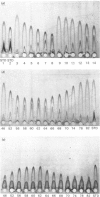
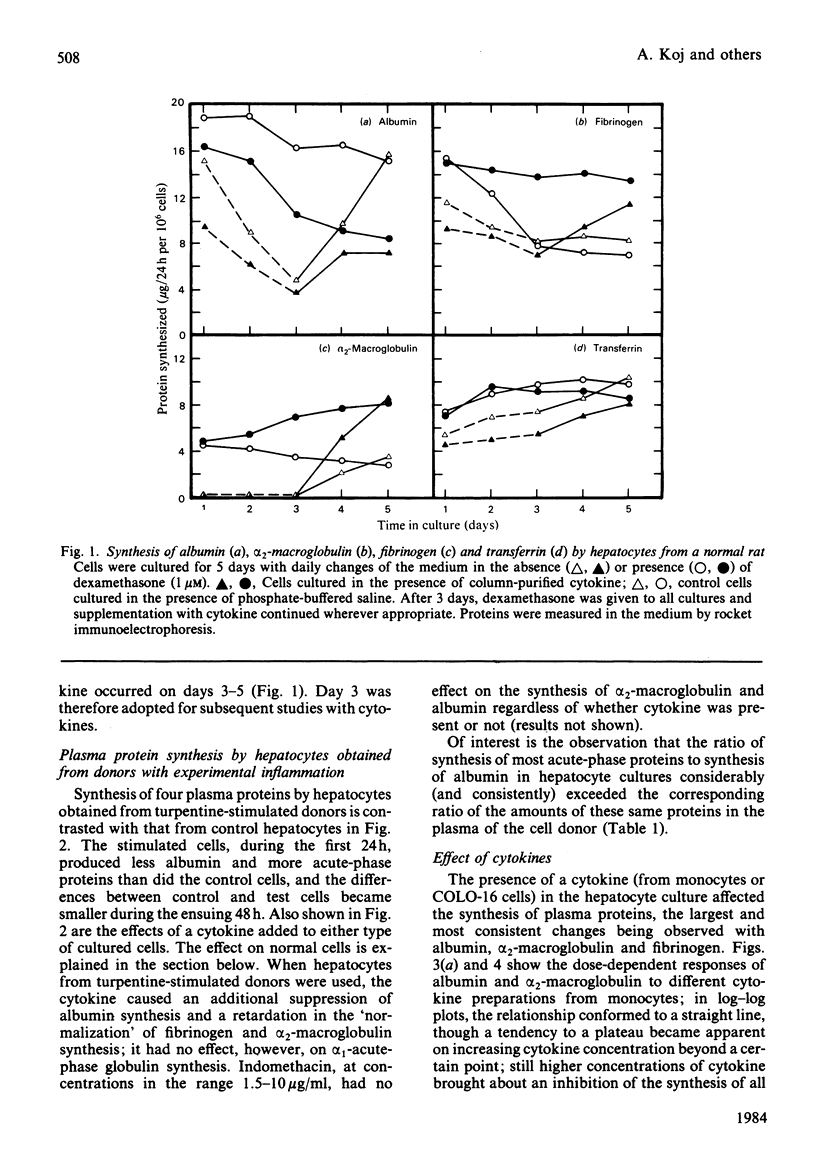
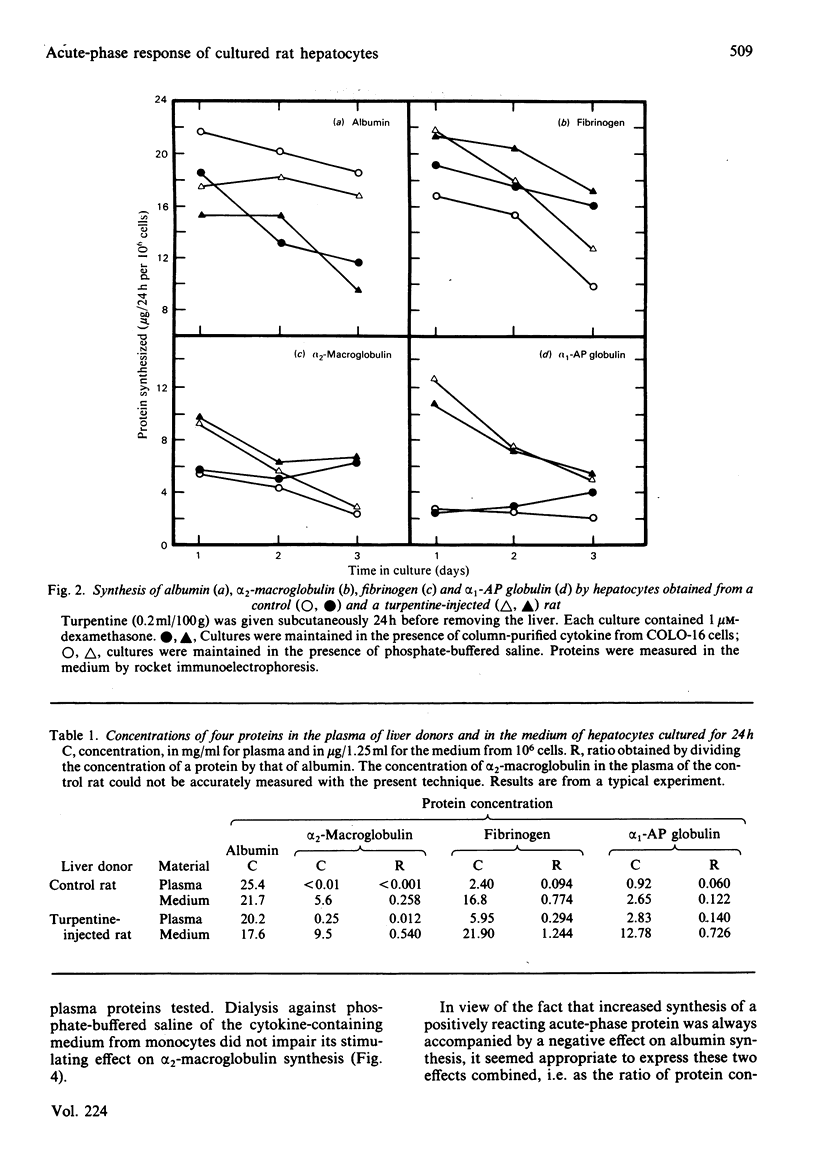
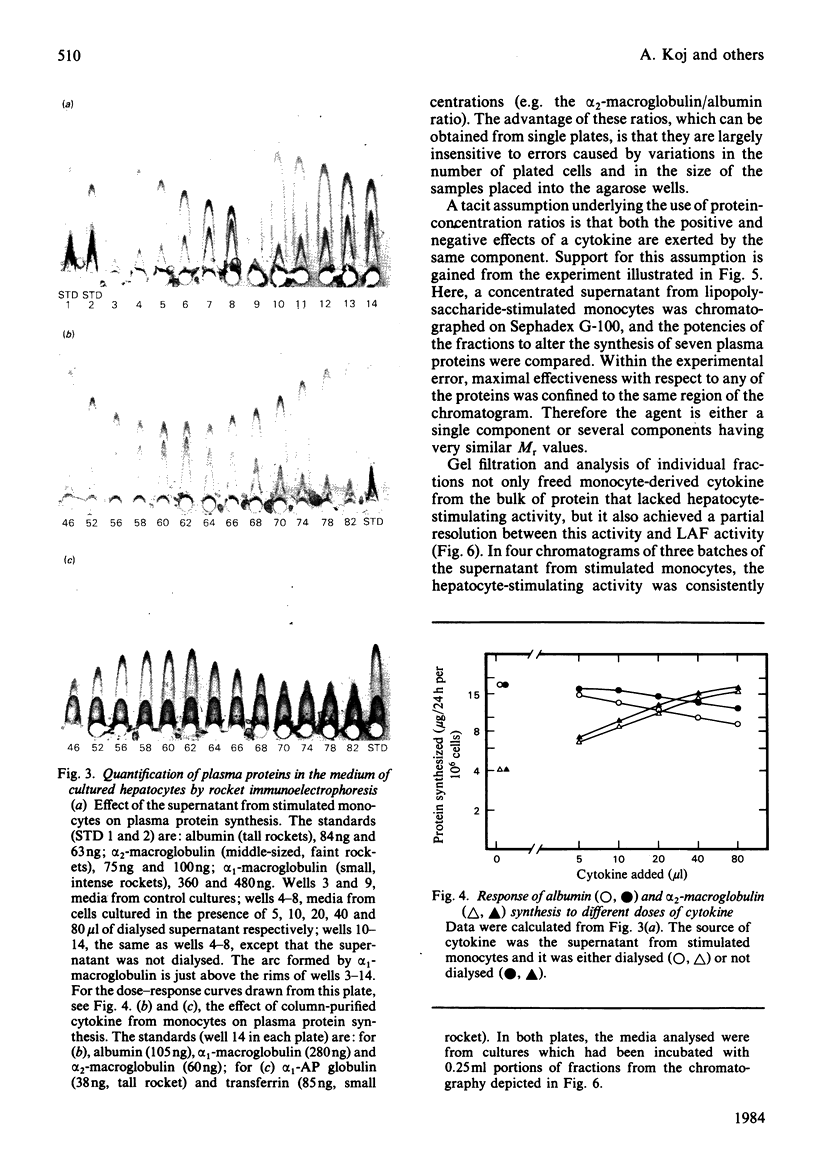
Abstract
Hepatocytes were isolated from adult livers and cultured for periods of up to 5 days as monolayers at an initial density of 10(6) cells/10cm2 in Williams E medium containing insulin, dexamethasone and 5% foetal-calf serum. The daily production of 11 plasma proteins was measured by electroimmunoassay and compared with the concentrations of the same proteins in the plasma of normal rats and of those with experimental inflammation. Hepatocytes from normal rats synthesized proteins in relative amounts which were similar to the relative proportions of the same proteins in the plasma of turpentine-injected animals. The pattern changed only slowly during 5 days in culture, but it did so profoundly either when the medium was devoid of dexamethasone or when human cytokines (from endotoxin-stimulated monocytes or unstimulated human squamous-carcinoma cell line COLO-16) were added. The cytokines consistently increased the synthesis of alpha 2-macroglobulin and fibrinogen and depressed that of albumin; variable increases in the synthesis of alpha 1-acute-phase globulin, alpha 1-acid glycoprotein, haptoglobin and alpha 1-proteinase inhibitor, and variable decreases in transferrin synthesis, were seen, whereas the synthesis of antithrombin III, alpha 1-macroglobulin and prothrombin remained virtually unaffected. The cytokine effects on protein synthesis required the presence of dexamethasone. The hepatocyte-stimulating activity derived from monocytes chromatographed on Sephadex G-100 corresponding to 30 000 Da, as opposed to the lymphocyte-activating factor, which was eluted as a molecule of approx. 15 000 Da. This suggests that both activities probably reside with distinct molecular species in the preparations of human cytokines.
Full text
PDF


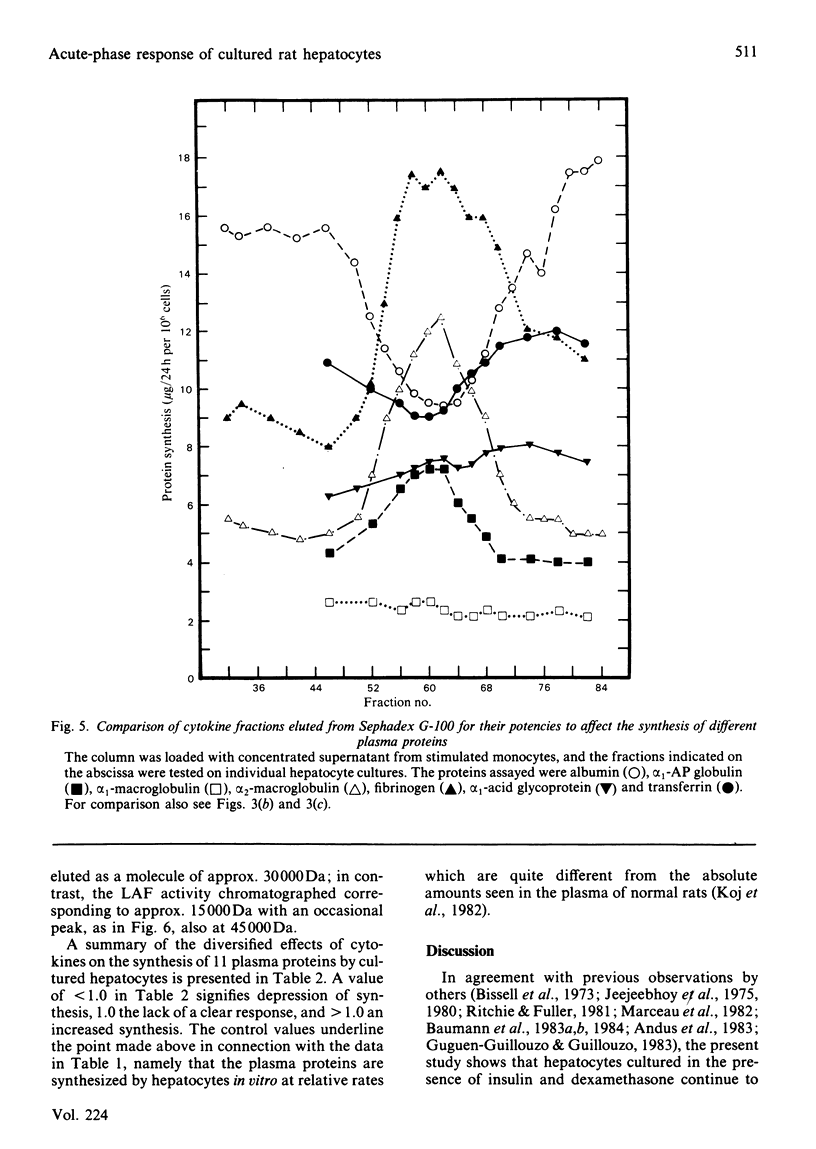
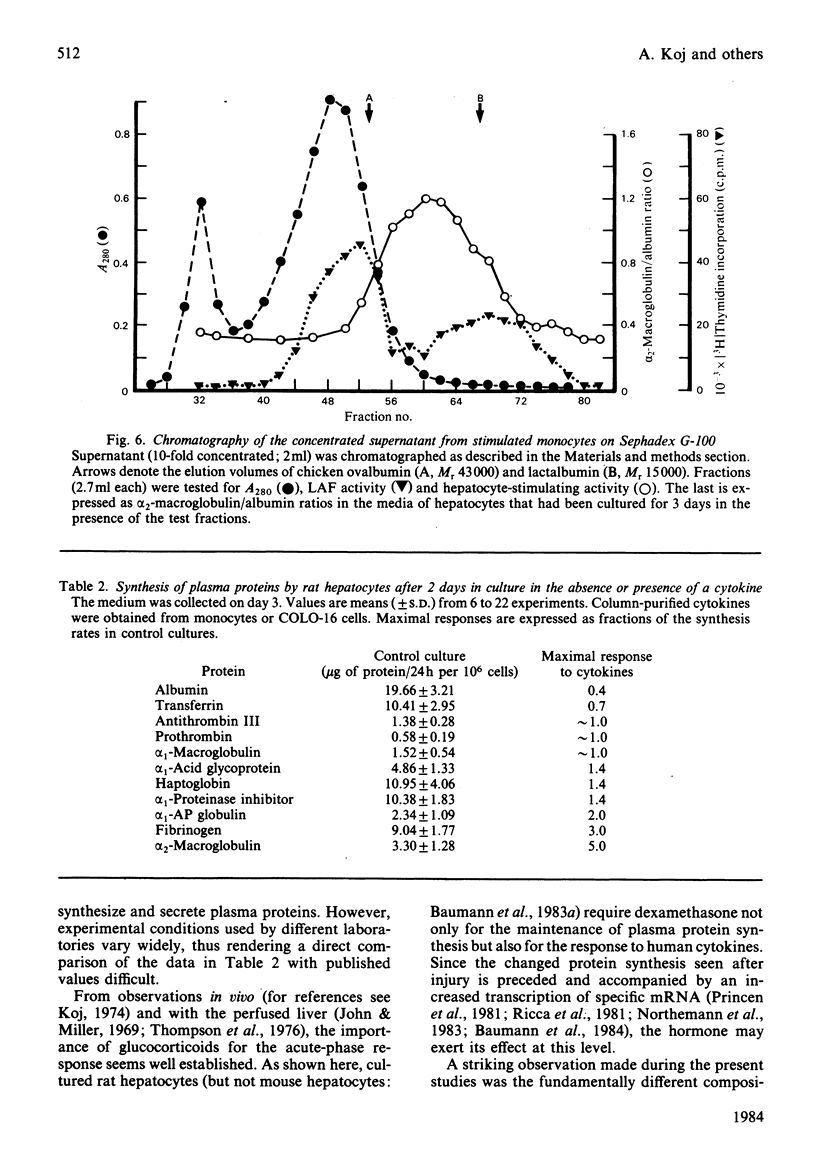


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Gross V., Tran-Thi T. A., Schreiber G., Nagashima M., Heinrich P. C. The biosynthesis of acute-phase proteins in primary cultures of rat hepatocytes. Eur J Biochem. 1983 Jul 1;133(3):561–571. doi: 10.1111/j.1432-1033.1983.tb07500.x. [DOI] [PubMed] [Google Scholar]
- Baumann H., Firestone G. L., Burgess T. L., Gross K. W., Yamamoto K. R., Held W. A. Dexamethasone regulation of alpha 1-acid glycoprotein and other acute phase reactants in rat liver and hepatoma cells. J Biol Chem. 1983 Jan 10;258(1):563–570. [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Gaines K. C. Synthesis and regulation of acute phase plasma proteins in primary cultures of mouse hepatocytes. J Cell Biol. 1983 Sep;97(3):866–876. doi: 10.1083/jcb.97.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Sauder D. N., Koj A. Human keratinocytes and monocytes release factors which regulate the synthesis of major acute phase plasma proteins in hepatic cells from man, rat, and mouse. J Biol Chem. 1984 Jun 10;259(11):7331–7342. [PubMed] [Google Scholar]
- Bernuau D., Rogier E., Feldmann G. Decreased albumin and increased fibrinogen secretion by single hepatocytes from rats with acute inflammatory reaction. Hepatology. 1983 Jan-Feb;3(1):29–33. doi: 10.1002/hep.1840030104. [DOI] [PubMed] [Google Scholar]
- Bissell D. M., Hammaker L. E., Meyer U. A. Parenchymal cells from adult rat liver in nonproliferating monolayer culture. I. Functional studies. J Cell Biol. 1973 Dec;59(3):722–734. doi: 10.1083/jcb.59.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Bendtzen K., Wolff S. M. Studies on the active site of human leukocytic pyrogen. Inflammation. 1982 Mar;6(1):63–78. doi: 10.1007/BF00910720. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Marnoy S. O., Rosenwasser L. J. Role of arachidonate metabolism in the immunoregulatory function of human leukocytic pyrogen/lymphocyte-activating factor/interleukin 1. J Immunol. 1983 Feb;130(2):890–895. [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. Molecular basis of fever in humans. Am J Med. 1982 May;72(5):799–819. doi: 10.1016/0002-9343(82)90548-4. [DOI] [PubMed] [Google Scholar]
- Gordon A. H., Limaos E. A. Human blood and rabbit peritoneal leucocytes as sources of endogenous mediators. Br J Exp Pathol. 1979 Aug;60(4):441–446. [PMC free article] [PubMed] [Google Scholar]
- Guguen-Guillouzo C., Guillouzo A. Modulation of functional activities in cultured rat hepatocytes. Mol Cell Biochem. 1983;53-54(1-2):35–56. doi: 10.1007/BF00225245. [DOI] [PubMed] [Google Scholar]
- Jeejeebhoy K. N., Ho J., Greenberg G. R., Phillips M. J., Bruce-Robertson A., Sodtke U. Albumin, fibrinogen and transferrin synthesis in isolated rat hepatocyte suspensions. A model for the study of plasma protein synthesis. Biochem J. 1975 Jan;146(1):141–155. doi: 10.1042/bj1460141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeejeebhoy K. N., Phillips M. J., Ho J., Bruce-Robertson A. Ultrastructural and functional studies of cultured adult rat hepatocytes. Ann N Y Acad Sci. 1980;349:18–27. doi: 10.1111/j.1749-6632.1980.tb29511.x. [DOI] [PubMed] [Google Scholar]
- John D. W., Miller L. L. Regulation of net biosynthesis of serum albumin and acute phase plasma proteins. Induction of enhanced net synthesis of fibrinogen, alpha1-acid glycoprotein, alpha2 (acute phase)-globulin, and haptoglobin by amino acids and hormones during perfusion of the isolated normal rat liver. J Biol Chem. 1969 Nov 25;244(22):6134–6142. [PubMed] [Google Scholar]
- Kampschmidt R. F., Upchurch H. F., Eddington C. L., Pulliam L. A. Multiple biological activities of a partially purified leukocytic endogenous mediator. Am J Physiol. 1973 Mar;224(3):530–533. doi: 10.1152/ajplegacy.1973.224.3.530. [DOI] [PubMed] [Google Scholar]
- Kampschmidt R. F., Upchurch H. F., Pulliam L. A. Characterization of a leukocyte-derived endogenous mediator responsible for increased plasma fibrinogen. Ann N Y Acad Sci. 1982;389:338–353. doi: 10.1111/j.1749-6632.1982.tb22148.x. [DOI] [PubMed] [Google Scholar]
- Kampschmidt R. F., Upchurch H. F., Worthington M. L., 3rd Further comparisons of endogenous pyrogens and leukocytic endogenous mediators. Infect Immun. 1983 Jul;41(1):6–10. doi: 10.1128/iai.41.1.6-10.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koj A., Dubin A., Kasperczyk H., Bereta J., Gordon A. H. Changes in the blood level and affinity to concanavalin A of rat plasma glycoproteins during acute inflammation and hepatoma growth. Biochem J. 1982 Sep 15;206(3):545–553. doi: 10.1042/bj2060545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Marceau N., Noël M., Deschênes J. Growth and functional activities of neonatal and adult rat hepatocytes cultured on fibronectin coated substratum in serum-free medium. In Vitro. 1982 Jan;18(1):1–11. doi: 10.1007/BF02796379. [DOI] [PubMed] [Google Scholar]
- McAdam K. P., Li J., Knowles J., Foss N. T., Dinarello C. A., Rosenwasser L. J., Selinger M. J., Kaplan M. M., Goodman R., Herbert P. N. The biology of SAA: identification of the inducer, in vitro synthesis, and heterogeneity demonstrated with monoclonal antibodies. Ann N Y Acad Sci. 1982;389:126–136. doi: 10.1111/j.1749-6632.1982.tb22131.x. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Broze G. J., Jr, Majerus P. W. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Anal Biochem. 1980 Jul 1;105(2):304–310. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- Northemann W., Andus T., Gross V., Nagashima M., Schreiber G., Heinrich P. C. Messenger RNA activities of four acute phase proteins during inflammation. FEBS Lett. 1983 Sep 19;161(2):319–322. doi: 10.1016/0014-5793(83)81033-3. [DOI] [PubMed] [Google Scholar]
- Princen J. M., Nieuwenhuizen W., Mol-Backx G. P., Yap S. H. Direct evidence of transcriptional control of fibrinogen and albumin synthesis in rat liver during the acute phase response. Biochem Biophys Res Commun. 1981 Sep 30;102(2):717–723. doi: 10.1016/s0006-291x(81)80191-x. [DOI] [PubMed] [Google Scholar]
- Regoeczi E., Hatton M. W., Long K. L. Studies of the metabolism of asialotransferrins: potentiation of the catabolism of human asialotransferrin in the rabbit. Can J Biochem. 1974 Mar;52(3):155–161. doi: 10.1139/o74-026. [DOI] [PubMed] [Google Scholar]
- Ricca G. A., Hamilton R. W., McLean J. W., Conn A., Kalinyak J. E., Taylor J. M. Rat alpha 1-acid glycoprotein mRNA. Cloning of double-stranded cDNA and kinetics of induction of mRNA levels following acute inflammation. J Biol Chem. 1981 Oct 25;256(20):10362–10368. [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. An in vitro bioassay for leukocytic endogenous mediator(s) using cultured rat hepatocytes. Inflammation. 1981 Dec;5(4):275–287. doi: 10.1007/BF00911093. [DOI] [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. Hepatocyte-stimulating factor: a monocyte-derived acute-phase regulatory protein. Ann N Y Acad Sci. 1983 Jun 27;408:490–502. doi: 10.1111/j.1749-6632.1983.tb23268.x. [DOI] [PubMed] [Google Scholar]
- Sauder D. N., Carter C. S., Katz S. I., Oppenheim J. J. Epidermal cell production of thymocyte activating factor (ETAF). J Invest Dermatol. 1982 Jul;79(1):34–39. doi: 10.1111/1523-1747.ep12510569. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Howlett G., Nagashima M., Millership A., Martin H., Urban J., Kotler L. The acute phase response of plasma protein synthesis during experimental inflammation. J Biol Chem. 1982 Sep 10;257(17):10271–10277. [PubMed] [Google Scholar]
- Selinger M. J., McAdam K. P., Kaplan M. M., Sipe J. D., Vogel S. N., Rosenstreich D. L. Monokine-induced synthesis of serum amyloid A protein by hepatocytes. Nature. 1980 Jun 12;285(5765):498–500. doi: 10.1038/285498a0. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., Vogel S. N., Ryan J. L., McAdam K. P., Rosenstreich D. L. Detection of a mediator derived from endotoxin-stimulated macrohpages that induces the acute phase serum amyloid A response in mice. J Exp Med. 1979 Sep 19;150(3):597–606. doi: 10.1084/jem.150.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney G. D., Garfield R. E., Jones K. G., Latham A. N. Studies using sedimentation velocity on heterogeneity of size and function of hepatocytes from mature male rats. J Lab Clin Med. 1978 Mar;91(3):432–443. [PubMed] [Google Scholar]
- Sztein M. B., Vogel S. N., Sipe J. D., Murphy P. A., Mizel S. B., Oppenheim J. J., Rosenstreich D. L. The role of macrophages in the acute-phase response: SAA inducer is closely related to lymphocyte activating factor and endogenous pyrogen. Cell Immunol. 1981 Sep 1;63(1):164–176. doi: 10.1016/0008-8749(81)90037-x. [DOI] [PubMed] [Google Scholar]
- Tatsuta E., Sipe J. D., Shirahama T., Skinner M., Cohen A. S. Different regulatory mechanisms for serum amyloid A and serum amyloid P synthesis by cultured mouse hepatocytes. J Biol Chem. 1983 May 10;258(9):5414–5418. [PubMed] [Google Scholar]
- Thompson W. L., Abeles F. B., Beall F. A., Dinterman R. E., Wannemacher R. W., Jr Influence of the adrenal glucocorticoids on the stimulation of synthesis of hepatic ribonucleic acid and plasma acute-phase globulins by leucocytic endogenous mediator. Biochem J. 1976 Apr 15;156(1):25–32. doi: 10.1042/bj1560025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, Pekarek R. S., Thompson W. L., Curnow R. T., Beall F. A., Zenser T. V., DeRubertis F. R., Beisel W. R. A protein from polymorphonuclear leukocytes (LEM) which affects the rate of hepatic amino acid transport and synthesis of acute-phase globulins. Endocrinology. 1975 Mar;96(3):651–661. doi: 10.1210/endo-96-3-651. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Gunn J. M. Long-term cell culture of adult rat liver epithelial cells. Exp Cell Res. 1974 Nov;89(1):139–142. doi: 10.1016/0014-4827(74)90196-7. [DOI] [PubMed] [Google Scholar]