Abstract
Background:
Since the beginning of the SARS-CoV-2 pandemic, there have been mutations caused by new SARS-CoV-2 variants, such as Alpha, Beta, Gamma, Delta, and Omicron, recognized as the VOC worldwide. These variants can affect vaccine efficacy, disease control, and treatment effectiveness. The present study aimed to evaluate the levels of total and neutralizing antibodies produced by PastoCoAd vaccine candidates against the VOC strains at different time points.
Methods:
Two vaccine candidates were employed against SARS-CoV-2 using adenoviral vectors: prime only (a mixture of rAd5-S and rAd5 RBD-N) and heterologous prime-boost (rAd5-S/SOBERANA vaccine). The immunogenicity of these vaccine candidates was assessed in mouse, rabbit, and hamster models using ELISA assay and virus neutralization antibody test.
Results:
The immunogenicity results indicated a significant increase in both total and neutralizing antibodies titers in the groups receiving the vaccine candidates at various time points compared to the control group (p < 0.05). The results also showed that the PastoCoAd vaccine candidates Ad5 S & RBD-N and Ad5 S/SOBERANA could neutralize the VOC strains in the animal models.
Conclusion:
The ability of vaccine candidate to neutralize the VOC strains in animal models by generating neutralizing antibodies at different time points may be attributed to the use of the platform based on the Adenoviral vector, the N proteins in the Ad5 S & RBD-N vaccine candidate, and the SOBERANA Plus booster in the Ad5 S/SOBERANA vaccine candidate.
Key Words: COVID-19 vaccines, Mutation, Nucleocapsid, Spike protein
INTRODUCTION
COVID-19 has been one of the significant challenges worldwide in recent decades[1,2]. Since the beginning of the SARS-CoV-2 pandemic, mutations have globally emerged by new SARS-CoV-2 variants such as Alpha, Beta, Gamma, Delta, and Omicron as the VOC. These variants limited the effect of vaccines and the efficacy of treatments[3,4].
Based on available evidence, S protein plays a critical role in the immune response and vaccine development[5]. Moreover, it is the main factor in the entry of SARS CoV-2 into the host cell and contains immunodominant epitopes. Studies have documented that the N protein successfully increases the production of specific anti-SARS-CoV-2 IgG antibodies in animal models, especially in the bronchus-associated lymphoid tissues of rats[6,7].
The simultaneous use of the S and N proteins as a platform may synergistically affect the immune responses and can be employed as a practical strategy against the new strains of SARS-CoV-2[8,9]. Johnson & Johnson, AstraZeneca, and Sputnik have utilized the replication-deficient adenoviral vector platform to develop the COVID-19 vaccine[10]. This platform is one of the most powerful platforms, remarkably influences the immune system[11]. Accordingly, the PastoCoAd candidate vaccines were developed as Ad5-based vaccines, containing the S and N genes, against the SARS-CoV-2. Their potency, toxicity, and protection efficacy were evaluated[9].
In the present study, the levels of total antibody and neutralizing antibodies against the Wuhan, Beta, Delta, and Omicron strains of SARS-CoV-2 were evaluated using PastoCoAd candidate vaccines at different time points.
MATERIALS AND METHODS
Animal studies
All animal models (mouse, rabbit, and hamster) were assigned to four groups. Groups 1 and 2 included mock (PBS) and Ad5 empty (negative control), respectively. Group 3 consisted of Ad5 S/Boster SOBERANA, two-dose regimen (heterologous prime-boost), with the first dose of the rAd5-S candidate vaccine and the second dose of the SOBERANA vaccine (recombinant dimeric RBD protein)[12] in a 28-day interval. The last group comprised Ad5 mix S & RBD-N single dose, a single-dose regimen (prime only) including a combination of rAd5-S and rAd5 RBD-N. The use of a recombinant protein-based vaccine as a second dose significantly raised antibody levels; thus, the SOBERANA vaccine was used as a recombinant dimeric RBD protein in some groups (Table 1). In each group, there were seven mice, six rabbits, and seven hamsters. All animals, including female and male mice (aged 6-8 weeks), female rabbits (weighing 2.5-3.5 kg), and golden Syrian hamsters (aged 9-11 weeks), were purchased from the Animal Production Center at the Pasteur Institute of Iran (Tehran). The animals were fully vaccinated with the PastoCoAd candidate vaccines. The hamster blood samples were obtained 28 and 42 days post-immunization. The mice's blood samples were also collected from the venous sinus 3 and 6 months post-immunization. The rabbits’ blood samples were taken 3 and 6 months post immunization for follow-up and 28 days after the booster injection in the ninth month. The blood samples were centrifuged at 1000 ×g for 5 min, and the sera were stored at -20 °C for further studies.
Table 1.
Animal groups and dosage of PastoCoAd vaccines
| Group | Mouse | Rabbit | Hamster | |||||
|---|---|---|---|---|---|---|---|---|
| Name | DC |
DC2
prime |
DC2 boost |
DC2
prime |
DC2 boost | DC2 boost | DC2 prime | DC2 boost |
| G1 | PBS (Mock) | PBS | -- | PBS | -- | -- | PBS | -- |
| G2 | rAd5 empty | 108 VP | -- | 109 VP | -- | -- | 1010 VP | -- |
| G3 | rAd5-S / SOBERANA |
108 VP | 10 µg | 109 VP | 30 µg | 30 µg | 1010 VP | 20 µg |
| G4 | rAd5-S & rAd5RBD-N mix | 5× 107 VP & 5× 107 VP | -- | 5× 108 VP & 5× 108 VP | -- | -- | 5× 109 VP & 5× 109 VP | -- |
DC: dose content; VP: viral particles; G: group
Preparation of viral strains
Viral strains of SARS-CoV-2 were prepared as described previously[8]. All procedures with the viruses were conducted using a biosafety level 3 facility according to the World Health Organization’s recommendations.
Immunogenicity studies
ELISA assay was used in the third and sixth months in the mice group and in the third, sixth, and ninth months in the rabbit group to evaluate total IgG antibody titters. Since the SARS-CoV-2 virus genome is one of the most mutable genomes, Alpha, Beta, Gamma, Delta, and Omicron variants were isolated shortly after our initial study[9]. In the following, the serum samples were tested on Beta, Delta, and Omicron variants (Table 2). These strains were received from the COVID-19 National Reference Laboratory at the Pasteur Institute of Iran. In brief, 2.5 μg/mL of recombinant S and N proteins (purchased from Sina Biotechnology Knowledge-Based Company, Iran) were used for plate coating and incubated at 4 °C for 18 hours. The blocking step was then performed by adding 5% skim milk (dissolved in PBS; pH 7.4) at 37 °C for one hour. Next, the diluted sera (1:100 and 1:500) in PBS containing 5% skim milk were added and incubated at 37 °C for one hour. Afterwards, horseradish peroxidase-conjugated anti-mouse IgG was added and incubated at room temperature for one hour. Following washing the plate with PBS-Tween, PBST and TMB substrates were added and incubated for 10 min. Finally, the reaction was stopped by adding sulfuric acid (H2SO4), and the optical density was measured at 450 and 630 nm.
Table 2.
The timeline of evaluating the humoral immune responses against the PastoCoAd vaccines in animal groups
|
Animal
|
Mouse | Rabbit | Hamster | ||||
|---|---|---|---|---|---|---|---|
| Timeline (day) | 90 | 180 | 90 | 180 | 270 | 28 | 42 |
| Antibody type | |||||||
| Total IgG | + | + | + | + | + | - | - |
| IgG1/2a | + | + | + | + | + | - | - |
| SARS-CoV-2 variants | |||||||
| Beta | - | - | - | - | - | + G (1, 2, 4) | + G (3) |
| Delta | + | + | + | + | - | + G (1, 2, 4) | + G (3) |
| Omicron | - | - | - | + | + | - | - |
G: group; +: test done, -: test not done
SARS-CoV-2 neutralizing antibody
The conventional VNT titer was used to confirm the presence of neutralizing antibodies in the serum of animals. The serum of the hamsters (on day 28 for groups 1, 2, and 4 and on day 42 for group 3) was examined against the Beta and Delta variants of SARS-CoV-2. The sera of the mice and rabbit groups were examined against the Delta variant at three and six months. Moreover, the sera of the rabbits at months 6 and 10 were examined against the Omicron variant after a booster dose in the ninth month. In summary, the serum samples were incubated at 56 °C for 30 minutes to inactivate complement system. After preparing the two-fold serial dilution of the serum samples and exposing them to an equal amount of the 50% Tissue Culture Infectious Dose of SARS-CoV-2, the resulting mixture was placed in an incubator with 5% CO2 at 37 °C for 90 min. Subsequently, 100 µL of the mixture from the previous step was added to monolayer Vero cells at 37 °C for one hour. An hour after the infection, the inoculum was replenished with fetal bovine serum-free DMEM supplemented with penicillin-streptomycin and then incubated for 72 hours. The test was repeated three times in each dilution, and antibody neutralization was assessed based on the virus-induced cytopathic effect.
Statistical analysis
The statistical analysis was performed using GraphPad Prism software version 8.12 (GraphPad Software Inc., San Diego, USA). Moreover, one-way ANOVA, Mann-Whitney U test, and Spearman's correlation tests were used to compare the follow-up criteria among the animal groups and the neutralizing antibody data. The significance level was p < 0.05 at a 95% confidence interval.
RESULTS
Immunogenicity results
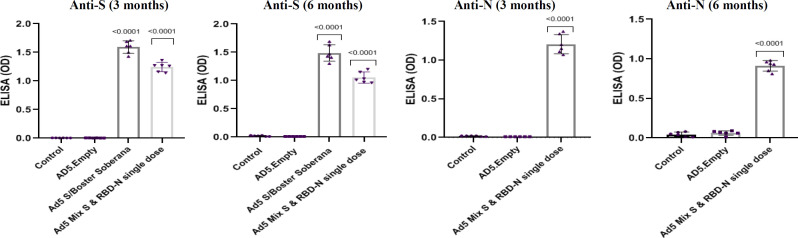
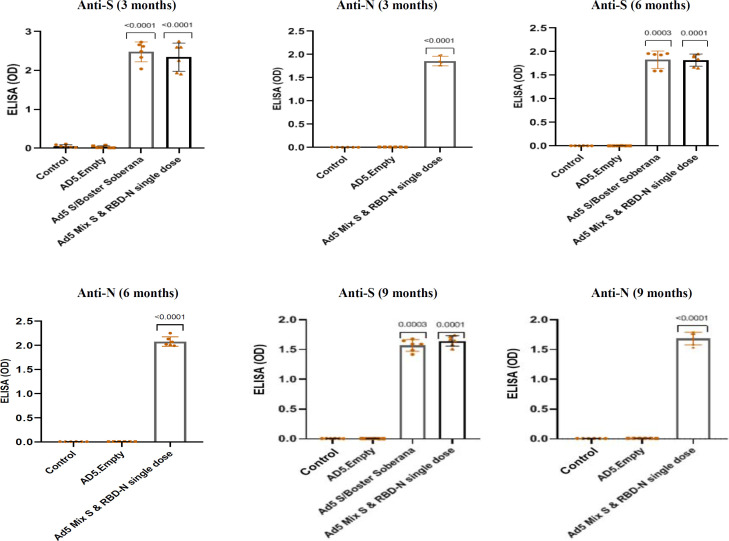
The total anti-spike IgG and total anti-nucleocapsid IgG were investigated using ELISA assay at three and six months for the mice and three, six, and nine months for rabbits. The results showed a significant increase in total IgG and antibody titer in groups receiving the vaccine, compared to the two control groups (placebo and Ad5 empty; p < 0.05). The mice and rabbits were vaccinated with a single dose of Ad5-S/booster SOBERANA and Ad5 mix-S & RBD-N. The vaccination resulted in a significantly stronger IgG response compared to the control groups. Figures 1 and 2 illustrate the results of sera diluted at 1:100 for the mice and 1:500 for rabbits.
Fig. 1.
Humoral immune responses in the vaccinated BALB/c mice. ELISA was performed 3 and 6 months after the inoculation of Ad5-S or RBD-N and SOBERANA as a booster dose. S-specific and N-total antibodies were assessed. Serum samples be diluted at least 1:100. Each dotted graph represents a replicate sample in the mice group (p < 0.05, two-sided Mann-Whitney U test). AD5.Empty (rAd5 empty), Ad5 S/Boster Soberana (rAd5 S/SOBERANA), Ad5 Mix S & RBD-N (rAd5-S & rAd5 RBD-N mix); OD: optical density
Fig. 2.
Humoral immune responses in the vaccinated rabbits. ELISA was performed 3, 6, and 9 months after the inoculation of Ad5-S or RBD-N and SOBERANA as a booster dose. S-specific and N-total antibodies were assessed. Serum samples be diluted at least 1:100. Each dotted graph represents a replicate sample in the rabbit group (p<0.05, two-sided Mann-Whitney U test). AD5.Empty (rAd5 empty), Ad5 S/Boster Soberana (rAd5 S/SOBERANA), Ad5 Mix S & RBD-N (rAd5-S & rAd5 RBD-N mix); OD: optical density
Virus neutralization antibody test
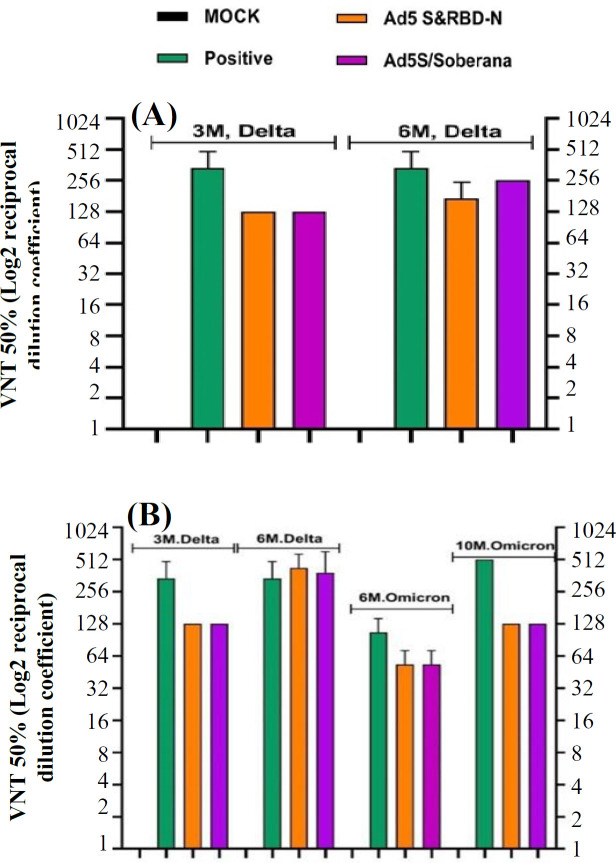
The VNT test results showed that the control groups were unable to neutralize the virus and induce cytopathic effects in all dilutions. In hamsters, virus neutralization (the Beta variant) was observed in all vaccinated groups at the 1:32 dilution for AD5 S/N (rAd5-S/rAd5 RBD-N), and the 1:64 dilution for AD5 S/SOBERANA (rAd5 S/SOBERANA), and AD5 S &RBD-N (rAd5-S & rAd5 RBD-N mixture). Furthermore, the serum dilutions of 1:64 in groups receiving rAd5 S/rAd5 RBD-N and a mixture of rAd5-S/rAd5 RBD-N and the serum dilution of 1:256 in rAd5 S/RBD SOBERANA neutralized the Delta variant of virus (Fig. 3). In mice, virus neutralization (the Delta variant) by neutralizing antibodies was observed in both prime-only and prime-boost groups at the 1:128 dilution in month three, as well as at the 1:128 and 1:256 dilutions in the same groups at three and six months, respectively (Fig. 4A). In the rabbits, the serum dilutions of 1:128 and 1:256 neutralized the Delta variant in months three and six, respectively. However, in the Omicron strain, virus neutralization by neutralizing antibodies was observed in both vaccinated groups at the 1:64 dilution at month 6 and 1:128 dilution at month 10 (28 days after the booster dose; Fig. 4B).
Fig. 3.
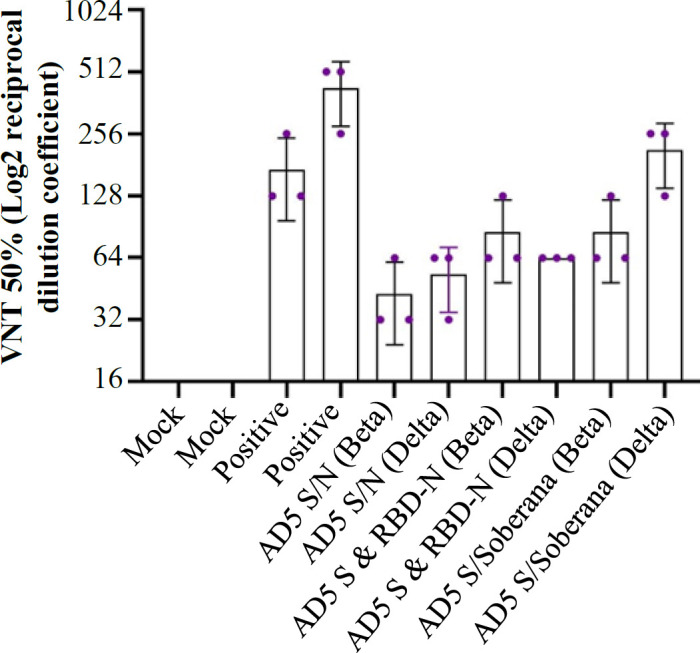
VNT test results for the Beta and Delta variants in different dilutions for all groups of hamsters 4 weeks after the first injection, with negative and positive control. AD5 (rAd5 empty), AD5 S/N (rAd5-S/rAd5 RBD-N), AD5 S/SO (rAd5 S/SOBERANA), and AD5 S & N (rAd5-S & rAd5 RBD-N mix)
Fig. 4.
VNT test results for the Omicron and Delta variants in different dilutions for all groups of mice (A) and rabbits (B) 3 and 6 months after the first injection, with negative and positive controls. AD5 (rAd5 empty), AD5 S/SO (rAd5-S/SOBERANA), and AD5 S&N (rAd5-S & rAd5 RBD-N mix)
Discussion
Mutation in the SARS-CoV-2 genome can lead to the virus evasion of neutralizing antibodies[13], which in turn affects vaccine efficacy and virus circulation in the community[14,15]. In the initial vaccine study using the hamster model, a neutralizing antibody test was conducted for the Wuhan strain. The results revealed that the virus was neutralized at the 1:64 serum dilution in all three groups studied[9]. The VNTs on the Beta and Delta strains were performed using the same hamster serums in our previous study[9].
The results of our study demonstrated that the titer of neutralizing antibodies decreased by 1:32 for Beta and Delta variants in the Ad5 S/N group. In the Ad5 S & RBD-N single-dose and Ad5 S/SOBERANA cases, the neutralizing antibody titer against the Beta and Delta strains was about 1:64, similar to the titer used against Wuhan strain, which indicates no reduction in the neutralizing antibody titer. The observations from the Ad5 S/SOBERANA group showed that the neutralizing antibody titer against the Beta strain was about 1:64, which was the same as the original strain. However, it increased to 1:256 against the Delta strain, suggesting the effectiveness of the vaccine candidate against the Delta strain compared to Ad5 S & RBD-N and mock groups. In mouse groups receiving Ad5 S & RBD-N and Ad5 S/SOBERANA, the VNT against the Delta strain in the third and sixth months showed that the Ad5 S & RBD-N recipient group neutralized the Delta strain at a 1:128 dilution, similar to the original strain at the above-mentioned times. In the rabbit model, the VNT was performed against the Delta and Omicron strains in the Ad5 S & RBD-N and Ad5 S/SOBERANA groups in the third, sixth, and tenth months. In the two groups, Ad5 S & RBD-N and Ad5 S/SOBERANA, the Delta virus was neutralized at a dilution of 1:128 in the third month and at a dilution of 1:256 in the sixth month. In these groups, the 1:64 serum dilution neutralized the Omicron strain virus in the sixth month, indicating a decrease in the production of neutralizing antibodies against the Omicron strain compared to the Delta strain in this month. However, it has still possessed an acceptable protective effect against the Omicron strain. Moreover, the serum samples collected from these groups 28 days after the booster dose injection (nine months after the first injection) could neutralize Omicron at the 1:128 dilution. This increase is due to the administration of a booster dose in the ninth month. These findings indicate that the vaccine candidates in animal models could still neutralize new strains after several months. No animal study has yet investigated the long-term effect of existing COVID-19 vaccines on total antibody and neutralizing antibody titers.
Various studies have determined the titer of neutralizing antibodies against the new strains of SARS-CoV-2 such as Alpha, Beta, and Omicron in human sera following administration of existing vaccines. Ikegame et al. collected the serum of Sputnik V vaccine recipients in Argentina one month after the second dose. They documented that the serum of the Sputnik V vaccine effectively neutralized the Wild and Alpha variants. Moreover, the sera exhibited moderate and significant neutralizing antibodies titers against mutant species E484K and Beta, respectively[16]. Jongeneelen et al. collected the sera of the recipients who received a single dose of the Johnson vaccine 71 days after the clinical trial. The sera effectively neutralized the Wild, Beta, and Gamma variants. However, a slight decrease was recorded in the neutralization potency against the Delta variant[17]. Planas et al. compared the neutralization efficacy of nine monoclonal antibodies in the serum samples obtained from individuals who received two doses of the Pfizer or AstraZeneca vaccines (collected five months after completing vaccination) and also from the recovered patients (collected six or 12 months after recovery). The researchers concluded that Omicron was completely or partially resistant to the tested monoclonal antibodies[18]. Sera from recipients of the Pfizer or AstraZeneca vaccines struggled to control Omicron; however, the serum from individuals who had recovered were barely able to neutralize the activity of Omicron compared to Delta. Moreover, Omicron was neutralized by the serum of individuals who recovered and received a single booster dose[18]. In a study on serum samples of 20 volunteers prepared two or four weeks after the second dose of the BNT162b2 vaccine, Liu et al. showed that serum antibodies could inhibit the Wild, Kappa, Delta, and Eta strains[19]. Planas et al. used monoclonal antibodies such as Bamlanivimab (LY-CoV555), Atsui Wimab (LY-CoV016), Casirivimab (REGN10933), and Imduimab (REGN10987). These antibodies were collected from the recovered individuals 6 to 12 months after recovery. They also collected serum from the vaccinated individuals who had received 1 or 2 doses of the Pfizer or AstraZeneca vaccine four weeks before serum preparation. Their findings revealed that the Delta variant was resistant to neutralization by some anti-N terminal domain and anti-RBD monoclonal antibodies such as bamlanivimab. The antibodies were unable to bind to the S protein. The antibodies of the recovered individuals were four times more effective against the Alpha and Beta variants than the Delta variant. The serum of the vaccinated individuals receiving a single dose of the Pfizer or AstraZeneca vaccine showed a barely detectable inhibitory effect on the Delta variant. Two doses asministration of the vaccine resulted in a 95% neutralization response in individuals, which was three to five times lower in the Delta variant compared to the Alpha and Beta variants[20].
CONCLUSION
The findings of the present study demonstrate that the PastoCoAd vaccine candidates Ad5 S & RBD-N and Ad5 S/SOBERANA have the ability to neutralize the VOC strains in animal models by generating neutralizing antibodies at different time points. This outcome may be attributed to the use of the platform based on the Adenoviral vector, the N proteins in the Ad5 S & RBD-N vaccine candidate, and the SOBERANA Plus booster in the Ad5 S/SOBERANA vaccine candidate.
DECLARATIONS
Acknowledgments
The authors declare that they did not use artificial intelligence-assisted in the production of the submitted work.
Ethical approval
All the experimental procedures in this study were conducted in accordance with the Institutional Animal Care guidelines of the Pasteur Institute’s Animal House and approved by the Ethics Committee of the Pasteur Institute of Iran (ethical code: IR.PII.REC.1399.057).
Consent to participate
Not applicable.
Consent for publication
All authors reviewed the results and approved the final version of the manuscript.
Authors’ contributions
MP: writing–original draft preparation and investigation; AT: formal analysis; SE, HA, and AM: investigation; SYH: writing-review and editing; PE: investigation; AB: conceptualization; KB: conceptualization and project administration.
Data availability
All relevant data can be found within the manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was financially supported by the Pasteur Institute of Iran, Tehran.
Supplementary information
The online version does not contain supplementary material.
References
- 1.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87(4):281–6. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu Y, Jin H, Xiang H, Wang N. Optimal lockdown policy for vaccination during COVID-19 pandemic. Financ Res Lett. 2022;45:102123. doi: 10.1016/j.frl.2021.102123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu J, Peng P, Cao X, Wu K, Chen J, Wang K, et al. Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol Immunol. 2022;19(2):293–5. doi: 10.1038/s41423-021-00836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wratil PR, Stern M, Priller A, Willmann A, Almanzar G, Vogel E, et al. Three exposures to the spike protein of SARS-CoV-2 by either infection or vaccination elicit superior neutralizing immunity to all variants of concern. Nat Med. 2022;28(3):496–503. doi: 10.1038/s41591-022-01715-4. [DOI] [PubMed] [Google Scholar]
- 5.Mamedov T, Yuksel D, Ilgın M, Gürbüzaslan İ, Gulec B, Mammadova G, et al. Engineering, production and characterization of spike and nucleocapsid structural proteins of SARS–CoV-2 in Nicotiana benthamiana as vaccine candidates against COVID-19. Biorxiv. 2020 [Google Scholar]
- 6.Silva EKVB, Bomfim CG, Barbosa AP, Noda P, Noronha IL, Fernandes BHV, et al. Immunization with SARS-CoV-2 nucleocapsid protein triggers a pulmonary immune response in rats. PLoS one. 2022;17(5):e0268434. doi: 10.1371/journal.pone.0268434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smits VAJ, Hernández-Carralero E, Paz-Cabrera MC, Cabrera E, Hernández-Reyes Y, Hernández-Fernaud JR, et al. The nucleocapsid protein triggers the main humoral immune response in COVID-19 patients. Biochem Biophys Res Commun. 2021;543:45–9. doi: 10.1016/j.bbrc.2021.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salehi M, Hosseini H, Jamshidi HR, Jalili H, Tabarsi P, Mohraz M, et al. Assessment of BIV1-CovIran inactivated vaccine–elicited neutralizing antibody against the emerging SARS-CoV-2 variants of concern. Clin Microbiol Infect. 2022;28(6):882.e1–882. doi: 10.1016/j.cmi.2022.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan PM, Ali T, Saber E, Asghar A, Delaram D, Mostafa SV, et al. Potency, toxicity and protection evaluation of PastoCoAd candidate vaccines: Novel preclinical mix and match rAd5 S, rAd5 RBD-N and SOBERANA dimeric-RBD protein. Vaccine. 2022;40(20):2856–68. doi: 10.1016/j.vaccine.2022.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanaparthy R, Mohan G, Vasireddy D, Atluri P. Review of COVID-19 viral vector-based vaccines and COVID-19 variants. Infez Med. 2021;29(3):328–38. doi: 10.53854/liim-2903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garofalo M, Staniszewska M, Salmaso S, Caliceti P, Pancer KW, Wieczorek M, et al. Prospects of replication-deficient adenovirus based vaccine development against SARS-CoV-2. Vaccines (Basel). 2020;8(2):293. doi: 10.3390/vaccines8020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang-Monteagudo A, Ochoa-Azze R, Climent-Ruiz Y, Macías-Abraham C, Rodríguez-Noda L, Valenzuela-Silva C, et al. A single dose of SARS-CoV-2 FINLAY-FR-1A vaccine enhances neutralization response in COVID-19 convalescents, with a very good safety profile: An open-label phase 1 clinical trial. Lancet Reg Heal Am. 2021;4:100079. doi: 10.1016/j.lana.2021.100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyoshima Y, Nemoto K, Matsumoto S, Nakamura Y, Kiyotani K. SARS-CoV-2 genomic variations associated with mortality rate of COVID-19. J Hum Genet. 2020;65:1075–82. doi: 10.1038/s10038-020-0808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–24. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JS, Jang JH, Kim JM, Chung YS, Yoo CK, Han MG. Genome-wide Identification and characterization of point mutations in the SARS-CoV-2 genome. Osong Public Heal Res Perspect. 2020;11(3):101–11. doi: 10.24171/j.phrp.2020.11.3.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikegame S, Siddiquey MNA, Hung CT, Haas G, Brambilla L, Oguntuyo KY, et al. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Nat Commun. 2021;12:4598. doi: 10.1038/s41467-021-24909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jongeneelen M, Kaszas K, Veldman D, Huizingh J, van der Vlugt R, Schouten T, et al. Ad26 COV2 S elicited neutralizing activity against Delta and other SARS-CoV-2 variants of concern. BioRxiv. 2021 [Google Scholar]
- 18.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276–80. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Liu Y, Xia H, Zou J, Weaver SC, Swanson KA, et al. BNT162b2-elicited neutralization of B 1 617 and other SARS-CoV-2 variants. Nature. 2021;596(7871):273–5. doi: 10.1038/s41586-021-03693-y. [DOI] [PubMed] [Google Scholar]
- 20.Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671–5. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data can be found within the manuscript.