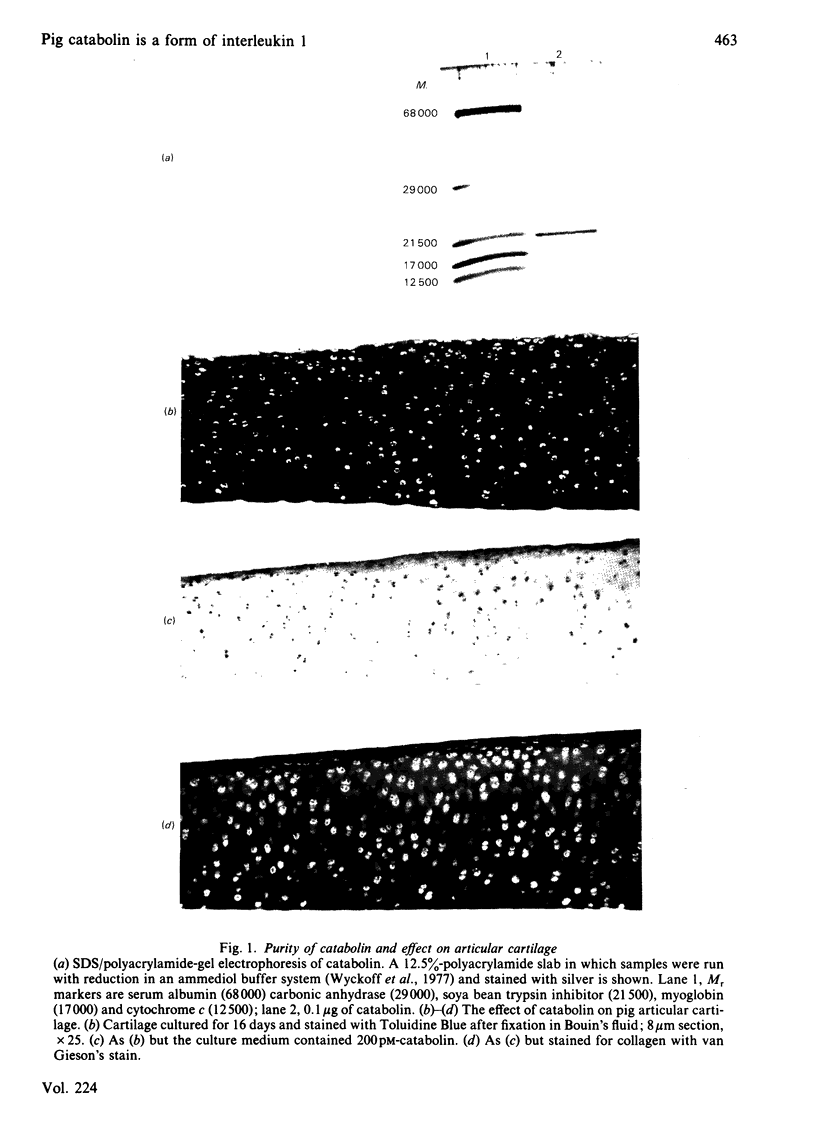
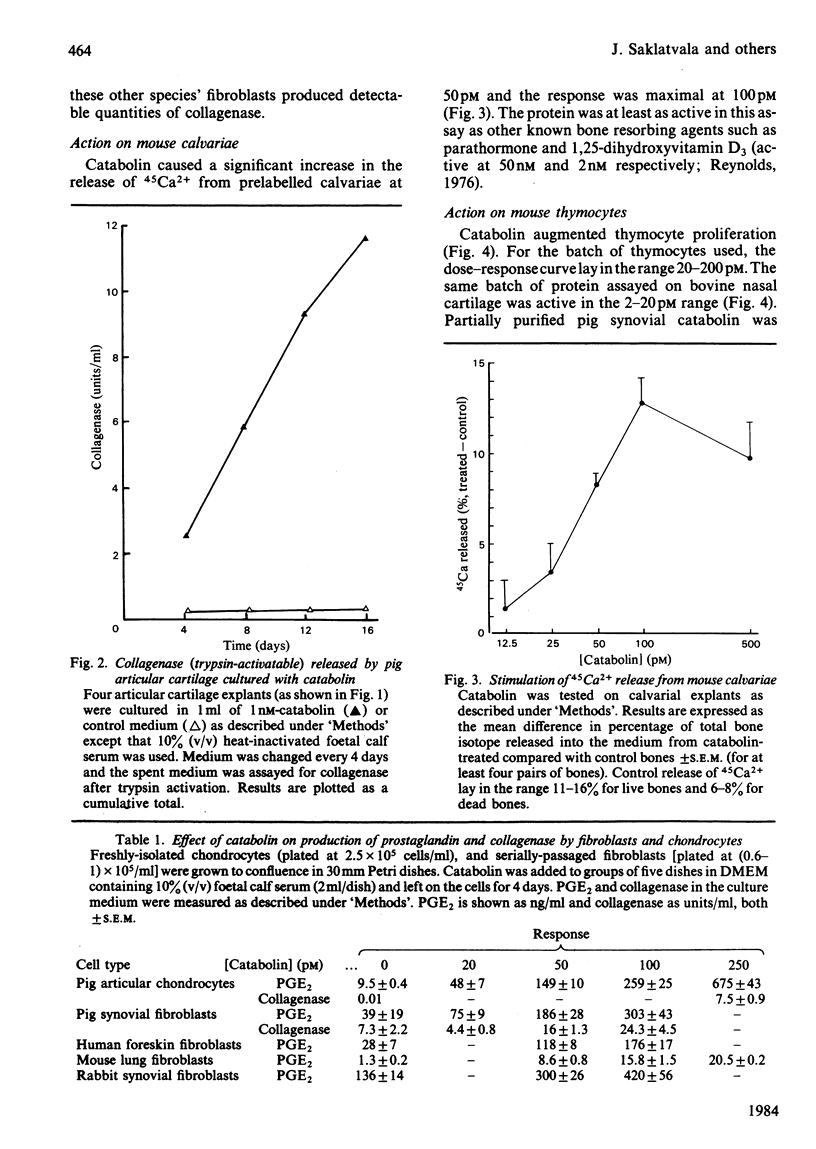
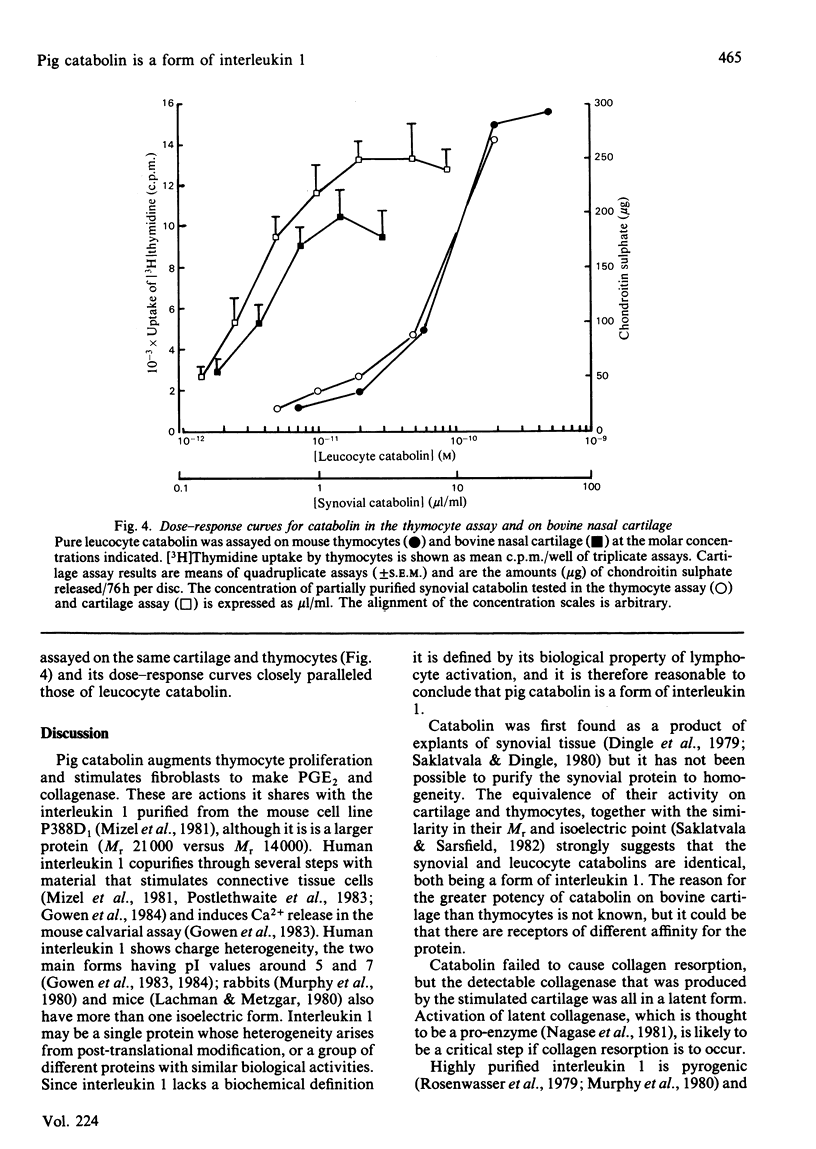
Abstract
Homogeneous catabolin from pig leucocytes induced proteoglycan breakdown, but not collagen breakdown, in explants of articular cartilage. It augmented lectin-induced proliferation of mouse thymocytes, stimulated production of prostaglandin E2 and collagenase by fibroblasts and chondrocytes, and increased Ca2+ release from mouse calvarial explants, all at concentrations down to 50 pM. In view of these effects it was concluded that pig catabolin is a form of interleukin 1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Bréard J., Chess L., Krane S. M. Participation of monocyte-macrophages and lymphocytes in the production of a factor that stimulates collagenase and prostaglandin release by rheumatoid synovial cells. J Clin Invest. 1979 Nov;64(5):1386–1392. doi: 10.1172/JCI109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Saklatvala J., Hembry R., Tyler J., Fell H. B., Jubb R. A cartilage catabolic factor from synovium. Biochem J. 1979 Oct 15;184(1):177–180. doi: 10.1042/bj1840177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., Meats J. E., Russell R. G. Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim Biophys Acta. 1984 Feb 14;797(2):186–193. doi: 10.1016/0304-4165(84)90121-1. [DOI] [PubMed] [Google Scholar]
- Iribe H., Koga T., Kotani S., Kusumoto S., Shiba T. Stimulating effect of MDP and its adjuvant-active analogues on guinea pig fibroblasts for the production of thymocyte-activating factor. J Exp Med. 1983 Jun 1;157(6):2190–2195. doi: 10.1084/jem.157.6.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman L. B., Metzgar R. S. Characterization of high and low molecular weight lymphocyte-activating factor (interleukin I) from P388D and J774.1 mouse macrophage cell lines. J Reticuloendothel Soc. 1980 Jun;27(6):621–629. [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Luger B. M., Mathieson B. J., Mage M., Schmidt J. A., Oppenheim J. J. Murine epidermal cell-derived thymocyte-activating factor resembles murine interleukin 1. J Immunol. 1982 May;128(5):2147–2152. [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Mizel D. Purification to apparent homogeneity of murine interleukin 1. J Immunol. 1981 Mar;126(3):834–837. [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Nagase H., Jackson R. C., Brinckerhoff C. E., Vater C. A., Harris E. D., Jr A precursor form of latent collagenase produced in a cell-free system with mRNA from rabbit synovial cells. J Biol Chem. 1981 Dec 10;256(23):11951–11954. [PubMed] [Google Scholar]
- Okai Y., Tashiro H., Yamashita U. 3T3 fibroblasts are stimulated by 12-O-tetradecanoyl-phorbol-13-acetate to produce thymocyte-activating factors. FEBS Lett. 1982 Jun 1;142(1):93–95. doi: 10.1016/0014-5793(82)80226-3. [DOI] [PubMed] [Google Scholar]
- Pilsworth L. M., Saklatvala J. The cartilage-resorbing protein catabolin is made by synovial fibroblasts and its production is increased by phorbol myristate acetate. Biochem J. 1983 Nov 15;216(2):481–489. doi: 10.1042/bj2160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Lachman L. B., Mainardi C. L., Kang A. H. Interleukin 1 stimulation of collagenase production by cultured fibroblasts. J Exp Med. 1983 Feb 1;157(2):801–806. doi: 10.1084/jem.157.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A., Rosenthal A. S. Adherent cell function in murine T-lymphocyte antigen recognition. IV. Enhancement of murine T-cell antigen recognition by human leukocytic pyrogen. J Exp Med. 1979 Sep 19;150(3):709–714. doi: 10.1084/jem.150.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Characterization of catabolin, the major product of pig synovial tissue that induces resorption of cartilage proteoglycan in vitro. Biochem J. 1981 Dec 1;199(3):705–714. doi: 10.1042/bj1990705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Curry V. A., Sarsfield S. J. Purification to homogeneity of pig leucocyte catabolin, a protein that causes cartilage resorption in vitro. Biochem J. 1983 Nov 1;215(2):385–392. doi: 10.1042/bj2150385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Dingle J. T. Identification of catabolin, a protein fro synovium which induces degradation of cartilage in organ culture. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1225–1231. doi: 10.1016/0006-291x(80)90082-0. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Sarsfield S. J. Lymphocytes induce resorption of cartilage by producing catabolin. Biochem J. 1982 Jan 15;202(1):275–278. doi: 10.1042/bj2020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala G., Allavena P., Djeu J. Y., Kasahara T., Ortaldo J. R., Herberman R. B., Oppenheim J. J. Human large granular lymphocytes are potent producers of interleukin-1. Nature. 1984 May 3;309(5963):56–59. doi: 10.1038/309056a0. [DOI] [PubMed] [Google Scholar]
- Sellers A., Reynolds J. J. Identification and partial characterization of an inhibitor of collagenase from rabbit bone. Biochem J. 1977 Nov 1;167(2):353–360. doi: 10.1042/bj1670353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztein M. B., Vogel S. N., Sipe J. D., Murphy P. A., Mizel S. B., Oppenheim J. J., Rosenstreich D. L. The role of macrophages in the acute-phase response: SAA inducer is closely related to lymphocyte activating factor and endogenous pyrogen. Cell Immunol. 1981 Sep 1;63(1):164–176. doi: 10.1016/0008-8749(81)90037-x. [DOI] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]



