Abstract
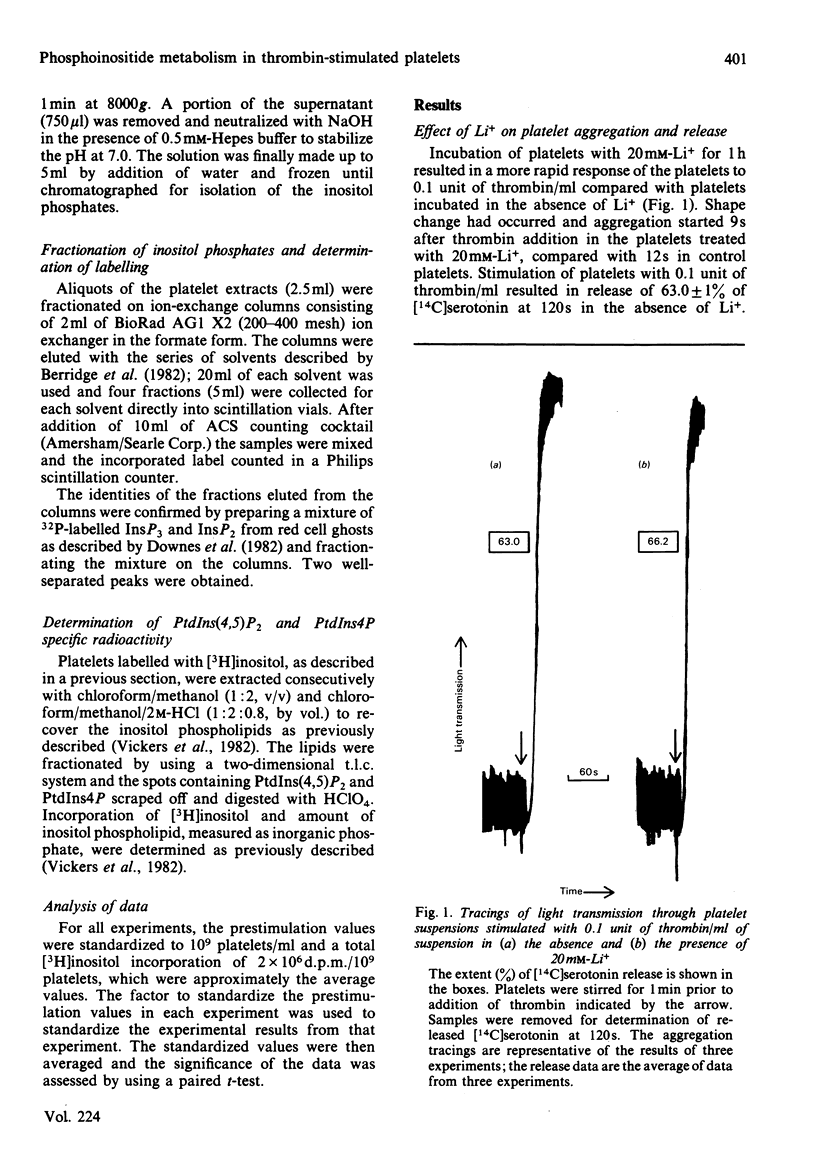
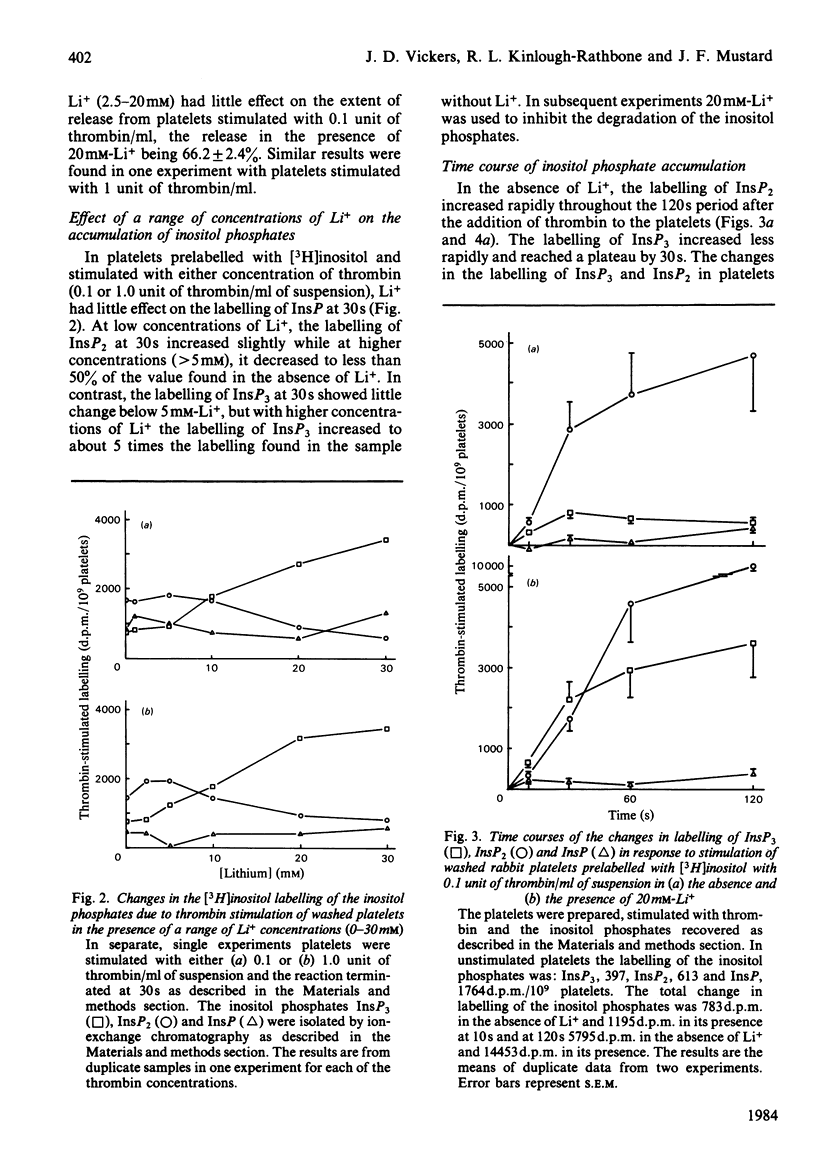
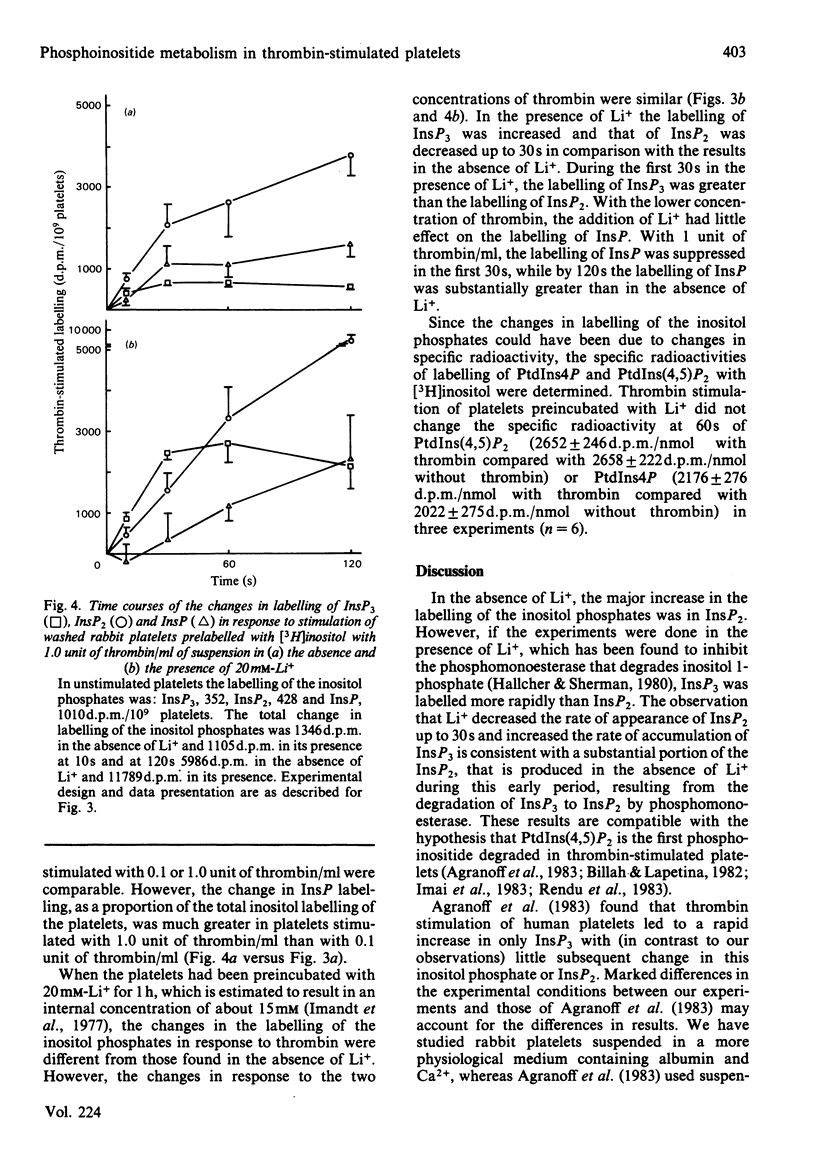
Experiments with washed rabbit platelets demonstrate that stimulation with a low concentration of thrombin (0.1 unit/ml), that causes maximal aggregation and partial release of amine granule contents, also causes increased accumulation of [3H]inositol-labelled inositol trisphosphate (InsP3) in the presence of 20 mM-Li+. This concentration of Li+ was found to inhibit the degradation of inositol phosphates by phosphomonoesterases. This result indicates that phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] is degraded early after platelet stimulation with thrombin, although in a previous study we had found no decrease in amount. In the absence of Li+, the labelling of inositol bisphosphate (InsP2) increased more rapidly than that of InsP3, consistent with rapid degradation of InsP3 by phosphomonoesterase. After 30s the increase in InsP2 was augmented by Li+. This increase in InsP2 could have been due to increased degradation of phosphatidylinositol 4-phosphate or inhibition of breakdown of InsP2 to InsP with a lesser inhibition of breakdown of InsP3 to InsP2. The effect on InsP3 and InsP2 of stimulation of the platelets with 1.0 unit of thrombin/ml was comparable with the effect of the lower concentration of thrombin. Inositol phosphate (InsP) labelling did not increase in response to 0.1 unit of thrombin/ml, but increased when the platelets were stimulated with 1.0 unit of thrombin/ml. Whether the increase in InsP was due to increased degradation of phosphatidylinositol or a greater rate of breakdown of InsP2 to InsP than InsP to inositol cannot be determined in these experiments. These results indicate that degradation of PtdIns(4,5)P2 is an early event in platelet activation by thrombin and that formation of inositol phosphates and 1,2-diacylglycerol rather than a decrease in PtdIns(4,5)P2 may be the important change.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Akhtar R. A., Hawthorne J. N. Acetylcholine increases the breakdown of triphosphoinositide of rabbit iris muscle prelabelled with [32P] phosphate. Biochem J. 1977 Jan 15;162(1):61–73. doi: 10.1042/bj1620061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Ardlie N. G., Perry D. W., Packham M. A., Mustard J. F. Influence of apyrase on stability of suspensions of washed rabbit platelets. Proc Soc Exp Biol Med. 1971 Apr;136(4):1021–1023. doi: 10.3181/00379727-136-35419. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J. M., Butler A. M., Peterson D. A., White J. G. Phosphatidic acid releases calcium from a platelet membrane fraction in vitro. Prostaglandins Med. 1978 Nov;1(5):387–396. doi: 10.1016/0161-4630(78)90125-8. [DOI] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Holmsen H., Dangelmaier C. A., Holmsen H. K. Thrombin-induced platelet responses differ in requirement for receptor occupancy. Evidence for tight coupling of occupancy and compartmentalized phosphatidic acid formation. J Biol Chem. 1981 Sep 25;256(18):9393–9396. [PubMed] [Google Scholar]
- Imai A., Nakashima S., Nozawa Y. The rapid polyphosphoinositide metabolism may be a triggering event for thrombin-mediated stimulation of human platelets. Biochem Biophys Res Commun. 1983 Jan 14;110(1):108–115. doi: 10.1016/0006-291x(83)91267-6. [DOI] [PubMed] [Google Scholar]
- Imandt L., Genders T., Wessels H., Haanen C. The effect of lithium on platelet aggregation and platelet release reaction. Thromb Res. 1977 Sep;11(3):297–308. doi: 10.1016/0049-3848(77)90183-9. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Mustard J. F. Changes in 32P-content of phosphatidic acid and the phosphoinositides of rabbit platelets during aggregation induced by collagen or thrombin. Br J Haematol. 1974 Feb;26(2):243–253. doi: 10.1111/j.1365-2141.1974.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Packham M. A., Guccione M. A., Greenberg J. P., Kinlough-Rathbone R. L., Mustard J. F. Release of 14C-serotonin during initial platelet changes induced by thrombin, collagen, or A23187. Blood. 1977 Nov;50(5):915–926. [PubMed] [Google Scholar]
- Packham M. A., Guccione M. A., Nina M., Kinlough-Rathbone R. L., Mustard J. F. Effects of tris on responses of human and rabbit platelets to aggregating agents. Thromb Haemost. 1984 Apr 30;51(2):140–144. [PubMed] [Google Scholar]
- Putney J. W., Jr, Burgess G. M., Halenda S. P., McKinney J. S., Rubin R. P. Effects of secretagogues on [32P]phosphatidylinositol 4,5-bisphosphate metabolism in the exocrine pancreas. Biochem J. 1983 May 15;212(2):483–488. doi: 10.1042/bj2120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendu F., Marche P., Maclouf J., Girard A., Levy-Toledano S. Triphosphoinositide breakdown and dense body release as the earliest events in thrombin-induced activation of human platelets. Biochem Biophys Res Commun. 1983 Oct 31;116(2):513–519. doi: 10.1016/0006-291x(83)90553-3. [DOI] [PubMed] [Google Scholar]
- Rittenhouse S. E. Human platelets contain phospholipase C that hydrolyzes polyphosphoinositides. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5417–5420. doi: 10.1073/pnas.80.17.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse S. E. Inositol lipid metabolism in the responses of stimulated platelets. Cell Calcium. 1982 Oct;3(4-5):311–322. doi: 10.1016/0143-4160(82)90019-7. [DOI] [PubMed] [Google Scholar]
- Vickers J. D., Kinlough-Rathbone R. L., Mustard J. F. Changes in phosphatidylinositol-4,5-bisphosphate 10 seconds after stimulation of washed rabbit platelets with ADP. Blood. 1982 Dec;60(6):1247–1250. [PubMed] [Google Scholar]
- Vickers J. D., Kinlough-Rathbone R. L., Mustard J. F. Changes in the platelet phosphoinositides during the first minute after stimulation of washed rabbit platelets with thrombin. Biochem J. 1984 Apr 1;219(1):25–31. doi: 10.1042/bj2190025. [DOI] [PMC free article] [PubMed] [Google Scholar]


