol. 2020;22:1963–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabater L Ausania F Bakker OJ, et al. Evidence-based guidelines for the management of exocrine pancreatic insufficiency after pancreatic surgery. Ann Surg. 2016;264:949–958. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez-Munoz JE Drewes AM Lindkvist B, et al. Recommendations from the United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis. Pancreatology. 2018;18:847–854. [DOI] [PubMed] [Google Scholar]
- 15.Dominguez-Munoz JE, Iglesias-Garcia J. Oral pancreatic enzyme substitution therapy in chronic pancreatitis: is clinical response an appropriate marker for evaluation of therapeutic efficacy? JOP. 2010;11:158–162. [PubMed] [Google Scholar]
- 16.Dominguez-Munoz JE Iglesias-Garcia J Vilarino-Insua M, et al. 13C-mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2007;5:484–488. [DOI] [PubMed] [Google Scholar]
- 17.Barkin JA Westermann A Hoos W, et al. Frequency of appropriate use of pancreatic enzyme replacement therapy and symptomatic response in pancreatic cancer patients. Pancreas. 2019;48:780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsmark CE Tang G Xu H, et al. The use of pancreatic enzyme replacement therapy in patients with a diagnosis of chronic pancreatitis and pancreatic cancer in the US is infrequent and inconsistent. Aliment Pharmacol Ther. 2020;51:958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivoleti P Dolan RD Yang AL, et al. Provider differences in monitoring and management of exocrine pancreatic insufficiency in chronic pancreatitis. Pancreas. 2022;51:25–27. [DOI] [PubMed] [Google Scholar]
- 20.Chittajallu V Abou Saleh M Lindsey AS, et al. Exocrine pancreatic insufficiency treatment in chronic pancreatitis remains suboptimal and is significantly lower in minorities patients. Pancreas. 2020;49:1402–1403. [Google Scholar]
- 21.Chittajallu V Abou Saleh M Lindsey AS, et al. Treatment of exocrine pancreatic insufficiency in pancreatic cancer is low and disproportionally affects older and African American patients. Pancreas. 2020;49:1403. [Google Scholar]
- 22.Inspire™. Available at: https://www.inspire.com. Accessed November 18, 2021.
- 23.Johansson V Islind AS Lindroth T, et al. Online communities as a driver for patient empowerment: systematic review. J Med Internet Res. 2021;23:e19910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrell B, French Merkley V, Ingar N. Reducing pill burden and helping with medication awareness to improve adherence. Can Pharm J (Ott). 2013;146:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Lazaro CI García-González JM Adams DP, et al. Adherence to treatment and related factors among patients with chronic conditions in primary care: a cross-sectional study. BMC Fam Pract. 2019;20:132. [DOI] [PMC free article] [PubMed] [Google Scholar]


