Abstract
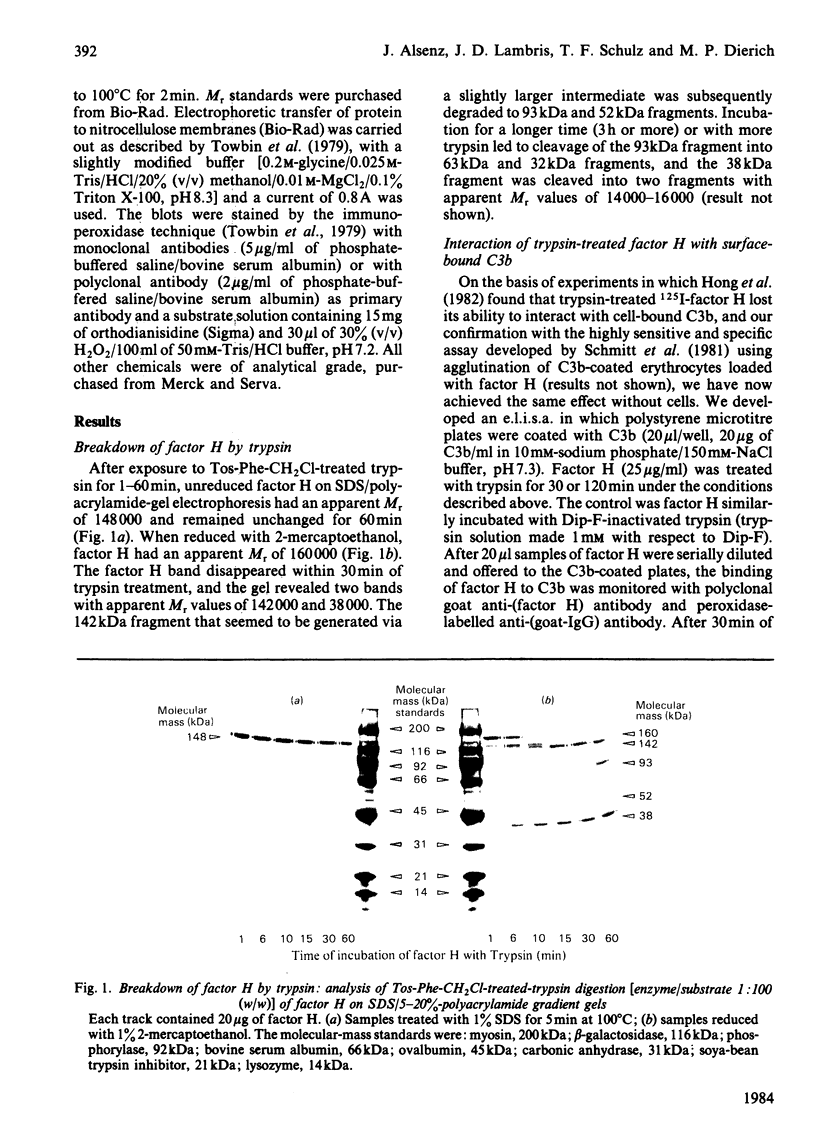
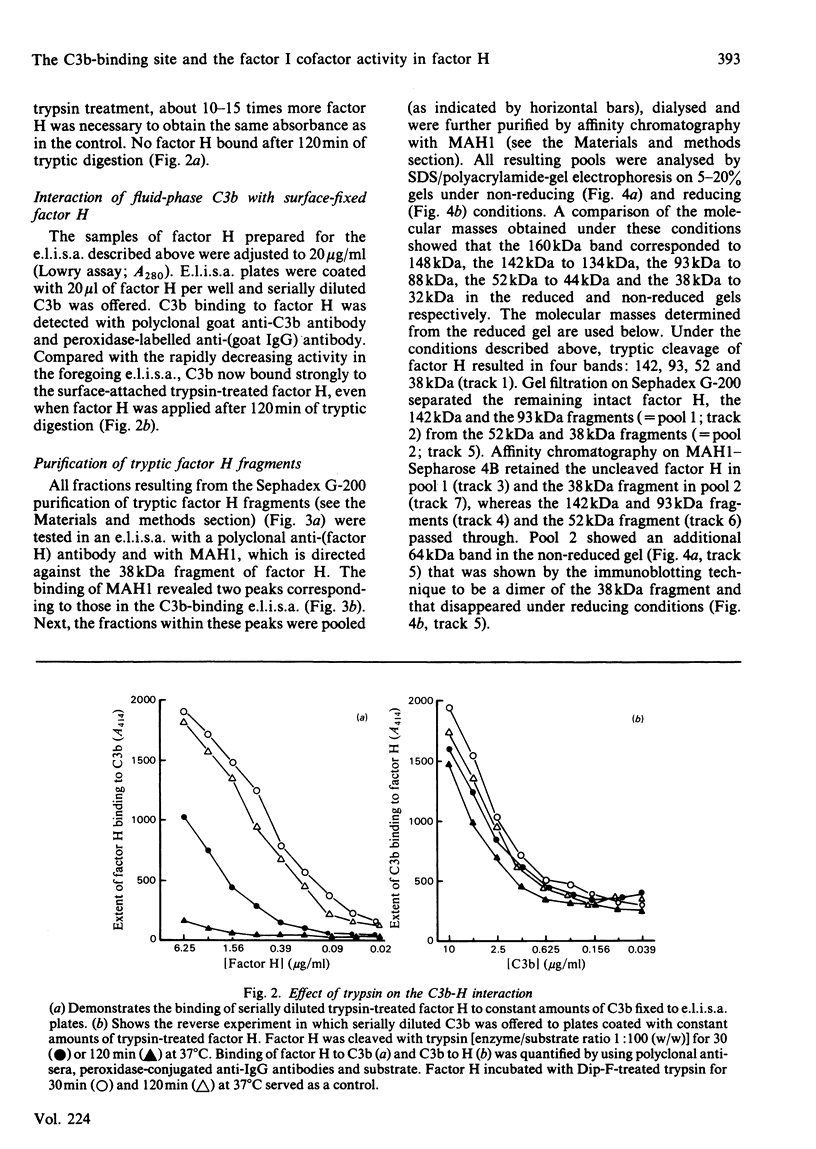
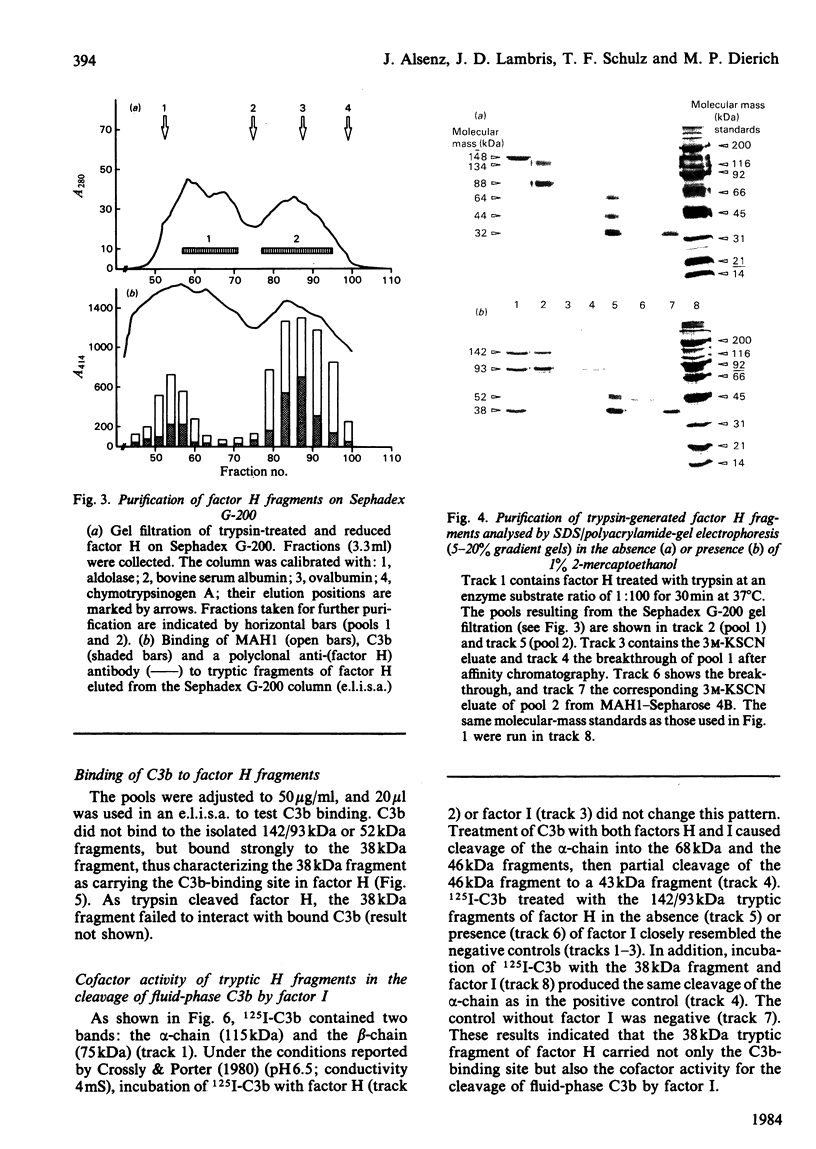
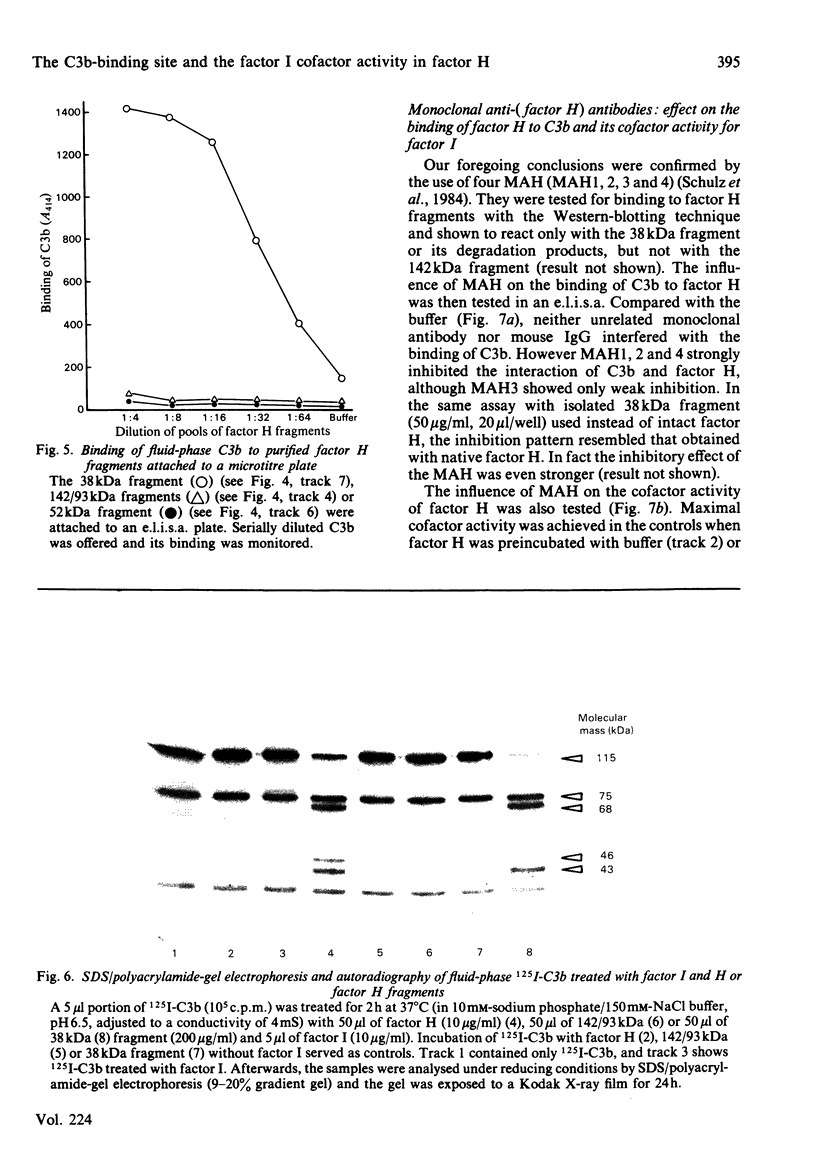
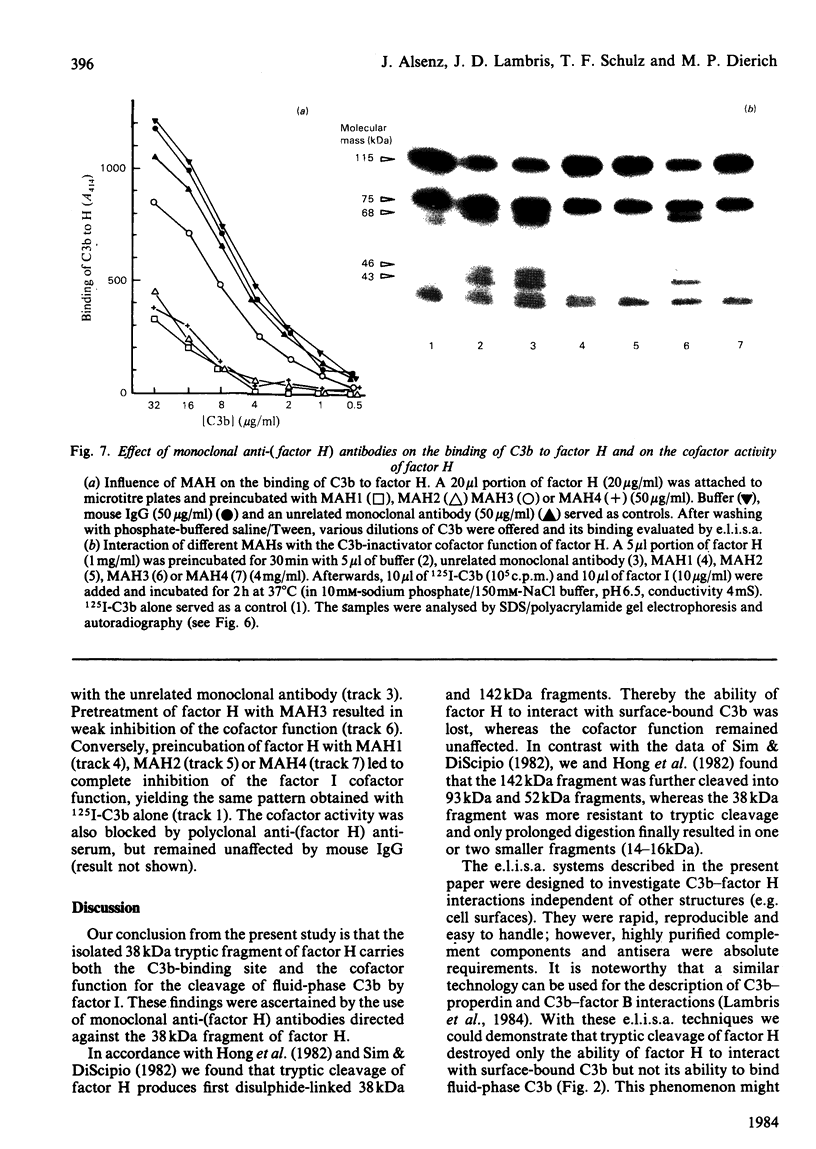
Trypsin treatment of human factor H (H160) [enzyme/substrate ratio 1:100 (w/w), 30 min, 37 degrees C] generated a 38 kDa (H38) and a 142 kDa (H142) fragment linked by disulphide bonds (H38/142). The fragments were purified by reduction with 2-mercapto-ethanol, gel filtration on a Sephadex G-200 column and affinity chromatography with monoclonal anti-(factor H) antibody coupled to Sepharose 4B. This monoclonal antibody bound to a site in the 38 kDa fragment. To localize the C3b binding site in factor H we used two enzyme-linked immunosorbent assays (e.l.i.s.a.). For the first test, e.l.i.s.a. plates were coated with C3b; H160, H38/142, H38 and H142 were added, and their binding was monitored by goat anti-(factor H) and peroxidase-labelled rabbit anti-goat antibodies. Only intact factor H bound to the C3b-coated plates. For the second test, e.l.i.s.a. plates were coated with comparable amounts of factor H or its fragments, and C3b was offered at several dilutions. In contrast with the results from the first assay, C3b bound to intact factor H, H38/142 and H38 but not to H142, thus characterizing H38 as the fragment carrying the C3b-binding site. To identify the fragment responsible for the cofactor activity of factor H (cleavage of fluid-phase C3b by factor I), 125I-C3b was incubated with either H38 or H142 and factor I. H142 had no cofactor activity, whereas H38 had the same cofactor function as intact H. To further investigate the relationship between the C3b-binding site and the site of factor H essential for its cofactor activity, we made use of monoclonal antibodies directed against the H38. Those antibodies inhibiting the binding of C3b to H160 also inhibited the cofactor function, whereas those without effect on the C3b binding also did not interfere with the cofactor activity. This suggests that the C3b-binding site and the site essential for the cofactor activity of factor H are both localized in the 38 kDa tryptic fragment of factor H in close proximity or are identical.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitter-Suermann D., Burger R., Hadding U. Activation of the alternative pathway of complement: efficient fluid-phase amplification by blockade of the regulatory complement protein beta1H through sulfated polyanions. Eur J Immunol. 1981 Apr;11(4):291–295. doi: 10.1002/eji.1830110405. [DOI] [PubMed] [Google Scholar]
- Bokisch V. A., Müller-Eberhard H. J., Cochrane C. G. Isolation of a fragment (C3a) of the third component of human complement containing anaphylatoxin and chemotactic activity and description of an anaphylatoxin inactivator of human serum. J Exp Med. 1969 May 1;129(5):1109–1130. doi: 10.1084/jem.129.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad D. H., Carlo J. R., Ruddy S. Interaction of beta1H globulin with cell-bound C3b: quantitative analysis of binding and influence of alternative pathway components on binding. J Exp Med. 1978 Jun 1;147(6):1792–1805. doi: 10.1084/jem.147.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley L. G., Porter R. R. Purification of the human complement control protein C3b inactivator. Biochem J. 1980 Oct 1;191(1):173–182. doi: 10.1042/bj1910173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierich M. P., Scheiner O., Mussel H. H., Hamman K. P., Schopf R. E., Schulz T. Characterization of complement receptors. Mol Immunol. 1982 Oct;19(10):1255–1265. doi: 10.1016/0161-5890(82)90291-7. [DOI] [PubMed] [Google Scholar]
- Discipio R. G., Hugli T. E. Circular dichroism studies of human factor H. A regulatory component of the complement system. Biochim Biophys Acta. 1982 Dec 6;709(1):58–64. doi: 10.1016/0167-4838(82)90421-6. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gardner W. D., White P. J., Hoch S. O. Identification of a major human serum DNA-binding protein as beta 1H of the alternative pathway of complement activation. Biochem Biophys Res Commun. 1980 May 14;94(1):61–67. doi: 10.1016/s0006-291x(80)80187-2. [DOI] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. An improved purification procedure for the third component of complement and beta 1H globulin from human serum. Mol Immunol. 1979 Oct;16(10):767–776. doi: 10.1016/0161-5890(79)90154-8. [DOI] [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. The physiological breakdown of the third component of human complement. Mol Immunol. 1980 Jan;17(1):9–20. doi: 10.1016/0161-5890(80)90119-4. [DOI] [PubMed] [Google Scholar]
- Hong K., Kinoshita T., Dohi Y., Inoue K. Effect of trypsinization on the activity of human factor H. J Immunol. 1982 Aug;129(2):647–652. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambris J. D., Alsenz J., Schulz T. F., Dierich M. P. Mapping of the properdin-binding site in the third component of complement. Biochem J. 1984 Jan 1;217(1):323–326. doi: 10.1042/bj2170323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris J. D., Dobson N. J., Ross G. D. Release of endogenous C3b inactivator from lymphocytes in response to triggering membrane receptors for beta 1H globulin. J Exp Med. 1980 Dec 1;152(6):1625–1644. doi: 10.1084/jem.152.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambris J. D., Ross G. D. Characterization of the lymphocyte membrane receptor for factor H (beta 1H-globulin) with an antibody to anti-factor H idiotype. J Exp Med. 1982 May 1;155(5):1400–1411. doi: 10.1084/jem.155.5.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Chemistry and reaction mechanisms of complement. Adv Immunol. 1968;8:1–80. doi: 10.1016/s0065-2776(08)60464-2. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Götze O. C3 proactivator convertase and its mode of action. J Exp Med. 1972 Apr 1;135(4):1003–1008. doi: 10.1084/jem.135.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Schreiber R. D. Molecular biology and chemistry of the alternative pathway of complement. Adv Immunol. 1980;29:1–53. doi: 10.1016/s0065-2776(08)60042-5. [DOI] [PubMed] [Google Scholar]
- NILSSON U. R., MUELLER-EBERHARD H. J. ISOLATION OF BETA IF-GLOBULIN FROM HUMAN SERUM AND ITS CHARACTERIZATION AS THE FIFTH COMPONENT OF COMPLEMENT. J Exp Med. 1965 Aug 1;122:277–298. doi: 10.1084/jem.122.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaki K., Iida K., Okubo M., Inai S. Reaction mechanisms of beta1H globulin. Int Arch Allergy Appl Immunol. 1978;57(3):221–232. doi: 10.1159/000232106. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Complement C3 convertase: cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977 Jul 1;146(1):257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Gagnon J. Human C4-binding protein: N-terminal amino acid sequence analysis and limited proteolysis by trypsin. FEBS Lett. 1982 Jan 11;137(1):75–79. doi: 10.1016/0014-5793(82)80318-9. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Schmitt M., Mussel H. H., Dierich M. P. beta 1H-hemagglutination assay: a highly sensitive test for human beta 1H and its uses. J Immunol Methods. 1981;47(1):75–85. doi: 10.1016/0022-1759(81)90258-1. [DOI] [PubMed] [Google Scholar]
- Schmitt M., Mussel H. H., Hammann K. P., Scheiner O., Dierich M. P. Role of beta 1H for the binding of C3b-coated particles to human lymphoid and phagocytic cells. Eur J Immunol. 1981 Oct;11(10):739–745. doi: 10.1002/eji.1830111002. [DOI] [PubMed] [Google Scholar]
- Schulz T. F., Scheiner O., Alsenz J., Lambris J. D., Dierich M. P. Use of monoclonal antibodies against factor H to investigate the role of a membrane-associated protein antigenically related to H in C3b-receptor function. J Immunol. 1984 Jan;132(1):392–398. [PubMed] [Google Scholar]
- Sim R. B., DiScipio R. G. Purification and structural studies on the complement-system control protein beta 1H (Factor H). Biochem J. 1982 Aug 1;205(2):285–293. doi: 10.1042/bj2050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of C3b hemolytic activity by a plasma protein distinct from C3b inactivator. Science. 1976 Sep 10;193(4257):1011–1013. doi: 10.1126/science.948757. [DOI] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]