Abstract
Background:
Hesitancy about vaccination during pregnancy posed challenges to SARS-CoV-2 vaccination efforts. We aimed to examine rates of SARS-CoV-2 vaccination among Ontario residents who gave birth in early 2022, and to compare rates of SARS-CoV-2 vaccine uptake with rates of tetanus, diphtheria, and pertussis (Tdap) and influenza vaccination during pregnancy in 2019, 2021, and 2022.
Methods:
We conducted a population-based retrospective cohort study to describe vaccination rates among pregnant and comparable nonpregnant populations in Ontario using linked administrative data. Provincially insured females who had a live, in-hospital birth from Jan. 1 to Mar. 31 in 2019, 2021, or 2022 were our primary cohort. Using log-binomial regression, we tested associations between SARS-CoV-2 (2022) and Tdap and influenza (2019, 2021, 2022) vaccination status, with birth group and covariates. We compared SARS-CoV-2 vaccination status with the status of a matched cohort of nonpregnant females and conducted subgroup analyses by age and prenatal clinician type.
Results:
Among birthing people, 78.7% received their first SARS-CoV-2 vaccine dose and 74.2% received a second dose. The rate was significantly higher among nonpregnant comparators (dose 1: relative risk [RR] 0.94, 95% confidence interval [CI] 0.93–0.94; dose 2: RR 0.91, 95% CI 0.90–0.91). However, the rate of SARS-CoV-2 vaccination uptake among birthing people was higher than uptake of Tdap or influenza vaccination. Tetanus, diphtheria, and pertussis vaccination increased over time from 22.2% in 2019 to 32.6% in 2022, and influenza vaccination rose to 35.3% in 2021 but returned to prepandemic levels in 2022 (27.7%). Vaccination rates were lower among pregnant people who were young, multiparous, or residents of rural or economically deprived areas for all 3 vaccines.
Interpretation:
Rates of SARS-CoV-2 vaccination were lower among pregnant people than among nonpregnant comparators but were higher than rates of routinely recommended Tdap and influenza vaccinations. Pandemic urgency may have overcome a great deal of hesitancy about vaccinating against SARS-CoV-2 during pregnancy in 2022, but uptake of routinely recommended vaccines in pregnancy remains a challenge.
Trial registration:
Although pregnancy is associated with a higher risk of severe COVID-19,1–8 pregnant people were not included in premarket clinical trials of SARS-CoV-2 vaccines.9–12 On the basis of international vaccination registry data indicating safety of the messenger RNA (mRNA) vaccines against SARS-CoV-2, vaccination in pregnancy was recommended by several national organizations, 13,14 and Ontario prioritized pregnant people for vaccine access beginning Apr. 23, 2021.15 Despite growing evidence of the vaccines’ safety and effectiveness during pregnancy,16–20 SARS-CoV-2 vaccine uptake was challenged by concerns about the safety of new vaccines and mRNA technology during pregnancy21,22 and fertility-focused misinformation, some of which specifically targeted marginalized communities.23–27 Early analysis of SARS-CoV-2 vaccination during pregnancy in Ontario indicated relatively low SARS-CoV-2 vaccination coverage among those giving birth in 2021, with a trend of steadily increasing coverage over the course of that year;28 many who initially did not receive the vaccination during pregnancy received it after their pregnancy.29 Additional research is required to investigate reduced uptake and disparities in uptake among pregnant people who are eligible for vaccination.
Uptake of SARS-CoV-2 vaccination in pregnancy may be informed by assessing the rates of other routinely recommended vaccinations during pregnancy (seasonal influenza and tetanus, diphtheria and pertussis [Tdap]), which have been low among pregnant people in Canada compared with rates among children or seniors,30 or pregnant populations in other countries. 31–33 Barriers to vaccination receipt during pregnancy may both reflect and widen social and health disparities.29,30,34,35 Strong pan-Canadian recommendations36 may be improving uptake,37 although this progress may have been disrupted by the COVID-19 pandemic.38,39 In 2007, the Canadian National Advisory Committee on Immunization (NACI) strengthened its recommendation for influenza vaccination during pregnancy to specify that vaccination for all pregnant people was “particularly recommended” because the group is “at high-risk of influenza-related complications.”40 Ontario publicly funds influenza vaccination during pregnancy through the province’s Universal Influenza Immunization Program, which began in 2000.41 In 2018, NACI and the Society of Obstetricians and Gynaecologists of Canada recommended pertussis vaccination (in the form of the Tdap vaccine) in every pregnancy;36 however, funding for this recommendation was not immediate in all provinces.42 Ontario started to publicly fund a routine dose of the Tdap vaccine in every pregnancy in April 2022.43
In this study, we aimed to examine whether Ontario residents who gave birth in early 2022, and who would have been eligible for vaccination during pregnancy, had lower receipt of SARS-CoV-2 vaccines than nonpregnant comparators, and to investigate whether they may have delayed vaccination until the postpartum period. Our secondary aim was to compare rates of Tdap and influenza vaccination among 2019, 2021, and 2022 birth groups, and to explore similarities and differences between Tdap and SARS-CoV-2 vaccination uptake. We hypothesized that rates of SARS-CoV-2 vaccination would be lower among pregnant people than among their comparators, that the 2019 group would have higher uptake of Tdap and influenza vaccination than the pandemic groups, and that pregnant people who received Tdap vaccination would be more likely to receive SARS-CoV-2 vaccination than those who did not receive Tdap vaccination.
Methods
We conducted a population-based retrospective cohort study to describe and compare rates of SARS-CoV-2, Tdap, and influenza vaccination among pregnant and comparable nonpregnant populations. This study is part of a convergent parallel mixed-methods study examining changes in health service use in pregnancy during the COVID-19 pandemic (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231522/tab-related-content); the protocol has been published44 and prospectively registered (NCT05663762). We report the results in accordance with the Reporting of Studies Conducted Using Observational Routinely-collected Health Data statement.45
Setting
We conducted the study in Ontario, Canada, which maintains multiple health administrative data holdings for publicly funded health services, including those for about 140 000 live births each year.46
Data sources and study population
We linked data sets using encoded identifiers and analyzed them at ICES. Data sets included a derived data set linking infants born in Ontario hospitals to birthing people (MOMBABY), Ontario’s central SARS-CoV-2 vaccination database (COVaxON), and the Ontario Health Insurance Plan (OHIP) claims database for Tdap and influenza vaccinations. Full data sources are listed in Appendix 2 (available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231522/tab-related-content); details of data sets and cohort creation have been published.44 Our primary cohort included provincially insured females who had a live, in-hospital birth between Jan. 1 and Mar. 31 in 2019, 2021, or 2022. The 3 birth years represent distinct periods of the COVID-19 pandemic with no overlapping gestational periods.44 Eligible patents had a valid ICES key number, delivery date, and birth date. We assigned date of childbirth as the index event; the lookback window spanned gestation (Appendix 3, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231522/tab-related-content). We established a comparator cohort of nonpregnant females aged 18–44 who received health care in Ontario between Jan. 1 and Mar. 31, 2022, for matched analysis.
Exposure
Primary exposures were antenatal periods for the 2019, 2021, and 2022 birth groups.44 Births between Jan. 1 and Mar. 31, 2019, were pregnancies and births before the COVID-19 pandemic; those between Jan. 1 and Mar. 31, 2021, occurred during the pandemic but before widespread vaccine availability; and those between Jan. 1 and Mar. 31, 2022, were patients who were pregnant and gave birth after widespread vaccine availability.
Outcomes
SARS-CoV-2, Tdap, and influenza vaccinations were the outcomes of interest. We assessed the SARS-CoV-2 outcome only for the 2022 birth group, who would have had access to vaccines during the entirety of their gestational period.15 We observed first and second dose coverage from the release of SARS-CoV-2 vaccines (December 2020) up to 3 months after the study’s latest birth date (June 2022), and observed timing of these doses in relation to pregnancy. We classified vaccination timing by comparing gestational age (reported or estimated for the n = 23 records without gestational age by subtracting 40 wk from childbirth date), birth date, and vaccination date. We assessed Tdap and influenza vaccination receipt during gestation for all patients in our primary cohort by examining the relevant physician billing claims in OHIP.
Covariates, potential confounders, and effect modifiers
Sociodemographic characteristics and health service use may affect vaccination status. We selected a priori 10 independent measures collected in administrative health data by consulting experts and reviewing literature (Appendix 2): parity, use of assisted reproductive technology, gestational length, singleton or multiple birth, perinatal care provider type, early perinatal care visits, delivery type, age, rurality, and socioeconomic “deprivation” (reported by dissemination area–level Canadian Index of Multiple Deprivation [CIMD] ethnocultural composition, residential instability, and economic dependency).47
Statistical analysis
Descriptive analysis
We report sociodemographic characteristics, use of perinatal health services, and birth outcomes using measures of general frequency (counts and percentages), central tendency (means and medians), and dispersion (standard deviations and interquartile ranges [IQRs]). To account for minimal missingness in rurality and CIMD values (< 0.5%), we performed a single imputation using predictive mean matching.
Inferential analysis
We tested associations between outcomes, primary exposure (birth group), and other covariates using log-binomial regression models with robust variance estimation to estimate relative risk (RR) and associated confidence intervals (CIs). For our primary outcome (SARS-CoV-2 vaccination in pregnant and nonpregnant groups), we used propensity score matching to minimize effects of confounding. 48 We restricted the 2022 birth group to ages 18–44 years and performed a propensity score–matched analysis with a cohort of similarly aged nonpregnant females. We estimated the propensity score by logistic regression using age, rurality, parity, and the 3 CIMD factors to estimate the probability of being in the pregnant female group. We greedy matched the 2 cohorts 1:1 using a caliper of 0.2 times the standard deviation of the logit of the propensity score. For the remaining multivariable analyses, we forced all factors into our models to reflect the conscientious process by which they were selected and our exploratory objectives. We performed all analyses using SAS software, version 9.4.
Sensitivity analysis
We conducted prespecified subgroup analyses to examine differences by 2 factors identified in our literature review as potentially influential: prenatal clinician type and age (which we categorized into birthing people aged < 25 yr v. ≥ 25 yr) following the same methods previously described. When comparing SARS-CoV-2 vaccinations by age, we re-ran the matching procedure with an additional hard match on age. We also stratified our 2022 group by birth month to identify any differences in SARS-CoV-2 vaccine uptake.
Ethics approval
Section 45 of Ontario’s Personal Health Information Protection Act allows ICES to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. Study approval was waived by the Hamilton Integrated Research Ethics Board and granted by the University of British Columbia Behavioural Research Ethics Board (H22-01905).
Results
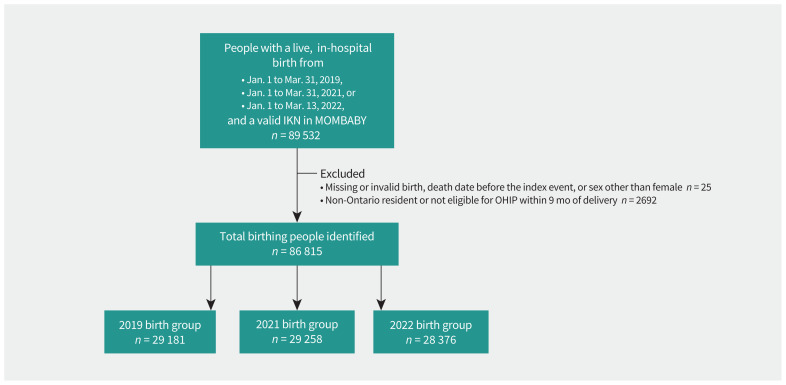
We identified 86 815 people who gave birth between Jan. 1 and Mar. 31 in 2019 (n = 29 181), 2021 (n = 29 258), and 2022 (n = 28 376) (Figure 1). The median age was 32 years (Table 1). Obstetricians most frequently provided prenatal care, and the median number of prenatal visits before 32 weeks of gestation was 5 (IQR 3–7). A total of 43.6% gave birth for the first time and 3.5% used assisted reproductive technology. The comparator cohort contained 2 576 817 eligible nonpregnant females aged 18–44 years; propensity score matching resulted in 28 177 people in each group (Table 2). The standardized differences of all descriptive factors were 0.1 or less, suggesting comparability between groups.
Figure 1:
Flow diagram showing primary cohort creation and exclusions. Note: IKN = ICES key number, MOMBABY = Ontario Mother–Baby linked data set, OHIP = Ontario Health Insurance Plan.
Table 1:
Sociodemographic characteristics, perinatal health services contact, and vaccination information among patients in the primary birth cohorts
| Variable | No. (%)* | |||
|---|---|---|---|---|
| Overall n = 86 815 |
2019 birth group n = 29 181 |
2021 birth group n = 29 258 |
2022 birth group n = 28 376 |
|
| Sociodemographic characteristics | ||||
| Age, yr, median (IQR) | 32 (28–35) | 31 (28–35) | 31 (28–35) | 32 (29–35) |
| Rural residence | 6202 (7.1) | 2094 (7.2) | 2066 (7.1) | 2042 (7.2) |
| Neighbourhood-level ethnocultural composition† | ||||
| 1 (least diversity) | 13 533 (15.6) | 4556 (15.6) | 4604 (15.7) | 4373 (15.4) |
| 2 | 13 997 (16.1) | 4600 (15.8) | 4795 (16.4) | 4602 (16.2) |
| 3 | 15 527 (17.9) | 5128 (17.6) | 5211 (17.8) | 5188 (18.3) |
| 4 | 19 044 (21.9) | 6387 (21.9) | 6361 (21.7) | 6296 (22.2) |
| 5 (greatest diversity) | 24 714 (28.5) | 8510 (29.2) | 8287 (28.3) | 7917 (27.9) |
| Neighbourhood-level economic dependency† | ||||
| 1 (least dependency) | 24 963 (28.8) | 8340 (28.6) | 8435 (28.8) | 8188 (28.9) |
| 2 | 18 099 (20.8) | 5980 (20.5) | 6162 (21.1) | 5957 (21.0) |
| 3 | 16 161 (18.6) | 5519 (18.9) | 5278 (18.0) | 5364 (18.9) |
| 4 | 14 839 (17.1) | 5001 (17.1) | 5055 (17.3) | 4783 (16.9) |
| 5 (greatest dependency) | 12 753 (14.7) | 4341 (14.9) | 4328 (14.8) | 4084 (14.4) |
| Neighbourhood-level residential instability† | ||||
| 1 (least instability) | 12 601 (14.5) | 4190 (14.4) | 4353 (14.9) | 4058 (14.3) |
| 2 | 16 387 (18.9) | 5374 (18.4) | 5604 (19.2) | 5409 (19.1) |
| 3 | 19 047 (21.9) | 6425 (22.0) | 6311 (21.6) | 6311 (22.2) |
| 4 | 18 645 (21.5) | 6159 (21.1) | 6274 (21.4) | 6212 (21.9) |
| 5 (greatest instability) | 20 135 (23.2) | 7033 (24.1) | 6716 (23.0) | 6386 (22.5) |
| Perinatal health services contact | ||||
| Use of assisted reproductive technology for current pregnancy | 3061 (3.5) | 1128 (3.9) | 595 (2.0) | 1338 (4.7) |
| Early prenatal care visits (count), median (IQR)‡ | 5 (3–7) | 6 (4–8) | 5 (3–7) | 5 (3–7) |
| Prenatal care provider type | ||||
| Obstetrician | 56 717 (65.3) | 18 931 (64.9) | 19 330 (66.1) | 18 456 (65.0) |
| Family physician | 12 308 (14.2) | 4459 (15.3) | 3966 (13.6) | 3883 (13.7) |
| Shared care§ | 2280 (2.6) | 741 (2.5) | 758 (2.6) | 781 (2.8) |
| Midwife | 14 372 (16.6) | 4761 (16.3) | 4794 (16.4) | 4817 (17.0) |
| No care or other | 1138 (1.3) | 289 (1.0) | 410 (1.4) | 439 (1.5) |
| Parity | ||||
| 0 | 37 846 (43.6) | 12 390 (42.5) | 12 973 (44.3) | 12 483 (44.0) |
| 1 | 31 365 (36.1) | 10 737 (36.8) | 10 416 (35.6) | 10 212 (36.0) |
| 2 | 11 276 (13.0) | 3874 (13.3) | 3747 (12.8) | 3655 (12.9) |
| ≥ 3 | 6328 (7.3) | 2180 (7.5) | 2122 (7.3) | 2026 (7.1) |
| ≥ 1 infant(s) aged ≤ 24 mo in household | 11 433 (13.2) | 3882 (13.3) | 3824 (13.1) | 3727 (13.1) |
| SARS-CoV-2 vaccination (2022 birth group only) | ||||
| First dose | ||||
| Overall¶ | – | – | – | 22 581 (79.6) |
| Timing | ||||
| Before conception | – | – | – | 11 264 (49.9) |
| During pregnancy | – | – | – | 10 435 (46.2) |
| After giving birth | – | – | – | 882 (3.9) |
| Second dose | ||||
| Overall§ | – | – | – | 21 425 (75.5) |
| Timing | ||||
| Before conception | – | – | – | 2929 (13.7) |
| During pregnancy | – | – | – | 17 595 (82.1) |
| After giving birth | – | – | – | 901 (4.2) |
| SARS-CoV-2 and Tdap vaccination (2022 birth group only) | ||||
| Received dose 1 and Tdap vaccine | – | – | – | 8347 (29.4) |
| Received dose 1 but not Tdap vaccine | – | – | – | 14 234 (50.2) |
| Received Tdap vaccine but not dose 1 | – | – | – | 916 (3.2) |
| Received neither Tdap vaccine nor dose 1 | – | – | – | 4879 (17.2) |
| Received dose 2 and Tdap vaccine | – | – | – | 8086 (28.5) |
| Received dose 2 but not Tdap vaccine | – | – | – | 13 339 (47.0) |
| Received Tdap vaccine but not dose 2 | – | – | – | 1177 (4.1) |
| Received neither Tdap vaccine nor dose 2 | – | – | – | 5774 (20.3) |
| Tdap and influenza vaccination | ||||
| Received both Tdap and influenza vaccine | 12 877 (14.8) | 3734 (12.8) | 5186 (17.7) | 3957 (13.9) |
| Received influenza but no Tdap vaccine | 13 676 (15.8) | 4614 (15.8) | 5152 (17.6) | 3910 (13.8) |
| Received Tdap but no influenza vaccine | 12 046 (13.9) | 2742 (9.4) | 3998 (13.7) | 5306 (18.7) |
| Received neither influenza nor Tdap vaccine | 48 216 (55.5) | 18 091 (62.0) | 14 922 (51.0) | 15 203 (53.6) |
Note: IQR = interquartile range; Tdap = tetanus, diphtheria and pertussis.
Unless stated otherwise.
Measured as quintiles.
Early prenatal care visits occurred before 32 weeks of gestation.
Includes patients with an equal number of minor prenatal assessment billings by family physicians and obstetricians between conception date and index date.
Vaccine records were examined for each birthing person between Dec. 1, 2020, and up to June 30, 2022.
Table 2:
Sociodemographic characteristics, perinatal health services contact, and receipt of SARS-COV-2 vaccination of matched birth cohort and nonpregnant comparator cohort
| Variable | No. (%)* | Standardized difference | |
|---|---|---|---|
| 2022 birth cohort n = 28 177 |
Matched nonpregnant cohort n = 28 177 |
||
| Sociodemographic characteristics | |||
| Age, yr, median (IQR) | 32 (29–35) | 32 (29–35) | 0.00 |
| Rural residence | 2023 (7.2) | 2012 (7.1) | 0.00 |
| Neighbourhood-level ethnocultural composition† | |||
| 1 (least diversity) | 4338 (15.4) | 4346 (15.4) | 0.00 |
| 2 | 4576 (16.2) | 4577 (16.2) | 0.00 |
| 3 | 5150 (18.3) | 5131 (18.2) | 0.00 |
| 4 | 6249 (22.2) | 6248 (22.2) | 0.00 |
| 5 (greatest diversity) | 7864 (27.9) | 7875 (27.9) | 0.00 |
| Neighbourhood-level economic dependency† | |||
| 1 (least dependency) | 8142 (28.9) | 8144 (28.9) | 0.00 |
| 2 | 5925 (21.0) | 5925 (21.0) | 0.00 |
| 3 | 5330 (18.9) | 5330 (18.9) | 0.00 |
| 4 | 4747 (16.8) | 4748 (16.9) | 0.00 |
| 5 (greatest dependency) | 4033 (14.3) | 4028 (14.3) | 0.00 |
| Neighbourhood-level residential instability† | |||
| 1 (least instability) | 4035 (14.3) | 4029 (14.3) | 0.00 |
| 2 | 5373 (19.1) | 5369 (19.1) | 0.00 |
| 3 | 6275 (22.3) | 6284 (22.3) | 0.00 |
| 4 | 6168 (21.9) | 6170 (21.9) | 0.00 |
| 5 (greatest instability) | 6326 (22.5) | 6325 (22.4) | 0.00 |
| Perinatal health services contact | |||
| Parity | |||
| 0 | 12 363 (43.9) | 12 361 (43.9) | 0.00 |
| 1 | 10 170 (36.1) | 10 172 (36.1) | 0.00 |
| 2 | 3640 (12.9) | 3640 (12.9) | 0.00 |
| ≥ 3 | 2004 (7.1) | 2004 (7.1) | 0.00 |
| SARS-CoV-2 vaccination | |||
| First dose‡ | 22 431 (79.6) | 23 282 (82.6) | NA |
| Second dose‡ | 21 289 (75.6) | 22 682 (80.5) | NA |
Note: IQR = interquartile range, NA = not applicable.
Unless stated otherwise.
Measured as quintiles.
Vaccine records were examined between Dec. 1, 2020, and June 30, 2022.
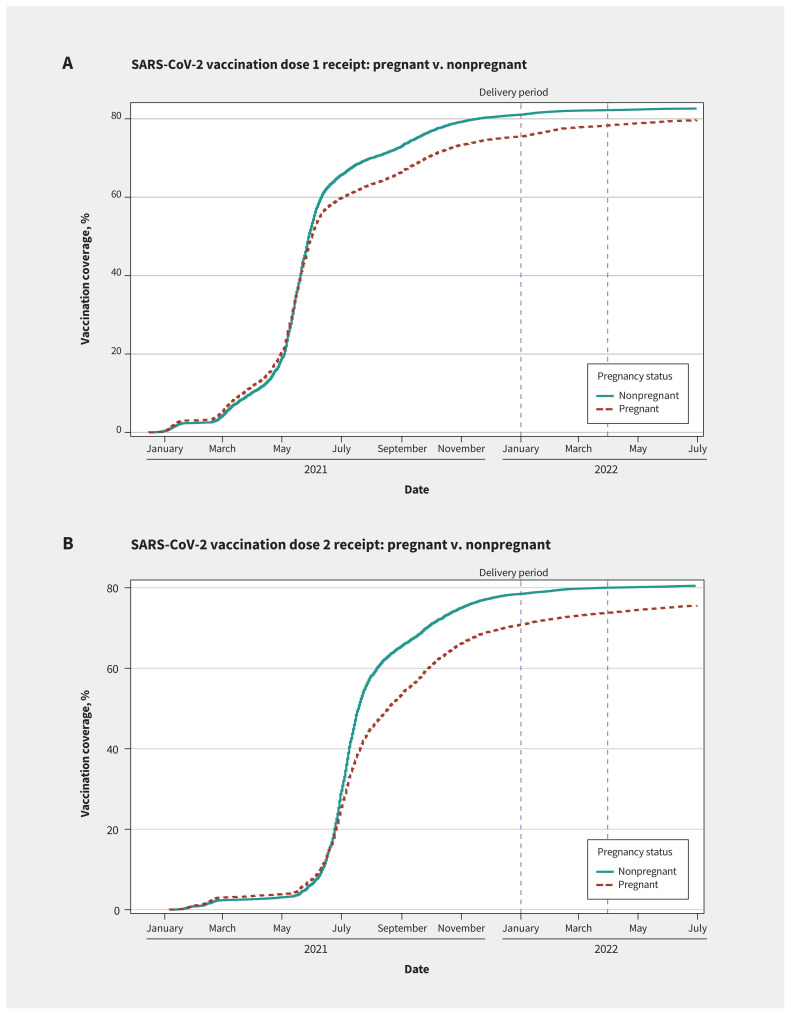
Among those in the 2022 birth group, 22 508 (79.6%) received an initial SARS-CoV-2 vaccine dose and 21 435 (75.5%) received a second dose by June 30, 2022. Of those who were vaccinated, nearly half (49.9%) received the first dose before pregnancy, and 3.9% delayed until after delivery. Among the nonpregnant matched cohort, 82.6% received an initial dose and 80.5% a second dose in the same period (Table 2); pregnant people were less likely than nonpregnant females to be vaccinated with either SARS-CoV-2 dose (dose 1: RR 0.94, 95% CI 0.93–0.94; dose 2: RR 0.91, 95% CI 0.90–0.91) (Table 3 and Figure 2). Rural residence and increasing neighbourhood-level economic dependency were negatively associated with receiving each SARS-CoV-2 vaccine dose during pregnancy (Table 3). We did not observe differences by birthing month (Appendix 4, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231522/tab-related-content).
Table 3:
Factors associated with SARS-CoV-2 vaccination among pregnant people compared with matched nonpregnant females
| Variable | Adjusted RR‡ (95% CI) | |
|---|---|---|
| First SARS-CoV-2 vaccine dose | Second SARS-Cov-2 vaccine dose | |
| SARS-CoV-2 vaccination | ||
| Pregnancy status* | ||
| Pregnant | 0.94 (0.93–0.94) | 0.91 (0.90–0.91) |
| Nonpregnant† | – | – |
| Sociodemographic characteristics | ||
| Age, yr | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Rural residence | 0.94 (0.94–0.94) | 0.94 (0.93–0.94) |
| Neighbourhood-level ethnocultural composition | ||
| 1 (least diversity)† | – | – |
| 2 | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| 3 | 1.01 (1.00–1.01) | 1.00 (1.00–1.01) |
| 4 | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| 5 (greatest diversity) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Neighbourhood-level economic dependency | ||
| 1 (least dependency)† | – | – |
| 2 | 0.98 (0.98–0.98) | 0.98 (0.98–0.98) |
| 3 | 0.98 (0.98–0.98) | 0.98 (0.97–0.98) |
| 4 | 0.97 (0.97–0.97) | 0.97 (0.97–0.97) |
| 5 (greatest diversity) | 0.98 (0.97–0.97) | 0.97 (0.96–0.98) |
| Neighbourhood-level residential instability | ||
| 1 (least instability)† | – | – |
| 2 | 1.00 (1.00–1.00) | 1.02 (0.99–1.05) |
| 3 | 0.99 (0.99–0.99) | 1.01 (0.98–1.04) |
| 4 | 0.99 (0.99–0.99) | 1.01 (0.98–1.04) |
| 5 (greatest instability) | 0.97 (0.97–0.97) | 0.99 (0.96–1.02) |
| Perinatal health services contact | ||
| Parity | ||
| 0† | – | – |
| 1 | 0.99 (0.98–0.99) | 0.98 (0.98–0.98) |
| 2 | 1.02 (1.02–1.02) | 1.02 (1.02–1.02) |
| ≥ 3 | 0.94 (0.94–0.95) | 0.93 (0.93–0.93) |
Note: CI = confidence interval, RR = relative risk.
Vaccine records were examined between Dec. 1, 2020, and June 30, 2022.
Reference group.
Adjusted for maternal age, rurality, Canadian Index of Multiple Deprivation measures (i.e., ethnocultural composition, economic dependency, and residential instability), and parity.
Figure 2:
Timing of SARS-CoV-2 vaccinations, by pregnant versus nonpregnant status. The vertical dashed lines represent the birthing windows of the cohort (i.e., Jan. 1 to Mar. 31).
Overall, 24 923 (28.7%) birthing people received Tdap vaccination during pregnancy; the proportion vaccinated increased between 2019 and 2022 (Table 1). After multivariable adjustment, the temporal effect remained significant (Table 4). All variables except assisted reproductive technology were significantly associated with Tdap vaccination.
Table 4:
Factors associated with tetanus, diphtheria, and pertussis vaccination among pregnant people
| Variable | Adjusted RR* (95% CI) |
|---|---|
| Birthing group | |
| 2019 birth† | – |
| 2021 birth | 1.46 (1.42–1.50) |
| 2022 birth | 1.50 (1.46–1.54) |
| Sociodemographic characteristics | |
| Age, yr | 1.02 (1.02–1.03) |
| Rural residence | 0.83 (0.79–0.88) |
| Neighbourhood-level ethnocultural composition | |
| 1 (least diversity)† | – |
| 2 | 1.08 (1.04–1.13) |
| 3 | 1.17 (1.13–1.22) |
| 4 | 1.19 (1.15–1.24) |
| 5 (greatest diversity) | 1.18 (1.14–1.23) |
| Neighbourhood-level economic dependency | |
| 1 (least dependency)† | – |
| 2 | 0.96 (0.93–0.99) |
| 3 | 0.91 (0.89–0.94) |
| 4 | 0.88 (0.85–0.91) |
| 5 (greatest dependency) | 0.86 (0.83–0.89) |
| Neighbourhood-level residential instability | |
| 1 (least instability)† | – |
| 2 | 0.98 (0.94–1.01) |
| 3 | 0.95 (0.91–0.98) |
| 4 | 0.91 (0.88–0.94) |
| 5 (greatest instability) | 0.89 (0.86–0.93) |
| Perinatal health services contact | |
| Use of assisted reproductive technology for current pregnancy | 0.95 (0.90–1.00) |
| Early prenatal care visits‡ | 1.04 (1.03–1.04) |
| Prenatal care provider type | |
| Obstetrician† | – |
| Family physician | 1.38 (1.34–1.42) |
| Shared care | 1.20 (1.13–1.27) |
| Midwife | 1.05 (1.01–1.09) |
| No care or other | 0.51 (0.43–0.60) |
| Parity | |
| 0† | – |
| 1 | 0.81 (0.79–0.83) |
| 2 | 0.62 (0.60–0.65) |
| ≥ 3 | 0.45 (0.42–0.47) |
Note: CI = confidence interval, RR = relative risk.
Adjusted for birth group, maternal age, rurality, Canadian Index of Multiple Deprivation measures (i.e., ethnocultural composition, economic dependency, and residential instability), use of assisted reproductive technology, early prenatal care visits, type of prenatal care provider, and parity.
Reference group.
Early prenatal care visits occurred before 32 weeks of gestation.
Pregnant people who received Tdap vaccination (n = 9263) were more likely to also receive either dose of SARS-CoV-2 vaccination (dose 1, n = 8347, 90.1%; dose 2, n = 8086, 87.3%) than those who did not receive Tdap vaccination (n = 19 113) (dose 1, n = 14 234, 74.5%; dose 2, n = 13 339, 69.8%).
Overall, influenza vaccination was received by 26 553 (30.6%) birthing persons, with higher uptake in 2021 than either 2019 or 2022 (Table 1).
The factors associated with SARS-CoV-2 vaccination for birthing people across type of prenatal clinician are shown in Table 5. Age was significantly associated with uptake of both doses across all clinician types. Among those cared for by family physicians, primary care model was not associated with significant differences.
Table 5:
Factors associated with SARS-CoV-2 vaccination among pregnant people, by type of prenatal care provider
| Variable | Obstetrician; adjusted RR* (95% CI) n = 18 456 |
Family physician; adjusted RR*† (95% CI) n = 3883 |
Midwife; adjusted RR* (95% CI) n = 4817 |
|||
|---|---|---|---|---|---|---|
| First dose | Second dose | First dose | Second dose | First dose | Second dose | |
| Sociodemographic characteristics | ||||||
| Age, yr | 1.01 (1.01–1.01) | 1.01 (1.01–1.01) | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.01 (1.01–1.01) | 1.01 (1.01–1.02) |
| Rural residence | 0.95 (0.92–0.99) | 0.93 (0.89–0.97) | 0.98 (0.95–1.01) | 0.98 (0.95–1.01) | 0.96 (0.88–1.04) | 0.95 (0.85–1.07) |
| Neighbourhood-level ethnocultural composition | ||||||
| 1 (least diversity)‡ | – | – | – | – | – | – |
| 2 | 0.99 (0.97–1.01) | 0.98 (0.96–1.01) | 0.99 (0.96–1.03) | 1.00 (0.97–1.03) | 1.00 (0.94–1.06) | 1.02 (0.94–1.10) |
| 3 | 0.99 (0.97–1.01) | 0.98 (0.96–1.01) | 0.99 (0.96–1.02) | 0.99 (0.96–1.02) | 1.00 (0.94–1.06) | 1.01 (0.9–1.09) |
| 4 | 1.00 (0.98–1.02) | 0.99 (0.96–1.01) | 0.99 (0.96–1.02) | 0.99 (0.95–1.02) | 0.98 (0.92–1.04) | 0.99 (0.91–1.07) |
| 5 (greatest diversity) | 1.01 (0.99–1.03) | 0.99 (0.97–1.01) | 0.98 (0.95–1.02) | 0.98 (0.94–1.01) | 0.97 (0.90–1.04) | 0.95 (0.87–1.05) |
| Neighbourhood-level economic dependency | ||||||
| 1 (least dependency)‡ | – | – | – | – | – | – |
| 2 | 0.98 (0.97–1.00) | 0.98 (0.96–1.00) | 0.99 (0.96–1.03) | 0.99 (0.96–1.02) | 1.01 (0.96–1.06) | 1.02 (0.96–1.08) |
| 3 | 0.98 (0.96–0.99) | 0.97 (0.95–0.99) | 0.99 (0.95–1.02) | 0.98 (0.95–1.01) | 0.97 (0.92–1.02) | 0.96 (0.90–1.029) |
| 4 | 0.97 (0.95–0.99) | 0.96 (0.94–0.98) | 0.98 (0.95–1.01) | 0.98 (0.95–1.01) | 0.95 (0.90–1.01) | 0.92 (0.85–1.00) |
| 5 (greatest dependency) | 0.97 (0.95–0.99) | 0.96 (0.94–0.98) | 0.98 (0.95–1.02) | 0.98 (0.94–1.01) | 0.96 (0.90–1.02) | 0.95 (0.88–1.03) |
| Neighbourhood-level residential instability | ||||||
| 1 (least instability)‡ | – | – | – | – | – | – |
| 2 | 1.01 (0.99–1.03) | 1.01 (0.99–1.04) | 0.99 (0.96–1.03) | 0.99 (0.96–1.02) | 1.02 (0.96–1.09) | 1.00 (0.93–1.08) |
| 3 | 1.01 (0.99–1.03) | 1.01 (0.99–1.03) | 0.99 (0.96–1.03) | 0.99 (0.96–1.02) | 1.03 (0.97–1.09) | 1.04 (0.97–1.12) |
| 4 | 0.99 (0.97–1.01) | 0.99 (0.97–1.02) | 1.00 (0.96–1.03) | 0.99 (0.96–1.03) | 1.05 (0.99–1.11) | 1.05 (0.98–1.13) |
| 5 (greatest instability) | 0.99 (0.97–1.01) | 0.99 (0.96–1.01) | 0.99 (0.95–1.03) | 0.99 (0.95–1.02) | 0.99 (0.93–1.06) | 0.99 (0.90–1.08) |
| Use of assisted reproductive technology for current pregnancy | 1.00 (0.98–1.02) | 0.99 (0.97–1.02) | 1.00 (0.92–1.09) | 1.00 (0.93–1.08) | 1.05 (0.95–1.15) | 1.01 (0.90–1.13) |
| Early prenatal care visits§ | 1.00 (1.00–1.01) | 1.01 (1.00–1.01) | 1.00 (1.00–1.01) | 1.01 (1.00–1.01) | 1.01 (0.99–1.02) | 1.01 (0.90–1.13) |
| Parity | ||||||
| 0‡ | – | – | – | – | – | – |
| 1 | 0.98 (0.97–0.99) | 0.97 (0.96–0.98) | 0.99 (0.96–1.01) | 0.98 (0.96–1.00) | 0.99 (0.95–1.03) | 0.99 (0.94–1.04) |
| 2 | 0.92 (0.90–0.93) | 0.88 (0.86–0.90) | 0.96 (0.93–0.99) | 0.95 (0.92–0.98) | 0.94 (0.89–1.01) | 0.86 (0.79–0.94) |
| ≥ 3 | 0.84 (0.82–0.87) | 0.77 (0.74–0.80) | 0.94 (0.90–0.98) | 0.92 (0.89–0.96) | 0.77 (0.68–0.88) | 0.64 (0.53–0.78) |
| Primary care contact | ||||||
| Rostered to family physician | – | – | 1.00 (0.97–1.02) | 1.00 (0.98–1.02) | – | – |
| Primary care model¶ | ||||||
| Capitation‡ | – | – | – | – | – | – |
| Comprehensive care model | – | – | 1.02 (0.95–1.09) | 1.00 (0.93–1.06) | – | – |
| Missing | – | – | 1.00 (0.97–1.04) | 1.00 (0.97–1.04) | – | – |
| Other | – | – | 1.01 (0.98–1.04) | 1.01 (0.98–1.04) | – | – |
Note: CI = confidence interval, RR = relative risk.
Adjusted for maternal age, rurality, Canadian Index of Multiple Deprivation measures (i.e., ethnocultural composition, economic dependency, and residential instability), use of assisted reproductive technology, early prenatal care visits, and parity.
Also adjusted for whether the pregnant person was attached or rostered to their family physician and the physicians’ practice model.
Reference group.
Early prenatal care visits occurred before 32 weeks of gestation.
Capitation-based models include teams of 6 or more physicians who are compensated primarily through capitation payments but also receive fee-for-service payments. Comprehensive care model physicians are compensated primarily through fee-for-service. Both capitation and comprehensive care model physicians are eligible for specific bonuses and premiums based on patient enrolment. Other patient enrolment models were grouped together owing to small cell sizes and include family health groups and specialized models (such as general practice– or focused practice–physicians with alternative funding plans).
Across all clinician types, Tdap vaccination increased from 2019 to 2022 (Table 6). In multivariable analysis, later birth group, older age, and more early prenatal care visits were associated with greater uptake, and rurality and parity with lower uptake. Among patients of family physicians, Tdap vaccination differed by primary care model
Table 6:
Crude and adjusted analyses of tetanus, diphtheria, and pertussis vaccination, by prenatal care provider type
| Type of prenatal care provider | Obstetrician n = 56 717 |
Family physician n = 12 308 |
Midwife n = 14 372 |
|---|---|---|---|
| Crude analysis | RR (95% CI) | ||
| Birthing group | |||
| 2019 birth* | – | – | – |
| 2021 birth | 1.46 (1.41–1.51) | 1.28 (1.21–1.36) | 1.42 (1.32–1.54) |
| 2022 birth | 1.54 (1.49–1.60) | 1.22 (1.15–1.30) | 1.55 (1.44–1.67) |
| Adjusted analysis | Adjusted RR § (95% CI) | ||
| Birthing group | |||
| 2019 birth* | – | – | – |
| 2021 birth | 1.49 (1.45–1.55) | 1.32 (1.24–1.40) | 1.46 (1.35–1.57) |
| 2022 birth | 1.56 (1.51–1.61) | 1.26 (1.09–1.33) | 1.56 (1.45–1.68) |
| Sociodemographic characteristics | |||
| Age, yr | 1.02 (1.02–1.03) | 1.02 (1.01–1.02) | 1.04 (1.03–1.04) |
| Rural residence | 0.82 (0.75–0.90) | 0.81 (0.75–0.88) | 0.77 (0.67–0.89) |
| Neighbourhood-level ethnocultural composition | |||
| 1 (least diversity)* | – | – | – |
| 2 | 1.06 (1.00–1.12) | 1.11 (1.03–1.20) | 1.13 (1.02–1.25) |
| 3 | 1.18 (1.11–1.24) | 1.09 (1.00–1.17) | 1.29 (1.17–1.42) |
| 4 | 1.21 (1.15–1.28) | 1.10 (1.01–1.19) | 1.28 (1.16–1.41) |
| 5 (greatest diversity) | 1.24 (1.17–1.30) | 1.03 (0.95–1.11) | 1.01 (0.90–1.13) |
| Neighbourhood-level economic dependency | |||
| 1 (least dependency)* | – | – | – |
| 2 | 0.98 (0.94–1.01) | 0.97 (0.91–1.04) | 0.87 (0.81–0.94) |
| 3 | 0.91 (0.88–0.95) | 1.00 (0.94–1.08) | 0.82 (0.76–0.90) |
| 4 | 0.88 (0.85–0.92) | 0.95 (0.88–1.02) | 0.82 (0.75–0.89) |
| 5 (greatest dependency) | 0.87 (0.83–0.91) | 0.92 (0.85–1.00) | 0.75 (0.68–0.83) |
| Neighbourhood-level residential instability | |||
| 1 (least instability)* | – | – | – |
| 2 | 0.97 (0.92–1.01) | 1.00 (0.92–1.08) | 0.98 (0.89–1.08) |
| 3 | 0.94 (0.90–0.98) | 0.96 (0.89–1.03) | 0.92 (0.84–1.01) |
| 4 | 0.92 (0.88–0.96) | 0.88 (0.82–0.95) | 0.95 (0.87–1.04) |
| 5 (greatest instability) | 0.89 (0.85–0.93) | 0.92 (0.85–1.00) | 0.89 (0.81–0.98) |
| Perinatal health services contact | |||
| Use of assisted reproductive technology for current pregnancy | 0.92 (0.87–0.98) | 0.96 (0.81–1.14) | 1.06 (0.92–1.22) |
| Early prenatal care visits† | 1.03 (1.03–1.04) | 1.05 (1.04–1.06) | 1.04 (1.03–1.05) |
| Parity | |||
| 0* | – | – | – |
| 1 | 0.80 (0.78–0.82) | 0.88 (0.84–0.93) | 0.78 (0.73–0.83) |
| 2 | 0.60 (0.57–0.62) | 0.75 (0.69–0.81) | 0.57 (0.51–0.64) |
| ≥ 3 | 0.43 (0.40–0.47) | 0.59 (0.23–0.67) | 0.39 (0.32–0.46) |
| Primary care contact | |||
| Rostered to family physician | – | 0.99 (0.94–1.04) | – |
| Primary care model‡ | |||
| Capitation* | – | – | – |
| Comprehensive care model | – | 0.67 (0.53–0.85) | – |
| Missing | – | 0.63 (0.56–0.70) | – |
| Other | – | 1.09 (1.03–1.16) | – |
Note: CI = confidence interval, RR = relative risk.
Reference group.
Early prenatal care visits occurred before 32 weeks of gestation.
Capitation-based models include teams of 6 or more physicians who are compensated primarily through capitation payments but also receive fee-for-service payments. Comprehensive care model physicians are compensated primarily through fee-for-service. Both capitation and comprehensive care model physicians are eligible for specific bonuses and premiums based on patient enrolment. Other patient enrolment models were grouped together owing to small cell sizes and include family health groups and specialized models (such as general practice– or focused practice–physicians with alternative funding plans).
Adjusted for birth group, maternal age, rurality, Canadian Index of Multiple Deprivation measures (i.e., ethnocultural composition, economic dependency, residential instability), use of assisted reproductive technology, early prenatal care visits, and parity.
The overall birthing cohort contained 7916 (9.1%) people younger than age 25 years. Of the 28 376 people who gave birth in the 2022 group, 2275 (8.0%) were younger than 25 years. Crude analysis showed that they were significantly less likely to receive either the first or second SARS-CoV-2 vaccine than birthing people aged 25 years and older. After model adjustment, this difference decreased but remained significant (Appendix 5, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231522/tab-related-content).
The comparator cohort contained 585 009 nonpregnant females whom we hard-matched by age to the cohort of pregnant people younger than 25 years, resulting in 2191 matches. This analysis showed that young people who gave birth were significantly less likely than matched comparators to receive either dose of SARS-CoV-2 vaccination in both the unadjusted and matched models (Appendix 6, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.231522/tab-related-content).
Interpretation
Among the first group of Ontario residents eligible for SARS-CoV-2 vaccination during their entire pregnancies, most were vaccinated (78.7%) and completed the 2-dose series (74.2%). This is a high uptake for a new vaccine or any vaccine during pregnancy, likely driven by a pandemic-related sense of urgency, including emerging evidence on the risks of SARS-CoV-2 infection to pregnant people and the possibility of benefit to the newborn from transplacental passage of antibodies after vaccination.22,49,50 As hypothesized, uptake of SARS-CoV-2 vaccination was slightly lower among pregnant people than among matched nonpregnant females in Ontario, despite priority access for pregnant people. That said, only 3.9% delayed vaccination until after delivery, suggesting that the decision not to take the vaccine was either not strongly tied to specific concerns about pregnancy or continued owing to hesitancy about vaccination while breastfeeding. Given that SARS-CoV-2 vaccination was largely administered in special vaccination clinics rather than during routine office visits in 2022, it is unsurprising that prenatal clinician type was not associated with vaccine receipt.
In contrast to the rate of SARS-CoV-2 vaccination, a minority of pregnant people received Tdap (28.7%) or influenza (30.6%) vaccination during pregnancy, suggesting that they weighed the benefits and risks of the vaccines differently. Contrary to our second hypothesis that the pandemic would have a negative impact on Tdap and influenza vaccination, Tdap uptake increased considerably, from 22.2% in 2019 to 31.4% in 2021 and 32.6% in 2022. Whereas an increase of Tdap vaccination has been observed by others using a similar data set from 2011/12 to 2019/20,37 the large increase from 2019 to 2021 may have been associated with increased attention to vaccination related to the release of SARS-CoV-2 vaccines, as influenza vaccination also rose in 2021. However, uptake of influenza vaccination did not follow the same trend as Tdap vaccination uptake, falling back to prepandemic levels in 2022. Our third hypothesis, that those who received Tdap vaccination would be more likely to receive SARS-CoV-2 vaccination was supported by this analysis, suggesting that those who did not receive routine vaccinations during pregnancy were at greater risk of also not receiving pandemic vaccinations. However, given different data sources for SARS-CoV-2 vaccination and Tdap and influenza vaccinations, we believe this finding requires further research.
As has been previously observed with Tdap and influenza vaccination during pregnancy,30 those seen by family physicians were more likely to receive Tdap vaccination than those attended by obstetricians or midwives,30,34,35 possibly owing to family practice offices being equipped to offer vaccination onsite. These findings suggest that stocking and administering recommended vaccinations in obstetric and midwifery practices could increase uptake among pregnant people. Pregnant people younger than 25 years, along with rural residents, those who had previously given birth, and those residing in neighbourhoods with greater economic dependency were significantly less likely to receive vaccination during pregnancy, suggesting that inequities that posed barriers to Tdap and influenza vaccination in the past continued during the COVID-19 pandemic despite greater focus on vaccination and increased overall receipt of vaccination during pregnancy. Addressing disparities in access to vaccination may help close this gap.
Future research should examine uptake of SARS-CoV-2 vaccination among pregnant people over time and compare jurisdictions with different vaccine implementation approaches and social or epidemiologic conditions. Qualitative research examining pregnant people’s experiences with vaccine decisions during the pandemic could help explain the patterns observed. People who are pregnant or planning to become pregnant should be included in clinical trials of vaccines intended for their use, to provide high-quality evidence to support clinical and public health communication and decision-making.51 Despite the challenges this may pose for safety surveillance of new vaccines, offering coadministration of vaccines (e.g., Tdap and influenza vaccination at the same time as SARS-CoV-2 vaccination, rather than potentially requiring patients to prioritize and make multiple appointments) could improve uptake. Because additional vaccines may be recommended in Canada during pregnancy (e.g., the newly approved vaccine against respiratory syncytial virus,52 potential future vaccines against group B streptococcal disease,53 or against future pandemic pathogens), issues around collecting safety surveillance data for new vaccines and coadministration with other vaccinations should be considered. Although the predictors of receiving 1 or 2 doses were similar in this analysis, future research should investigate whether there are observable differences (e.g., in timing during or in relation to pregnancy between the minority of vaccine recipients who received only 1 dose and the majority who received both doses, and seek to identify predictors of receiving subsequent doses among those who received a first dose. Given low uptake of additional doses that have been recommended beyond the initial 2-dose series, this may be increasingly of interest if SARS-CoV-2 vaccination is eventually recommended in every pregnancy, similar to Tdap and influenza vaccinations.
Limitations
Ascertaining Tdap and influenza vaccination status from OHIP billing claims likely underestimated the total number of vaccinated people.35,37 Prior estimates of OHIP vaccination data compared with self-report of influenza vaccination using the Canadian Community Health Survey found a sensitivity of around 50% and a specificity of 95.7%–98%.54,55 On the other hand, evidence suggests that the COVaxON database from which we obtained SARS-CoV-2 vaccination data is more complete.56 We were unable to assess some factors influencing vaccine receipt among pregnant people, such as individual- or community-level access to vaccination and severity of COVID-19 outbreaks locally. People who gave birth in January 2022 may have had poorer access to SARS-CoV-2 vaccines than those who gave birth in March, although data (Appendix 3) suggest minimal overall impact of time within our 3-month window. Additionally, some people may have received vaccination before becoming pregnant or before realizing they were pregnant. In addition, the CIMD data were created from the 2016 Census, which may not reflect the birthing person’s actual residence or social position in 2019–2022, and there are known limitations of using area-level CIMD quintiles to make individual-level inferences.47,57 However, with our large data set and propensity score–matching methods, we were able to control for many known confounders.
Conclusion
Among pregnant people in Ontario in early 2022, 78.7% received 1 dose of SARS-CoV-2 vaccine and 74.2% received a second dose. Although this was a lower rate than among nonpregnant comparators, it was higher than the rate of either Tdap (32.6%) or influenza (27.7%) vaccination in the same year. Rates of Tdap vaccination rose considerably from 2019 to 2022. Influenza vaccination was higher in 2021 but returned to 2019 levels the following year. Vaccination rates were lower among pregnant people who were young, multiparous, or lived in rural or economically deprived areas. Pandemic urgency may have overcome a great deal of hesitancy about vaccinating against SARS-CoV-2 during pregnancy in 2022, but uptake of routinely recommended vaccines in pregnancy remains a challenge.
Acknowledgements
The authors acknowledge research coordination assistance from Marina Sadik, Andrea Carruthers, and Cassandra Kuyvenhoven, as well as contributions from collaborators on the larger grant.
Footnotes
Competing interests: Devon Greyson reports support for other research from the Canadian Institutes of Health Research (CIHR), British Columbia Immunization Committee, Canadian Immunization Research Network, BC Children’s Hospital Research Canucks for Kids Fund, University of British Columbia (UBC) Hampton Fund, UBC Faculty of Medicine, and Social Sciences and Humanities Research Council. Dr. Greyson also reports honoraria from the University of Sydney and University of Oulu, and unpaid membership with the Association for Information Science and Technology Special Interest Group on Health Information Executive Committee. Meredith Vanstone reports CIHR salary support from Canada Research Chairs program, Banting Postdoctoral Fellowships, Michael Smith Health Research BC Scholar Award, and CIHR/Public Health Agency of Canada (PHAC) Applied Public Health Chair.
This article has been peer reviewed.
Contributors: Devon Greyson, Meredith Vanstone, Rebecca Correia, Elizabeth Darling and Michelle Howard designed the study. Rebecca Correia, Devon Greyson, David Kirkwood, and Meredith Vanstone conducted the data analysis. All authors contributed to interpretation of data. Devon Greyson and Rebecca Correia drafted the manuscript. All authors critically revised it, gave approval of the final version to be published, and agreed to be accountable for all aspects of the work.
Funding: This work was supported by the CIHR (grant no. 179921). Meredith Vanstone is supported by a Tier 2 Canada Research Chair. Monica Molinaro has been supported by a Banting Postdoctoral Fellowship. Devon Greyson is supported by a Michael Smith Health Research BC Scholar Award and a CIHR/PHAC Applied Public Health Chair.
Data sharing: The administrative data set for this study is held securely in coded form at ICES. While legal data-sharing agreements between these data stewards and data providers prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access. Readers are welcome to contact the research team for further information. Data queries to ICES can be directed to Data and Analytic Services at das@ices.on.ca.
Disclaimer: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH), the Ministry of Long-Term Care, and the Ontario Health Data Platform (OHDP), a Province of Ontario initiative to support Ontario’s ongoing response to COVID-19 and its related impacts. This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under licence from Canada Post Corporation and Statistics Canada. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information and Ontario MOH. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. No endorsement by the OHDP, its partners, or the Province of Ontario is intended or should be inferred.
References
- 1.Woodworth KR, O’Malley Olsen E, Neelam V, et al. CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team; COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT). Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy: SET-NET, 16 Jurisdictions, March 29–October 14, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zambrano LD, Ellington S, Strid P, et al. CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team. Update: Characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status: United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei SQ, Bilodeau-Bertrand M, Liu S, et al. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 2021;193: E540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTER-COVID multinational cohort study. JAMA Pediatr 2021;175:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allotey J, Stallings E, Bonet M, et al. PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mark EG, McAleese S, Golden WC, et al. Coronavirus disease 2019 in pregnancy and outcomes among pregnant women and neonates: a literature review. Pediatr Infect Dis J 2021;40:473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandt JS, Fell DB. SARS-CoV-2 infection in pregnancy: lessons learned from the first pandemic wave. Paediatr Perinat Epidemiol 2021;35:34–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor MM, Kobeissi L, Kim C, et al. Inclusion of pregnant women in COVID-19 treatment trials: a review and global call to action. Lancet Glob Health 2021;9: e366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kons KM, Wood ML, Peck LC, et al. Exclusion of reproductive-aged women in COVID-19 vaccination and clinical trials. Womens Health Issues 2022;32:557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salloum M, Paviotti A, Bastiaens H, et al. The inclusion of pregnant women in vaccine clinical trials: an overview of late-stage clinical trials’ records between 2018 and 2023. Vaccine 2023;41:7076–83. [DOI] [PubMed] [Google Scholar]
- 12.Regan AK, Fell DB, Wise LA, et al. Challenges and opportunities for the epidemiological evaluation of the effects of COVID-19 vaccination on reproduction and pregnancy. Vaccine 2023;41:5931–5. [DOI] [PubMed] [Google Scholar]
- 13.Archived 9: National Advisory Committee on Immunization — Summary of updated vaccine statement of May 3, 2021. Ottawa: Public Health Agency of Canada; modified 2021 Dec. 23. Available: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines/summary-updated-statement-may-3-2021.html (accessed 2023 July 10). [Google Scholar]
- 14.Poliquin V, Castillo E, Boucoiran I, et al. SOGC statement on COVID-19 vaccination in pregnancy. Ottawa: The Society of Obstetricians and Gynaecologists of Canada (SOGC); 2020, revised 2022 Mar. 14:1–12. Available: https://sogc.org/common/Uploaded%20files/Latest%20News/SOGC_Statement_COVID-19_Vaccination_in_Pregnancy.pdf (accessed 2023 July 10). [Google Scholar]
- 15.Report #5: COVID-19 vaccination during pregnancy in Ontario — December 14, 2020 to May 31, 2022. Ottawa: BORN Ontario. Available: https://www.bornontario.ca/en/whats-happening/resources/Documents/COVID-19-Vaccination-during-pregnancy-in-Ontario_Report_Dec2020-May2022.pdf (accessed 2023 July 10). [Google Scholar]
- 16.Magnus MC, Gjessing HK, Eide HN, et al. COVID-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med 2021;385:2008–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, D’Souza R, Kharrat A, et al. COVID-19 pandemic and population-level pregnancy and neonatal outcomes: a living systematic review and meta-analysis. Acta Obstet Gynecol Scand 2021;100:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fell DB, Dhinsa T, Alton GD, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA 2022;327:1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fell D, Dimanlig-Cruz S, Török E, et al. Risk of preterm birth, small-for-gestational-age at birth, and stillbirth after receiving a booster dose of COVID-19 vaccine during pregnancy. J Obstet Gynaecol Can 2023;45:353. [Google Scholar]
- 20.Jorgensen SCJ, Drover SSM, Fell DB, et al. Newborn and early infant outcomes following maternal COVID-19 vaccination during pregnancy. JAMA Pediatr 2023; 177:1314–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skjefte M, Ngirbabul M, Akeju O, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol 2021;36:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramlawi S, Muldoon KA, Dunn SI, et al. Worries, beliefs and factors influencing perinatal COVID-19 vaccination: a cross-sectional survey of preconception, pregnant and lactating individuals. BMC Public Health 2022;22:2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misinformation about COVID-19 vaccines and pregnancy is widespread, including among women who are pregnant or planning to get pregnant [news release]. San Francisco: Kaiser Family Foundation; 2022. May 27. Available: https://www.kff.org/coronavirus-covid-19/press-release/misinformation-about-covid-19-vaccines-and-pregnancy-is-widespread-including-among-women-who-are-pregnant-or-planning-to-get-pregnant/ (accessed 2023 July 10). [Google Scholar]
- 24.Eldeib D. How misinformation about COVID vaccines and pregnancy took root early on and why it won’t go away. New York: ProPublica; 2022. Available: https://www.propublica.org/article/covid-misinformation-pregnancy-vaccine-testing (accessed 2023 July 10). [Google Scholar]
- 25.Schraer R. Covid: pregnant women targeted with false vaccine claims. BBC News 2022. Aug. 31. Available: https://www.bbc.com/news/health-62739554 (accessed 2023 July 10).
- 26.Hsu AL, Johnson T, Phillips L, et al. Sources of vaccine hesitancy: pregnancy, infertility, minority concerns, and general skepticism. Open Forum Infect Dis 2022; 9:ofab433. doi: 10.1093/ofid/ofab433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isaacs-Thomas B. There’s no evidence COVID-19 vaccines hurt fertility. Here’s what’s fueling the myth. PBS News 2021. Sept. 16. Available: https://www.pbs.org/newshour/health/how-anti-vaccine-scare-tactics-exploit-our-fertility-anxieties (accessed 2023 July 10).
- 28.Fell DB, Török E, Sprague AE, et al. Temporal trends and determinants of COVID-19 vaccine coverage and series initiation during pregnancy in Ontario, Canada, December 2020 to December 2021: a population-based retrospective cohort study. Vaccine 2023;41:1716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Török E, Dhinsa T, Dimanlig-Cruz S, et al. Temporal trends and determinants of COVID-19 vaccine series initiation after recent pregnancy. Hum Vaccin Immunother 2023;19:2215150. doi: 10.1080/21645515.2023.2215150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poliquin V, Greyson D, Castillo E. A systematic review of barriers to vaccination during pregnancy in the Canadian context. J Obstet Gynaecol Can 2019;41: 1344–55. [DOI] [PubMed] [Google Scholar]
- 31.Regan AK, Mak DB, Hauck YL, et al. Trends in seasonal influenza vaccine uptake during pregnancy in Western Australia: implications for midwives. Women Birth 2016;29:423–9. [DOI] [PubMed] [Google Scholar]
- 32.Cleary BJ, Rice Ú, Eogan M, et al. 2009 A/H1N1 influenza vaccination in pregnancy: uptake and pregnancy outcomes: a historical cohort study. Eur J Obstet Gynecol Reprod Biol 2014;178:163–8. [DOI] [PubMed] [Google Scholar]
- 33.Barber A, Muscoplat MH, Fedorowicz A. Coverage with tetanus, diphtheria, and acellular pertussis vaccine and influenza vaccine among pregnant women: Minnesota, March 2013–December 2014. MMWR Morb Mortal Wkly Rep 2017; 66:56–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halperin BA, MacKinnon-Cameron D, McNeil S, et al. Maintaining the momentum: key factors influencing acceptance of influenza vaccination among pregnant women following the H1N1 pandemic. Hum Vaccin Immunother 2014; 10:3629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Results of the survey on vaccination during pregnancy 2021. Ottawa: Public Health Agency of Canada; modified 2022 Dec. 13. Available: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/survey-vaccination-during-pregnancy-2021.html (accessed 2023 July 10). [Google Scholar]
- 36.Brophy J, Baclic O, Tunis M; National Advisory Committee on Immunization (NACI). Summary of the NACI update on immunization in pregnancy with tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis (Tdap) vaccine. Can Commun Dis Rep 2018;44:91–4 Available: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2018-44/issue-3-4-march-1-2018/article-5-update-immunization-pregnancy-vaccine-2018.html (accessed 2023 Apr. 14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fakhraei R, Fung SG, Petrcich W, et al. Trends and characteristics of Tdap vaccination during pregnancy in Ontario, Canada: a retrospective cohort study. CMAJ Open 2022;10:E1017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sell H, Assi A, Driedger SM, et al. Continuity of routine immunization programs in Canada during the COVID-19 pandemic. Vaccine 2021;39:5532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.COVID-19 pandemic fuels largest continued backslide in vaccinations in three decades [news release]. Geneva: World Health Organization; 2022. July 15, 2022. Available: https://www.who.int/news/item/15-07-2022-covid-19-pandemic-fuels-largest-continued-backslide-in-vaccinations-in-three-decades (accessed 2023 July 10). [Google Scholar]
- 40.Updated guidance on influenza vaccination during pregnancy. Public Health Agency of Canada; modified 2023 Dec. 18:1–81. Available: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-updated-guidance-influenza-vaccination-during-pregnancy.html (accessed 2024 May 25). [Google Scholar]
- 41.Sander B, Kwong JC, Bauch CT, et al. Economic appraisal of Ontario’s Universal Influenza Immunization Program: a cost–utility analysis. PLoS Med 2010;7:e1000256. doi: 10.1371/journal.pmed.1000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mijović H, Greyson D, Gemmell E, et al. Perinatal health care providers’ approaches to recommending and providing pertussis vaccination in pregnancy: a qualitative study. CMAJ Open 2020;8:E377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tdap (tetanus, diphtheria, pertussis) Vaccine Program. Toronto: Ministry of Health and Long-Term Care:1-5. Available: https://www.health.gov.on.ca/en/public/programs/immunization/docs/tdap_fs_en.pdf (accessed 2023 Aug. 4). [Google Scholar]
- 44.Vanstone M, Correia R, Howard M, et al. How do perceptions of COVID-19 risk impact pregnancy-related health decisions? A convergent parallel mixed-methods study protocol. PLoS One 2023;18:e0288952. doi: 10.1371/journal.pone.0288952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benchimol EI, Smeeth L, Guttmann A, et al. RECORD Working Committee. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 2015;12:e1001885. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Statistics Canada, Canadian Vital Statistics: Birth database (CVSB). Ottawa: Statistics Canada; 2022. Sept. 27. Available: https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&Id=1456141 (accessed 2024 Mar. 8). [Google Scholar]
- 47.The Canadian Index of Multiple Deprivation: user guide. Ottawa: Statistics Canada; 2019. Available: https://publications.gc.ca/site/eng/9.871994/publication.html (accessed 2023 Aug. 14). [Google Scholar]
- 48.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flannery DD, Gouma S, Dhudasia MB, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr 2021;175:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jorgensen SCJ, Burry L, Tabbara N. Role of maternal COVID-19 vaccination in providing immunological protection to the newborn. Pharmacotherapy 2022; 42: 58–70. [DOI] [PubMed] [Google Scholar]
- 51.Committee on Ethics. ACOG Committee Opinion No. 646: Ethical considerations for including women as research participants. Obstet Gynecol 2015; 126: e100–7. [DOI] [PubMed] [Google Scholar]
- 52.Summary of NACI statement of May 17, 2024: statement on the prevention of respiratory syncytial virus disease in infants. Ottawa: Public Health Agency of Canada; updated 2024 June 21:1–6. Available: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/national-advisory-committee-immunization-summary-statement-prevention-respiratory-syncytial-virus-disease-infants.html (accessed 2024 Aug. 3). [Google Scholar]
- 53.Madhi SA, Anderson AS, Absalon J, et al. Potential for maternally administered vaccine for infant group B streptococcus. N Engl J Med 2023;389:215–27. [DOI] [PubMed] [Google Scholar]
- 54.Kwong JC, Manuel DG. Using OHIP physician billing claims to ascertain individual influenza vaccination status. Vaccine 2007;25:1270–4. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz KL, Jembere N, Campitelli MA, et al. Using physician billing claims from the Ontario Health Insurance Plan to determine individual influenza vaccination status: an updated validation study. CMAJ Open 2016;4:E463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hobbs JL, Paul LA, Buchan SA, et al. Methodological changes implemented over time to support accurate and timely COVID-19 vaccine coverage estimates: Ontario, Canada. Vaccine 2023;41:3328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pampalon R, Hamel D, Gamache P, et al. A deprivation index for health planning in Canada. Chronic Dis Can 2009;29:178–91. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.