VV ECMO is a relative contraindication in patients with TBI given concerns for intracranial bleeding. However, we demonstrated there was no difference in survival between trauma VV ECMO subjects with versus without TBI (72% v 64%, p=0.45).
KEY WORDS: Brain injuries, traumatic, venovenous extracorporeal membrane oxygenation, respiratory failure
Abstract
BACKGROUND
Venovenous extracorporeal membrane oxygenation (VV ECMO) can support trauma patients with severe respiratory failure. Use in traumatic brain injury (TBI) may raise concerns of worsening complications from intracranial bleeding. However, VV ECMO can rapidly correct hypoxemia and hypercarbia, possibly preventing secondary brain injury. We hypothesize that adult trauma patients with TBI on VV ECMO have comparable survival with trauma patients without TBI.
METHODS
A single-center, retrospective cohort study involving review of electronic medical records of trauma admissions between July 1, 2014, and August 30, 2022, with discharge diagnosis of TBI who were placed on VV ECMO during their hospital course was performed.
RESULTS
Seventy-five trauma patients were treated with VV ECMO; 36 (48%) had TBI. Of those with TBI, 19 (53%) had a hemorrhagic component. Survival was similar between patients with and without a TBI (72% vs. 64%, p = 0.45). Traumatic brain injury survivors had a higher admission Glasgow Coma Scale (7 vs. 3, p < 0.001) than nonsurvivors. Evaluation of prognostic scoring systems on initial head computed tomography demonstrated that TBI VV ECMO survivors were more likely to have a Rotterdam score of 2 (62% vs. 20%, p = 0.03) and no survivors had a Marshall score of ≥4. Twenty-nine patients (81%) had a repeat head computed tomography on VV ECMO with one incidence of expanding hematoma and one new focus of bleeding. Neither patient with a new/worsening bleed received anticoagulation. Survivors demonstrated favorable neurologic outcomes at discharge and outpatient follow-up, based on their mean Rancho Los Amigos Scale (6.5; SD, 1.2), median Cerebral Performance Category (2; interquartile range, 1–2), and median Glasgow Outcome Scale—Extended (7.5; interquartile range, 7–8).
CONCLUSION
In this series, the majority of TBI patients survived and had good neurologic outcomes despite a low admission Glasgow Coma Scale. Venovenous extracorporeal membrane oxygenation may minimize secondary brain injury and may be considered in select patients with TBI.
LEVEL OF EVIDENCE
Prognostic and Epidemiological; Level IV.
The management of patients with traumatic brain injury (TBI) and acute lung injury can be particularly challenging. Patients with TBI are at increased risk for the development of acute respiratory distress syndrome (ARDS) and subsequent increased risk of mortality.1,2 A permissive hypercapnia strategy to allow for lower tidal volumes combined with higher positive end-expiratory pressure (PEEP) and delivered oxygen on the ventilator may typically be used to manage a patient with severe ARDS.3 In TBI, brain hypoxia and hypercapnia-induced vasodilation leading to increased cerebral blood volume and intracranial pressure (ICP) may result from typical ARDS ventilatory strategies, leading to secondary brain injury that can worsen morbidity and mortality.4,5 Furthermore, high intrathoracic pressures related to elevated positive PEEP can reduce central venous return and potentially alter cerebral perfusion, further contributing to secondary brain injury.6 While some studies have not demonstrated adverse effects of elevated PEEP on intracranial physiology, brain tissue oxygenation may be severely impaired with concomitant ARDS.7,8 Thus, the maintenance of adequate oxygenation and ventilation with minimal ventilator settings becomes imperative in patients with TBI. Since venovenous extracorporeal membrane oxygenation (VV ECMO) can correct hypoxia and hypercarbia and facilitate “lung rest” ventilator settings without significantly altering cerebral blood flow or ICP, it may serve a key role in the management of severe respiratory dysfunction in a population of patients with TBI.9,10
Although there is potential benefit, VV ECMO remains contraindicated in patients with TBI given concerns for intracranial bleeding.11 Current recommendations from the Extracorporeal Life Support Organization include both ARDS and thoracic trauma as indications for VV ECMO, but intracranial hemorrhage (ICH) remains a relative contraindication.12 However, alternative heparin dosing strategies, such as low-dose heparin, heparinized circuits, or heparin-free circuits, may mitigate the perceived risks of bleeding complications.13–15
In this study, we examine the outcomes of TBI patients on VV ECMO. We hypothesize that trauma patients with TBI who were managed with VV ECMO would have similar survival rates as a non-TBI VV ECMO population. We also hypothesize that those patients who survive to discharge will have good independent-functioning neurologic outcomes given the minimization of secondary causes of brain injury that VV ECMO allows. Finally, we describe bleeding and clotting complications of TBI patients on VV ECMO.
PATIENTS AND METHODS
Study Design
This is a single-center, retrospective cohort study. The original study, titled “Veno-venous Extracorporeal Membrane Oxygenation in Traumatic Brain Injury,” was approved by the institutional review board (HP-00103431) on October 13, 2022, and the need for written consent was waived.
Subject Selection
All subjects who were admitted to the trauma service and were subsequently placed on VV ECMO from January 1, 2014, to August 30, 2022, were screened. Inclusion criteria included the following: (1) discharge diagnosis of any type of TBI (2) cannulated for VV ECMO during admission.
Our institution uses a multidisciplinary approach for VV ECMO candidate selection. An intensivist from the critical care resuscitation unit,16 the lung rescue unit,17 and a trauma surgeon discuss and apply institutional guidelines for selection. The critical care resuscitation unit is a receiving intensive care unit at our trauma institution and the lung rescue unit is a dedicated VV ECMO intensive care unit.
Criteria for consideration for VV ECMO included the following: hypercapnia with respiratory acidosis (partial pressure of carbon dioxide [PaCO2] >60 mm Hg with a pH <7.25); inability to ventilate adequately with plateau pressure ≤30 cm H2O; and severe hypoxemia (ratio of arterial oxygen partial pressure to fractional inspired oxygen [PaO2/FiO2] <50 with FiO2 >80% for >3 hours or PaO2/FiO2 ratio <80 with FiO2 >80% for >6 hours) despite maximal ventilatory support and use of adjunctive therapies such as prone positioning, neuromuscular blockade, and inhaled pulmonary vasodilators. Ultimately, these are guidelines, and cannulation decisions are made on a case-by-case basis.
Throughout the study period, typical management goals for trauma VV ECMO patients included heparin infusion with goal partial thromboplastin time of 45 to 55 seconds and platelets of >40,000 μL. The decision to withhold heparin at the time of cannulation was at the discretion of the proceduralist, while the decision to hold a continuous heparin infusion was decided between the managing intensivist and trauma teams. These decisions were largely dependent on injury patterns, thrombotic risk, and bleeding risk.
Data Storage and Analysis
Study data were collected and managed using Research Electronic Data Capture tools hosted at our institution.18,19 The STrengthening the Reporting of OBservational studies in Epidemiology checklist (Supplemental Digital Content, Supplementary Data 1, http://links.lww.com/TA/D282), as part of the Enhancing the QUAlity and Transparency of health Research guidelines, was used for this cohort study. Demographics, pre-ECMO data, ECMO data, and subject outcomes were analyzed. All scoring systems were calculated on admission or pre-ECMO cannulation. The primary outcome was survival to hospital discharge. The secondary outcome was discharge and follow-up neurologic outcomes collected retrospectively from office visits. Initial head computed tomography (CT) scans obtained before VV ECMO cannulation were reviewed by a critical care–trained neurologist, blinded to clinical and outcome data, who tabulated Marshall and Rotterdam CT scores.20,21 The Marshall score categorizes TBI into diffuse versus focal CT head injury patterns and classifies injury severity based on the degree of midline shift, basal cistern compression, and the presence of hemorrhage or contusions. The Rotterdam score was devised as an ordinal predictor of outcome following TBI and is based on a combination of individual CT features including presence of epidural lesion, midline shift, intraventricular or subarachnoid blood, and compression of the basal cisterns (Supplemental Digital Content, Supplementary Table 1, http://links.lww.com/TA/D283). In both scoring systems, higher scores have been shown to correlate with worse functional neurological outcomes and/or prognosis. Neurologic decline (while on VV ECMO) was defined as 2-point decline in Glasgow Coma Scale (GCS), loss of pupillary or corneal activity, or >2 mm pupillary asymmetry.22 Neurologic outcomes were determined retrospectively from scores available at discharge and outpatient follow-up after discharge, using the Rancho Los Amigos Scale, Cerebral Performance Category, and Glasgow Outcome Scale—Extended (GOS-E) (Supplemental Digital Content, Supplementary Table 2, http://links.lww.com/TA/D283). Utilization of these neurologic and cognitive scoring systems following brain injury is useful in prognosticating functionality during recovery. There were no missing data from the cohort during admission, and outpatient follow-up data were available for all subjects. In addition, there were no patients excluded from those initially screened.
Data were analyzed with descriptive statistics. Parametric or nonparametric statistics were used based on the nature of the data. Normality was assessed with the Shapiro-Wilk test and examination of stem-and-leaf as well as q-q plots. The Student's t test was used to assess differences with parametric continuous data, and the Kruskall-Wallis and Wilcoxon rank-sum tests were used to analyze nonparametric data. Normally distributed data were presented with mean and SD, while nonnormally distributed data were presented with median and quartiles (Q1–Q3). χ2 tests were used to analyze categorical data. All tests were two-tailed, and a p value of <0.05 was used to define statistical significance. An unadjusted logistic regression was compared with a fixed-effects method. Fixed-effects methods control for potential confounding effects of unobserved, time-invariant variables and can be used to minimize sparse-data bias.23,24 A conditional (fixed-effects) logistic regression analysis was performed with survival as the dependent variable and presence or absence of TBI as the grouping variable.25 Independent laboratory and hemodynamic variables were chosen based on significance in the univariable analysis. All tests were performed in Stata version 17 (StataCorp 2021, Stata Statistical Software: Release 17; StataCorp LLC, College Station, TX) and GraphPad Prism 7.0 for Mac (GraphPad Software, La Jolla, CA).
RESULTS
Seventy-five subjects received VV ECMO while on the trauma service during the study period. Of those, 36 subjects were diagnosed with a TBI. There was no difference in survival between trauma VV ECMO subjects who had a TBI and who did not have a TBI (72% vs. 64%, p = 0.45). Of the subjects with TBI, there was no difference in age, race/ethnicity, sex, past medical history, body mass index, or time from admission to cannulation between survivors and nonsurvivors (Table 1). Survivors were placed on continuous renal replacement therapy during VV ECMO less frequently than nonsurvivors (45% vs. 70%, p = 0.14) and were less likely to have a cardiac arrest before VV ECMO (31% vs. 60%, p = 0.11), although these results were not statistically significant. Most subjects (69%) were cannulated via the internal jugular and femoral veins with no difference in cannulation strategy between survivors and nonsurvivors. Survivors had a longer VV ECMO course (240 hours vs. 72 hours, p = 0.04), hospital length of stay (45 days vs. 6.5 days, p < 0.001), and time on the ventilator (23.5 days vs. 4 days, p < 0.001) than nonsurvivors. Twenty-four survivors (94%) were discharged to a rehabilitation facility, and all subjects were liberated from the ventilator at hospital discharge.
TABLE 1.
Demographics and Characteristics of Subjects With TBI on VV ECMO
| All Subjects (n = 36) | Survivors (n = 26) | Nonsurvivors (n = 10) | p | |
|---|---|---|---|---|
| Age, median (IQR), y | 29 (23.5–39) | 28.5 (21–40) | 30.5 (24–37) | 0.86 |
| Race/ethnicity, n (%) | 0.53 | |||
| White | 16 (44) | 13 (50) | 3 (30) | |
| Hispanic | 2 (6) | 1 (4) | 1 (10) | |
| African American | 16 (44) | 10 (38) | 6 (60) | |
| Other | 2 (6) | 2 (8) | (0) | |
| Sex, n (%) | ||||
| Male | 29 (81) | 21 (51) | 8 (80) | 0.96 |
| BMI, median (IQR) | 27.2 (23.45–32.35) | 27.2 (22.4–30.1) | 28.3 (23.9–35.4) | 0.45 |
| Past medical history, n (%) | — | |||
| Asthma/COPD | 1 (3) | 1 (4) | 0 (0) | |
| DM | 1 (3) | 1 (4) | 0 (0) | |
| CAD | 2 (6) | 1 (4) | 1 (10) | |
| Liver disease | 1 (3) | 1 (4) | 0 (0) | |
| Substance abuse | 4 (11) | 3 (12) | 1 (10) | |
| VV ECMO cannulation strategy, n (%) | 0.83 | |||
| IJ/fem | 25 (69) | 18 (69) | 7 (70) | |
| Fem/fem | 10 (28) | 7 (27) | 3 (30) | |
| Dual lumen | 1 (3) | 1 (4) | 0 (0) | |
| Time from arrival to cannulation, median (IQR), h | 16 (3.5–72) | 13 (3–72) | 19 (4–72) | 0.41 |
| Decannulated, n (%) | 27 (75) | 26 (100) | 1 (10) | — |
| Use of CRRT during ECMO run, n (%) | 18 (50) | 11 (42) | 7 (70) | 0.14 |
| Cardiac arrest before cannulation, n (%) | 14 (39) | 8 (31) | 6 (60) | 0.11 |
| Time from admission to cannulation, median (IQR), h | 16 (3.5–72) | 13 (3–72) | 19 (4–72) | 0.76 |
| Hours on ECMO, median (IQR) | 212.5 (120–348) | 240 (167–336) | 72 (46–360) | 0.04 |
| Hospital LOS, median (IQR) | 33.5 (11.5–51.5) | 45 (32–57) | 6.5 (3–15) | <0.001 |
| Ventilator days, median (IQR) | 19 (9–34.5) | 23.5 (18–36) | 4 (3–14) | <0.001 |
| Discharge location, n (%) | — | — | — | |
| Home | 2 (6) | |||
| Rehab | 24 (94) | |||
| Ventilator requirements at discharge, n (%) | — | — | — | |
| Room air | 17 (65) | |||
| Trach collar | 9 (35) | |||
| Survival to discharge, n (%) | 26 (72) | — | — | — |
BMI, body mass index; COPD, chronic obstructive lung disease; CRRT, continuous renal replacement therapy; CAD, coronary artery disease; DM, diabetes mellitus; ECMO, extracorporeal membrane oxygenation; fem, femoral; IJ, internal jugular; IQR, interquartile range; LOS, length of stay; rehab, rehabilitation.
Most subjects suffered blunt traumatic injury (97%) with motor vehicle crash being the most common mechanism (42%). There was no difference in mechanism of injury between the survivor and nonsurvivor groups (Table 2). Injury Severity Score (27 vs. 34.5, p = 0.04) and head Abbreviated Injury Score (2 vs. 4, p = 0.02) was lower, and Trauma Score and Injury Severity Score was higher in the survivor group (0.875 vs. 0.426, p = 0.006).
TABLE 2.
Scoring Systems and Traumatic Injuries in TBI VV ECMO Subjects
| All Subjects (n = 36) | Survivors (n = 26) | Nonsurvivors (n = 10) | p | |
|---|---|---|---|---|
| Mechanism of injury, n (%) | 0.81 | |||
| Blunt | 35 (97) | 26 (100) | 9 (90) | |
| MVC | 15 (42) | 12 (46) | 3 (30) | |
| MCC | 6 (17) | 4 (16) | 2 (20) | |
| Fall | 7 (19) | 5 (19) | 2 (20) | |
| Pedestrian struck | 7 (19) | 5 (19) | 2 (20) | |
| Penetrating | 1 (3) | — | 1 (10) | |
| GSW | 1 (3) | 1 (10) | ||
| Drowning, n (%) | 5 (14) | 3 (12) | 2 (20) | 0.66 |
| SBP on admission, mean (SD), mm Hg | 107 (32) | 128 (101–130) | 75 (70–101) | 0.004 |
| RR on admission, median (IQR) | 25 (20–29.5) | 25 (20–28) | 25 (15–28) | 0.49 |
| ISS | 29.5 (20–38) | 27 (17–34) | 34.5 (29–50) | 0.04 |
| TRISS | 0.746 (0.572–0.913) | 0.875 (0.594–0.958) | 0.426 (0.212–0.667) | 0.006 |
| AIS | ||||
| Head | 3 (0–4) | 2 (0–3) | 4 (3–5) | 0.02 |
| Neck | 0 (0–0) | 0 (0–0) | 0 (0–2) | 0.14 |
| Spine | 0 (0–2) | 0 (0–2) | 1 (0–2) | 0.82 |
| Thorax | 3 (1.5–3.5) | 2 (1–3) | 3 (2–4) | 0.27 |
| Abdomen/pelvis | 2 (0–3) | 1.5 (0–3) | 2 (1–4) | 0.24 |
| Extremity | 1 (0–3) | 1 (0–3) | 1.5 (0–2) | 0.72 |
| Marshall score, n (%) | 0.003 | |||
| 1 | 16 (44) | 14 (54) | 2 (20) | |
| 2 | 13 (36) | 9 (35) | 4 (40) | |
| 3 | 4 (11) | 3 (12) | 1 (10) | |
| 4 | 1 (3) | 0 (0) | 1 (10) | |
| 5 | 1 (3) | 0 (0) | 1 (10) | |
| 6 | 1 (3) | 0 (0) | 1 (10) | |
| Rotterdam score, n (%) | 0.004 | |||
| 1 | 1 (3) | 1 (4) | 0 (0) | |
| 2 | 17 (47) | 15 (58) | 2 (20) | |
| 3 | 12 (33) | 8 (31) | 4 (40) | |
| 4 | 2 (6) | 1 (4) | 1 (10) | |
| 5 | 3 (8) | 1 (4) | 2 (20) | |
| 6 | 1 (3) | 0 (0) | 1 (10) | |
| GCS, n (%) | 0.03 | |||
| 14–15 | 0 (0) | 0 (0) | 0 (0) | |
| 9–13 | 5 | 5 (19) | 0 (0) | |
| 3–8 | 31 (86) | 21 (81) | 10 (100) |
AIS, Abbreviated Injury Scale; ECMO, extracorporeal membrane oxygenation; GCS, Glasgow Coma Scale; GSW, gunshot wound; ISS, Injury Severity Score; IQR, interquartile range; MVC, motor vehicle collision; MCC, motorcycle collision; RR, respiratory rate; SBP, systolic blood pressure; TRISS, Trauma Score and Injury Severity Score.
Although all subjects had a reduced GCS on admission, GCS was higher in survivors than nonsurvivors (7 vs. 3, p < 0.001). All survivors had a discharge GCS of 15.
All TBI subjects had a head CT before initiation of VV ECMO. Nineteen TBI subjects (53%) had a hematoma or hemorrhage, 12 (33%) had cerebral edema, 12 (33%) had diffuse axonal injury, and 4 (11%) had evidence of contusion (Supplemental Digital Content, Supplementary Table 3, http://links.lww.com/TA/D283). Subarachnoid hemorrhage was the most common type of ICH, seen in 11 subjects (31%). There was no difference in TBI type between survivors and nonsurvivors. Significantly more survivors had a Rotterdam score from an initial head CT of 2 (58% vs. 20%, p = 0.03). Of the four subjects (11%) with a Rotterdam score of ≥5, only one (25%) survived. No subjects with a Marshall score of ≥4 survived. Although survivors mainly presented with severe TBI based on initial GCS, CT-based Marshall and Rotterdam scores indicated that many of these patients sustained mild anatomic injury. While on VV ECMO, 29 subjects (81%) had a repeat head CT, and 4 subjects (14%) had a new finding from pre-ECMO imaging. One subject had multiple findings, and there was one expansion of a previously seen hemorrhage, one new hemorrhagic contusion, and three instances of the development of cerebral edema. Survivors and nonsurvivors had similar rates of new findings on head CT during VV ECMO. Neurologic decline rates on VV ECMO were not different between groups (15% vs. 10%, p = 0.66).
No neurosurgical procedures were performed while on VV ECMO. Before VV ECMO initiation, eight subjects (22%) had an external ventricular drain (EVD) placed, and two subjects (6%) had craniectomies. Among the individuals who had EVDs inserted, four subjects received heparin. However, heparin was held for the duration of their ECMO run in all cases. None of the subjects with EVDs encountered any bleeding complications related to the device, and all of them survived. There was no difference in ICP measurements precannulation and postcannulation (12 vs. 10, p = 0.57).
Neurologic outcomes of survivors were assessed at discharge and outpatient follow-up. The mean Rancho Los Amigos Scale at discharge was 6.5 (SD, 1.2), and Cerebral Performance Category median was 2 (interquartile range, 1–2). The GOS-E was collected retrospectively at outpatient follow-up with a mean length of time from discharge to appointment of 125 days (SD, 57 days). The median GOS-E was 7.5 (interquartile range, 7–8).
Of those that died, 6 (60%) were made do not resuscitate after family consultation: three because of poor neurologic prognosis and three because of multiorgan failure with escalating vasopressor requirements (Table 3). Two subjects were formally declared brain dead, one of whom had a worsening hemorrhage on head CT on VV ECMO. Two subjects died from multiorgan failure.
TABLE 3.
Characteristics of TBI VV ECMO Nonsurvivors With Mortality Due to Neurologic Diagnosis
| Subject | Cause of Death | Pre-ECMO GCS | Pre-ECMO Rotterdam Score | Pre-ECMO Marshall Score | New Finding on Head CT While on VV ECMO | Characteristics of New Finding on VV ECMO | Neurologic Decline While on VV ECMO | Pre-ECMO Head CT Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | DNR due to poor neurologic prognosis | 3 | 2 | 1 | No | NA | Yes | Cerebral edema |
| 2 | DNR due to poor neurologic prognosis | 3 | 2 | 1 | No | NA | No | Cerebral edema |
| 3 | DNR due to poor neurologic prognosis | 3 | 5 | 6 | No | NA | No | IPH, IVH |
| 4 | Declared brain dead | 3 | 5 | 5 | Yes | Size of hemorrhage | No | SAH, SDH |
| 5 | Declared brain dead | 3 | 3 | 2 | No | NA | No | Cerebral edema, DAI, IVH |
DAI, diffuse axonal injury; DNR, do not resuscitate; IPH, intraparenchymal hemorrhage; IVH, intraventricular hemorrhage; SAH, subarachnoid hemorrhage; SDH, subdural hematoma.
Pulmonary contusion was the most common cause of acute lung injury leading to cannulation (42%) in all subjects with TBI. The decision to cannulate was made because of hypoxia in 35 subjects (97%) and hypercarbia in 16 subjects (44%). There was no difference between survivors and nonsurvivors in the cause of acute lung injury or reason for cannulation. There was also no difference in the precannulation ventilator settings used. In addition, PaO2/FiO2 ratios (68.5 vs. 64, p = 0.66) and Respiratory ECMO Survival Prediction scores (6 vs. 6, p = 0.91) were comparable between groups. Oxygen saturation was similar between survivors and nonsurvivors on admission (93% vs. 95%, p = 0.62) but was higher in survivors precannulation (85.5% vs. 76.5%, p = 0.03). Traumatic brain injury survivors were more likely to be cannulated within 3 hours of developing hypoxia, defined as a sustained oxygen saturation <90% (88% vs. 20%, p < 0.001). No survivors had hypoxia for ≥7 hours. As the length of hypoxia increased, probability of survival decreased (Fig. 1). A receiver operator characteristic curve was constructed with an area under the curve of 0.95 (95% confidence interval, 0.86–0.97). Analysis of the unadjusted logistic regression demonstrated poor goodness of fit and high deviance, likely because of small sample size. When comparing the fixed-effects method with the unadjusted logistic regression, the conditional model had improved predictive ability. Conditional logistic regression analysis demonstrated that length of time of hypoxia precannulation was predictive of mortality in TBI patients on VV ECMO (odds ratio, 2.08; 95% confidence interval, 1.34,2.35; p < 0.001).
Figure 1.
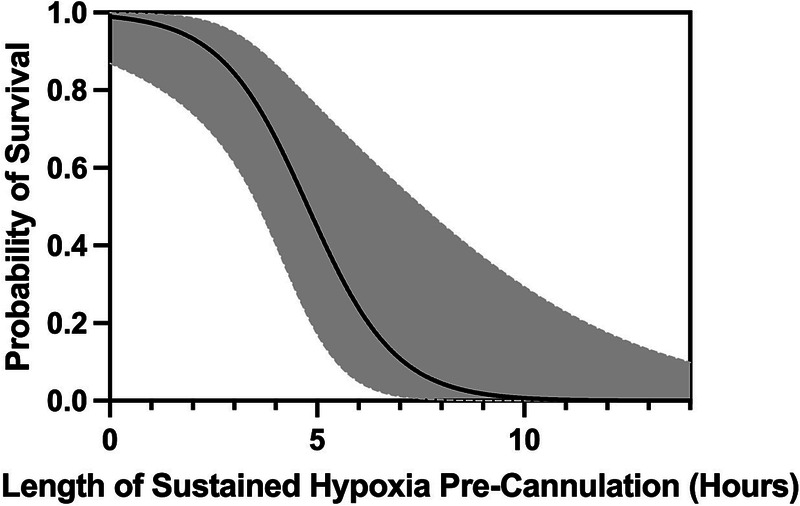
Probability of survival in TBI VV ECMO subjects by length of sustained hypoxia precannulation. Probability of survival is on the y axis with length of sustained hypoxia (hours) located on the x axis. Shaded area represents 95% confidence bands. Hypoxia is defined as oxygen saturation <90%.
Survivors had higher arterial blood gas pH levels on admission (7.27 vs. 6.97, p = 0.004), precannulation (7.28 vs. 7.13, p = 0.02), and postcannulation (7.38 vs. 7.31, p < 0.001). However, admission pCO2 (51 mm Hg vs. 60 mm Hg, p = 0.28) and pO2 (99.5 mm Hg vs. 76 mm Hg, p = 0.42), precannulation (53.5 mm Hg vs. 57.5 mm Hg, p = 0.37; 67.5 mm Hg vs. 64 mm Hg, p = 0.68), and postcannulation (41 mm Hg vs. 39 mm Hg, p = 0.7; 113 mm Hg vs. 113 mm Hg, p = 0.98) levels were comparable in survivors and nonsurvivors. When comparing admission, precannulation, and postcannulation arterial blood gas values within groups, there was a significant increase in pH and pO2, and decrease in pCO2 in both groups.
A heparin infusion was used for anticoagulation during VV ECMO in 18 subjects (50%) and was subsequently held in 13 (72%) of these subjects (Supplemental Digital Content, Supplementary Table 4, http://links.lww.com/TA/D283). Heparin was used more in survivors (62% vs. 20%, p = 0.03) (Fig. 2). Partial thromboplastin times were similar at admission (32 seconds vs. 38.5 seconds, p = 0.22) and during the VV ECMO course (48.5 seconds vs. 43 seconds, p = 0.49) in survivors and nonsurvivors. Platelets while on VV ECMO were higher in the survivor group (111 vs. 71, p = 0.02).
Figure 2.
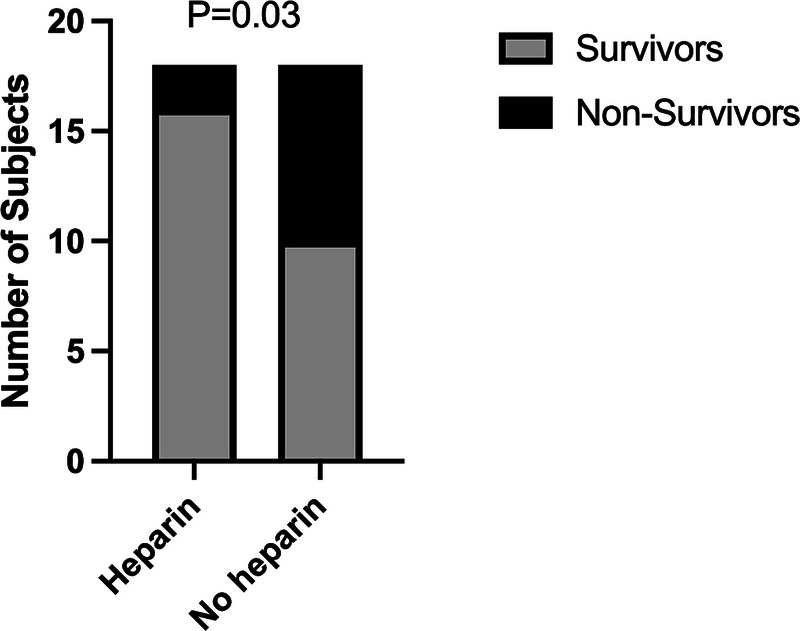
Traumatic brain injury VV ECMO heparin exposure by survivors and nonsurvivors. The x axis identifies exposure to heparin during the VV ECMO course. The y axis is the number of subjects in each group. The legend denotes survivors and nonsurvivors. p Values compare heparin exposure between survivors and nonsurvivors.
Seventeen subjects (47%) had a documented bleeding complication, with the most common being surgical or procedural site bleeding (47%). New internal bleeding was noted in 2 cases (12%). Survivors had similar rates of bleeding complications as nonsurvivors (10 vs. 7, p = 0.09). Ten subjects (28%) had documented clotting complications, all involving the oxygenator requiring exchange. Survivors had more clotting complications (9 vs. 1, p = 0.03). Two patients demonstrated hemorrhagic progression on CT after being placed on ECMO. One patient had a new hemorrhagic contusion and survived to hospital discharge. Another patient had worsening of a traumatic subarachnoid hemorrhage and ultimately died. Neither patient was on heparin during their ECMO course.
DISCUSSION
In our retrospective analysis of trauma patients placed on VV ECMO, there was no difference in survival between patients with or without TBI. While on VV ECMO, bleeding complications did not appear to significantly impact survival. Most importantly, there was a low incidence of worsening ICH while on VV ECMO. Survivors in our cohort had good neurologic outcomes both at hospital discharge and outpatient follow-up. Early utilization of VV ECMO in TBI patients with ARDS does not appear to affect mortality and may facilitate improved neurologic outcomes. Patients with TBI could have concomitant pulmonary injuries and are also at increased risk for the development of ARDS.1,6 Regardless of the pulmonary pathology, VV ECMO is used in patients with respiratory failure refractory to conventional management.12,26 While still a relatively new practice, multiple prior studies have explored the feasibility and safety of VV ECMO in the general trauma population.27–36
Our study demonstrates that patients with TBI on VV ECMO have similar observed mortality rates as trauma patients without TBI. Survivors in this patient population had largely independent-functioning neurologic outcomes despite presenting with severely depressed GCS. These findings may be due to several factors. First, most patients included had Marshall scores of 1 and 2 and Rotterdam scores of 2 and 3, representing mild TBI. Historically, these patients demonstrate good neurologic recovery. In addition, in our severely injured trauma population, GCS may be confounded by injury severity and shock states. Second, injurious ventilator settings can have deleterious effects on respiratory function in ARDS and can worsen TBI because of alteration in cerebral perfusion.37,38 Since VV ECMO facilitates “lung rest” settings, patients with TBI are not exposed to such settings and may avoid further neurologic insult. Lastly, VV ECMO can promote oxygen delivery while matching oxygen consumption in critically ill patients with refractory hypoxemia.39,40 In patients with neurologic injury, hypoxia is a significant secondary brain insult that can worsen morbidity and mortality.4 Our study demonstrates that mortality worsens in TBI patients with longer periods of hypoxia. Venovenous extracorporeal membrane oxygenation is an effective strategy to rapidly correct hypoxemia and minimize the incidence of ventilator associated lung injury.
Traumatic brain injury is a complex disease process, and determining the appropriate patient population to consider for VV ECMO remains unknown.14,15,41 Over half of our patient population had some form of hematoma or hemorrhage. Most survivors had lower Rotterdam and Marshall scores on initial head CT, and no survivors in our cohort had a Marshall score of ≥4. This is consistent with previous research that increasing Rotterdam and Marshall scores correlates with higher mortality.42–44 Our findings suggest that, in the presence of mild TBI, as determined by Rotterdam and Marshall scores, VV ECMO should be considered in patients with severe respiratory failure and may even provide a protective benefit in the setting of diffuse brain injury.
Our study demonstrates that VV ECMO can be used in patients with intracranial bleeds. Two patients had new hemorrhagic findings on repeat head CT despite neither receiving heparin during their ECMO course. Intracranial bleeding during VV ECMO without heparin use could be attributed to various other mechanisms. First, it must be considered that radiographic worsening and evolution of intracranial injuries can occur spontaneously after TBI.22 In addition, renal dysfunction/failure, rapid increase in PaO2, and rapid decrease in PaCO2 following ECMO initiation have been associated with increased likelihood of ICH development.45 Consumption of coagulation factors, acquired von Willebrand's syndrome, and hyperfibrinolysis have been observed in VV ECMO patients, which may increase the propensity for hemorrhagic conversion of brain lesions.46,47 The incidence of ICH development while on ECMO appears rare, occurring in less than 4% of cases.48,49
To better understand the utility of VV ECMO in patients with TBI, future studies could assess patients with TBI and concomitant severe lung injury, comparing outcomes of those managed with VV ECMO versus conventional ARDS ventilator strategies. Furthermore, ICP analysis in a similar study group could give insight into the possibility of a neuroprotective benefit of VV ECMO for TBI patients with ARDS.
Because this is a single-center retrospective analysis, there are several limitations to consider. First, confounding through unmeasured variables could influence survival after use of VV ECMO in TBI. Second, the size of the study may not be large enough to observe statistical significance between some variables. A post hoc sample size calculation demonstrated that comparisons were underpowered to detect a difference in TBI and non-TBI survival. Using a Wald test for detecting 10% difference in survival, approximately 126 subjects would have been required. Third, this study is subject to selection bias. While guidelines exist at our institution for VV ECMO selection, the decision to cannulate is made on a case-by-case basis, which may lead to variability in selection depending on the personnel making clinical decisions. This selection process may limit broader application of these results to other institutions. Selection bias likely also exists in the mortality versus heparin-use analysis (Fig. 2). More critically ill patients with particular injury patterns may not have received heparin because of concerns for bleeding, which may explain the higher mortality observed in the nonheparinized group. Lastly, variations in both VV ECMO and trauma care that developed over the 8-year period reviewed may have affected outcomes but were not studied in this analysis.
CONCLUSION
In this series, the majority of TBI patients survived and had good neurologic outcomes despite a low admissions GCS. Venovenous extracorporeal membrane oxygenation serves as an effective strategy to rapidly correct hypoxemia and respiratory acidosis. As a result, it has the potential to minimize the occurrence of secondary brain injuries. Our findings suggest that TBI patients with a hemorrhagic component can still be considered for VV ECMO initiation. Larger, multicenter, prospective studies that assess factors including injury and TBI severity, the management of anticoagulation therapy, bleeding complications, and overall mortality to determine a potential survival benefit to VV ECMO in TBI and which TBI patients will most benefit from VV ECMO would significantly enhance the depth and scope of our findings.
AUTHORSHIP
S.E.A. was involved in study design and conceptualization, data analysis and interpretation, literature search, and original manuscript writing and revisions. S.M.G. was involved in data analysis and interpretation, and manuscript review and editing. J.E.P. was involved in data analysis and interpretation, investigation, and manuscript review and editing. W.A.T. was involved in manuscript review and editing. R.K. was involved in manuscript review and editing. D.J.H. was involved in manuscript review and editing. B.S.T. was involved in manuscript review and editing. R.B. was involved in manuscript review and editing. D.M.S. was involved in manuscript review and editing. T.M.S. was involved in manuscript review and editing. E.K.P. was involved in study visualization and conceptualization, data curation, interpretation, and analysis, study investigation, methodology, resource utilization, software use, study supervision, study validation, and manuscript review and editing.
DISCLOSURE
Conflicts of Interest: Author Disclosure forms have been supplied and are provided as Supplemental Digital Content (http://links.lww.com/TA/D284).
Footnotes
Published online: October 27, 2023.
This study was presented at EuroELSO 2023, April 28, 2023, in Lisbon, Portugal.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jtrauma.com).
Contributor Information
Samuel E. Austin, Email: sammy.austin23@gmail.com.
Samuel M. Galvagno, Email: sgalvagno@som.umaryland.edu.
Jamie E. Podell, Email: jpodell@som.umaryland.edu.
William A. Teeter, Email: William.Teeter@som.umaryland.edu.
Rishi Kundi, Email: Rkundi@som.umaryland.edu.
Daniel J. Haase, Email: DHaase@som.umaryland.edu.
Bradley S. Taylor, Email: Bradley.Taylor@som.umaryland.edu.
Richard Betzold, Email: Rdbetzold@uabmc.edu.
Deborah M. Stein, Email: dstein@som.umaryland.edu.
Thomas M. Scalea, Email: tscalea@som.umaryland.edu.
Elizabeth K. Powell, Email: elizabeth.powell@som.umaryland.edu.
REFERENCES
- 1.Haddad SH, Arabi YM. Critical care management of severe traumatic brain injury in adults. Scand J Trauma Resusc Emerg Med. 2012;20:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aisiku IP Yamal JM Doshi P Rubin ML Benoit JS Hannay J, et al. The incidence of ARDS and associated mortality in severe TBI using the Berlin definition. J Trauma Acute Care Surg. 2016;80(2):308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acute Respiratory Distress Syndrome Network, Brower RG Matthay MA Morris A Schoenfeld D Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. [DOI] [PubMed] [Google Scholar]
- 4.Chesnut RM Marshall LF Klauber MR Blunt BA Baldwin N Eisenberg HM, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34(2):216–222. [DOI] [PubMed] [Google Scholar]
- 5.Humayun M, Premraj L, Shah V, Cho SM. Mechanical ventilation in acute brain injury patients with acute respiratory distress syndrome. Front Med (Lausanne). 2022;9:999885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Della Torre V Badenes R Corradi F Racca F Lavinio A Matta B, et al. Acute respiratory distress syndrome in traumatic brain injury: how do we manage it? J Thorac Dis. 2017;9(12):5368–5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemer SN Caldeira JB Santos RG Guimaraes BL Garcia JM Prado D, et al. Effects of positive end-expiratory pressure on brain tissue oxygen pressure of severe traumatic brain injury patients with acute respiratory distress syndrome: a pilot study. J Crit Care. 2015;30(6):1263–1266. [DOI] [PubMed] [Google Scholar]
- 8.Muench E Bauhuf C Roth H Horn P Phillips M Marquetant N, et al. Effects of positive end-expiratory pressure on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation. Crit Care Med. 2005;33(10):2367–2372. [DOI] [PubMed] [Google Scholar]
- 9.Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997;112(3):759–764. [DOI] [PubMed] [Google Scholar]
- 10.Kazmi SO, Sivakumar S, Karakitsos D, Alharthy A, Lazaridis C. Cerebral pathophysiology in extracorporeal membrane oxygenation: pitfalls in daily clinical management. Crit Care Res Pract. 2018;2018:3237810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peek GJ Clemens F Elbourne D Firmin R Hardy P Hibbert C, et al. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res. 2006;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonna JE Abrams D Brodie D Greenwood JC Rubio Mateo-Sidron JA Usman A, et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021;67(6):601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds HN, Cottingham C, McCunn M, Habashi NM, Scalea TM. Extracorporeal lung support in a patient with traumatic brain injury: the benefit of heparin-bonded circuitry. Perfusion. 1999;14(6):489–493. [DOI] [PubMed] [Google Scholar]
- 14.Muellenbach RM Kredel M Kunze E Kranke P Kuestermann J Brack A, et al. Prolonged heparin-free extracorporeal membrane oxygenation in multiple injured acute respiratory distress syndrome patients with traumatic brain injury. J Trauma Acute Care Surg. 2012;72(5):1444–1447. [DOI] [PubMed] [Google Scholar]
- 15.Parker BM Menaker J Berry CD Tesoreiero RB O'Connor JV Stein DM, et al. Single center experience with veno-venous extracorporeal membrane oxygenation in patients with traumatic brain injury. Am Surg. 2021;87(6):949–953. [DOI] [PubMed] [Google Scholar]
- 16.Scalea TM Rubinson L Tran Q Jones KM Rea JH Stein DM, et al. Critical care resuscitation unit: an innovative solution to expedite transfer of patients with time-sensitive critical illness. J Am Coll Surg. 2016;222(4):614–621. [DOI] [PubMed] [Google Scholar]
- 17.Menaker J Dolly K Rector R Kufera J Lee EE Tabatabai A, et al. The lung rescue unit-does a dedicated intensive care unit for venovenous extracorporeal membrane oxygenation improve survival to discharge? J Trauma Acute Care Surg. 2017;83(3):438–442. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA Taylor R Minor BL Elliott V Fernandez M O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall LF Marshall SB Klauber MR Van Berkum Clark M Eisenberg H Jane JA, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–S292. [PubMed] [Google Scholar]
- 21.Maas AIR, Hukkelhoven CWPM, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–1182 discussion 1173-82. [DOI] [PubMed] [Google Scholar]
- 22.Podell J Yang S Miller S Felix R Tripathi H Parikh G, et al. Rapid prediction of secondary neurologic decline after traumatic brain injury: a data analytic approach. Sci Rep. 2023;13(1):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosmer DWLS, Sturdivant RX. Applied Logistic Regression. 3rd ed. Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 24.Clayton D, Hills M. Statistical Models in Epidemiology. Oxford, United Kingdom: Oxford University Press; 2013. viii, 367. [Google Scholar]
- 25.Allison PD. Fixed Effects Regression Models. Los Angeles, CA: SAGE; 2009:x, 123. [Google Scholar]
- 26.Tiruvoipati R, Botha J, Peek G. Effectiveness of extracorporeal membrane oxygenation when conventional ventilation fails: valuable option or vague remedy? J Crit Care. 2012;27(2):192–198. [DOI] [PubMed] [Google Scholar]
- 27.Guirand DM Okoye OT Schmidt BS Mansfield NJ Aden JK Martin RS, et al. Venovenous extracorporeal life support improves survival in adult trauma patients with acute hypoxemic respiratory failure: a multicenter retrospective cohort study. J Trauma Acute Care Surg. 2014;76(5):1275–1281. [DOI] [PubMed] [Google Scholar]
- 28.Robba C Ortu A Bilotta F Lombardo A Sekhon MS Gallo F, et al. Extracorporeal membrane oxygenation for adult respiratory distress syndrome in trauma patients: a case series and systematic literature review. J Trauma Acute Care Surg. 2017;82(1):165–173. [DOI] [PubMed] [Google Scholar]
- 29.Biderman P, Einav S, Fainblut M, Stein M, Singer P, Medalion B. Extracorporeal life support in patients with multiple injuries and severe respiratory failure: a single-center experience? J Trauma Acute Care Surg. 2013;75(5):907–912. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad SB, Menaker J, Kufera J, O'Connor J, Scalea TM, Stein DM. Extracorporeal membrane oxygenation after traumatic injury. J Trauma Acute Care Surg. 2017;82(3):587–591. [DOI] [PubMed] [Google Scholar]
- 31.Bosarge PL Raff LA McGwin G Jr. Carroll SL Bellot SC Diaz-Guzman E, et al. Early initiation of extracorporeal membrane oxygenation improves survival in adult trauma patients with severe adult respiratory distress syndrome. J Trauma Acute Care Surg. 2016;81(2):236–243. [DOI] [PubMed] [Google Scholar]
- 32.Madershahian N Wittwer T Strauch J Franke UFW Wippermann J Kaluza M, et al. Application of ECMO in multitrauma patients with ARDS as rescue therapy. J Card Surg. 2007;22(3):180–184. [DOI] [PubMed] [Google Scholar]
- 33.Swol J Brodie D Napolitano L Park PK Thiagarajan R Barbaro RP, et al. Indications and outcomes of extracorporeal life support in trauma patients. J Trauma Acute Care Surg. 2018;84(6):831–837. [DOI] [PubMed] [Google Scholar]
- 34.Ried M Bein T Philipp A Muller T Graf B Schmid C, et al. Extracorporeal lung support in trauma patients with severe chest injury and acute lung failure: a 10-year institutional experience. Crit Care. 2013;17(3):R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SH, Huh U, Song S, Kim MS, Wang IJ, Tak YJ. Outcomes in trauma patients undergoing veno-venous extracorporeal membrane oxygenation for acute respiratory distress syndrome. Perfusion. 2023;38(5):1037–1044. [DOI] [PubMed] [Google Scholar]
- 36.Salas De Armas IA Akkanti B Doshi PB Patel M Kumar S Akay MH, et al. Traumatic respiratory failure and veno-venous extracorporeal membrane oxygenation support. Perfusion. 2022;37(5):477–483. [DOI] [PubMed] [Google Scholar]
- 37.Coles JP Minhas PS Fryer TD Smielewski P Aigbirihio F Donovan T, et al. Effect of hyperventilation on cerebral blood flow in traumatic head injury: clinical relevance and monitoring correlates. Crit Care Med. 2002;30(9):1950–1959. [DOI] [PubMed] [Google Scholar]
- 38.Mascia L Zavala E Bosma K Pasero D Decaroli D Andrews P, et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007;35(8):1815–1820. [DOI] [PubMed] [Google Scholar]
- 39.Ventetuolo CE, Muratore CS. Extracorporeal life support in critically ill adults. Am J Respir Crit Care Med. 2014;190(5):497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besen B, Romano TG, Zigaib R, Mendes PV, Melro LMG, Park M. Oxygen delivery, carbon dioxide removal, energy transfer to lungs and pulmonary hypertension behavior during venous-venous extracorporeal membrane oxygenation support: a mathematical modeling approach. Rev Bras Ter Intensiva. 2019;31(2):113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munoz-Bendix C, Beseoglu K, Kram R. Extracorporeal decarboxylation in patients with severe traumatic brain injury and ARDS enables effective control of intracranial pressure. Crit Care. 2015;19:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charry JD Falla JD Ochoa JD Pinzon MA Tejada JH Henriquez MJ, et al. External validation of the Rotterdam computed tomography score in the prediction of mortality in severe traumatic brain injury. J Neurosci Rural Pract. 2017;8(Suppl 1):S23–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munakomi S. A comparative study between Marshall and Rotterdam CT scores in predicting early deaths in patients with traumatic brain injury in a major tertiary care hospital in Nepal. Chin J Traumatol. 2016;19(1):25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishra R Ucros H Florez-Perdomo W, et al. Predictive value of Rotterdam score and Marshall score in traumatic brain injury: a contemporary review. Indian J Neurotrauma. 2022;19:9–077. [Google Scholar]
- 45.Luyt CE Brechot N Demondion P Jovanovic T Hekimian G Lebreton G, et al. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med. 2016;42(5):897–907. [DOI] [PubMed] [Google Scholar]
- 46.Yang S, Williams B, Kaczorowski D, Mazzeffi M. Overt disseminated intravascular coagulation with severe hypofibrinogenemia during veno-venous extracorporeal membrane oxygenation. J Extra Corpor Technol. 2022;54(2):148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boukobza M, Ehmer C, Baud F, Laissy JP. Unusual pattern of cerebral microbleeds and petechial hemorrhages after veno-arterial extracorporeal membrane oxygenation support. A report of 2 cases. J Stroke Cerebrovasc Dis. 2021;30(7):105792. [DOI] [PubMed] [Google Scholar]
- 48.Lorusso R Gelsomino S Parise O Di Mauro M Barili F Geskes G, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the extracorporeal life support organization database. Crit Care Med. 2017;45(8):1389–1397. [DOI] [PubMed] [Google Scholar]
- 49.Nasr DM, Rabinstein AA. Neurologic complications of extracorporeal membrane oxygenation. J Clin Neurol. 2015;11(4):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]


