Abstract
The uterine lining (endometrium) regenerates repeatedly over the life span as part of its normal physiology. Substantial portions of the endometrium are shed during childbirth (parturition) and, in some species, menstruation, but the tissue is rapidly rebuilt without scarring, rendering it a superlative model of regeneration in mammals. Nonetheless, following some assaults, including medical procedures and infections, the endometrium fails to regenerate and instead forms scars that may interfere with normal endometrial function and contribute to infertility. Thus, the endometrium provides an exceptional platform to answer a central question of regenerative medicine: Why do some systems regenerate while others scar? Here, we review our current understanding of the diverse set of endometrial injuries in humans, nonhuman primates, and rodents, and the associated mechanisms of regenerative success and failure. Elucidating the determinants of these disparate wound responses promises insights into fundamental mechanisms of mammalian regeneration with substantial implications for reproductive health.
Keywords: menstruation, pregnancy, Asherman syndrome, intrauterine adhesion, stem cell, parturition
1. INTRODUCTION
Throughout the human life span, the uterus displays a striking capacity for regeneration. The uterine lining (endometrium) undergoes extensive remodeling before, during, and after pregnancy, as well as programmed shedding and repair during each menstrual cycle. Consequently, the endometrium must regenerate approximately 400 times over the reproductive life span to restore tissue integrity and facilitate future pregnancy. Endometrial dysfunction contributes to infertility and a wealth of poorly understood health conditions, including endometrial cancer, endometriosis, and abnormal uterine bleeding, which respectively affect 1 in 32, 1 in 10, and >1 in 10 people with a uterus in their lifetimes (Howlader et al. 2021, Liu et al. 2007, Zondervan et al. 2020). Thus, unraveling the intricacies of endometrial regeneration holds tremendous promise for unlocking fundamental mechanisms of mammalian regeneration and improving the lives of hundreds of millions of individuals worldwide.
Uterine function relies on a complex architecture that must be restored after each menstruation or pregnancy (Figure 1a). The uterine cavity is lined with an epithelial monolayer on top of a supportive mesenchymal compartment (stroma) and underlying smooth muscle layer (myometrium). The surface epithelium (luminal epithelium) forms glandular offshoots (glandular epithelium) that project into the stroma and merge into a highly branched plexus running parallel to the myometrial border (Tempest et al. 2020, Yamaguchi et al. 2021). Recent single-cell transcriptomic approaches have revealed extensive heterogeneity among endometrial stromal and epithelial populations, illuminating the complexity of this important tissue (Garcia-Alonso et al. 2021; Kirkwood et al. 2021, 2022; Wang et al. 2020; Winkler et al. 2022).
Figure 1.
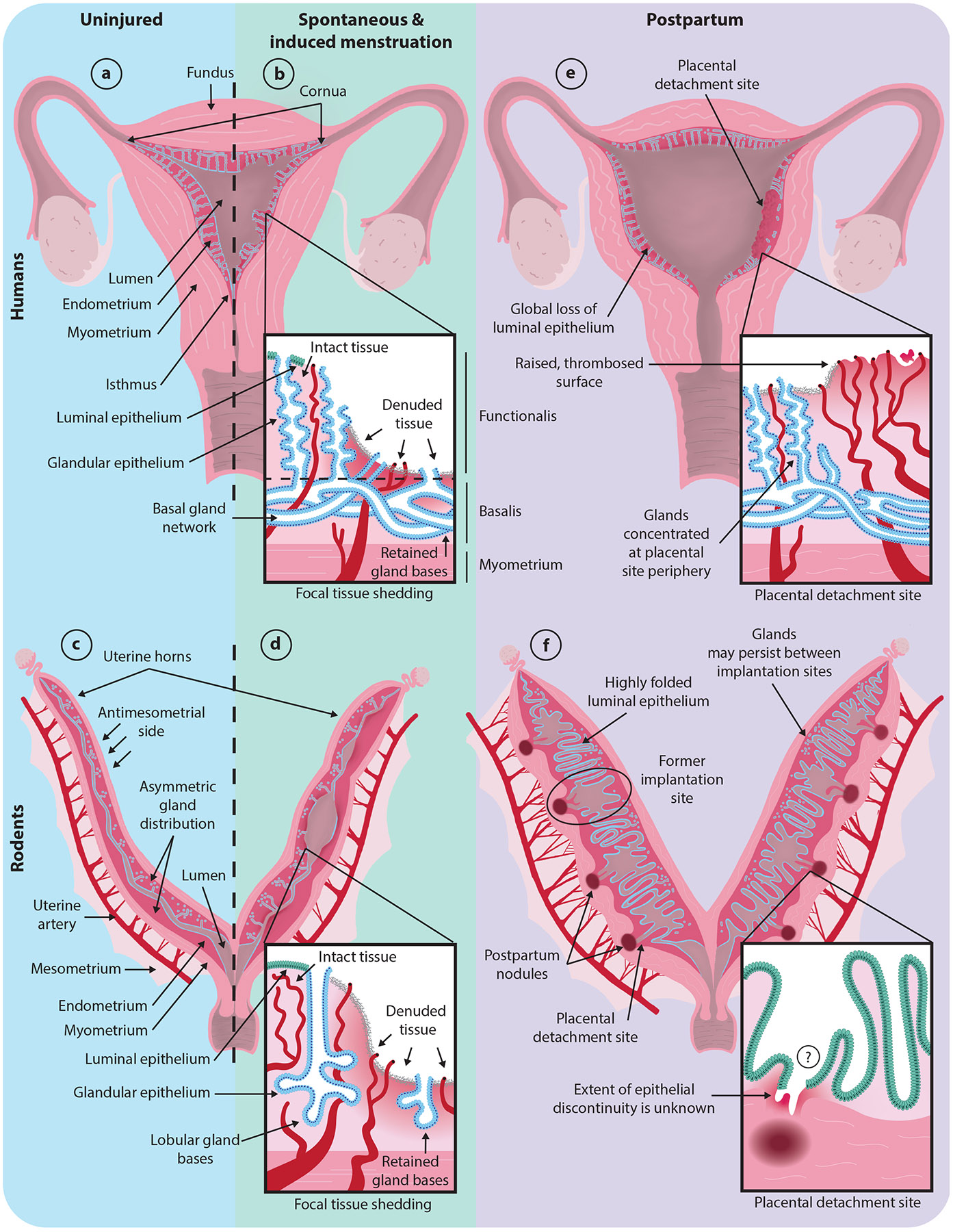
Working models of physiological disruption of the endometrium (dark magenta). Luminal epithelium is shown in green; glandular epithelium is shown in blue; and blood vessels are shown in dark red. The insets illustrate zoomed-in models of the indicated damaged regions. (a) Intact human uterus. (b) Human uterus undergoing menstruation. (c) Intact mouse uterus. (d) Mouse uterus undergoing experimentally induced tissue shedding as a model of menstruation. (e) Human uterus within one day of parturition. (f) Mouse uterus within one day of parturition. If or how postpartum gland bases connect to the lumen remains unclear. Note that the three-dimensional gland structure in the human and mouse postpartum uterus remains unknown. Mouse, but not human, uteri are approximately to scale across conditions.
A critical consideration for understanding endometrial regeneration is that the uterus is an organ defined by profound change. Over the menstrual cycle, fluctuations in estrogens (predominantly estradiol), progesterone, and androgens, as well as other hormones, alter cellular proliferation, cell type distributions, and tissue architecture (reviewed in Brenner & Slayden 2012, Gibson et al. 2020). Estrogen drives the expansion of the stroma and epithelium, resulting in thickening of the endometrium during the proliferative phase. Ovulation induces the transition to a progesterone-dominant stage (the secretory phase), during which glands become more tortuous and secrete products into their lumens (Gray et al. 2001, Tempest et al. 2020). If pregnancy is not established, the demise of the progesterone-producing corpus luteum leads to hormone withdrawal, resulting in focal shedding of the superficial layer of the endometrium (the functionalis) and exposure of the underlying layer (the basalis). If, instead, pregnancy occurs, the endometrium undergoes further remodeling to facilitate implantation and support fetal development. At the end of pregnancy, delivery, placental shearing, and subsequent necrosis remove large portions of the pregnant endometrium, which is termed the decidua (Isley 2021). Thus, endometrial function requires a capacity to regenerate from routine and variable tissue disruption.
In this review, we summarize current knowledge about the cellular responses to physiological events that breach the endometrium: menstruation and parturition. We highlight the evidence supporting numerous proposed mechanisms of endometrial regeneration, which may be differentially evoked in response to distinct damage. We provide an overview of a subset of nonphysiological injuries, particularly relating to medical procedures and infections, that are seemingly capable of overwhelming this regenerative repertoire, resulting in fibrosis. The examination of these fibrotic states, and the spontaneous reversion observed in some cases, provide an exciting opportunity to interrogate the determinants of regeneration, with substantial clinical implications. From this perspective, we discuss the possibility that several variables, ranging from the extent of tissue loss to hormone state, may contribute to conditions in which existing regenerative mechanisms are suppressed or diverted toward nonregenerative healing. A holistic approach to investigations of endometrial repair, considering both the foundational and clinical science of regenerative and nonregenerative healing, is critical to understanding and harnessing the remarkable regenerative potential of this tissue.
2. ENDOMETRIAL DISRUPTION DURING MENSTRUATION AND PARTURITION
The endometrium is rare among mammalian tissues in that it regularly experiences programmed tissue disruption. While both menstruation and parturition result in stereotyped tissue loss, they differ substantially in the localization and extent of damage, as well as the timescale of regeneration. Interrogating the extent to which diverse compartments are compromised is essential for understanding the regenerative burden imposed on the endometrium by these physiological events. In this section, we review the scale of damage induced by parturition, menstruation, and menstruation-like tissue shedding in primate and rodent models.
2.1. Menstruation
Menstruation is a carefully orchestrated, stepwise process of controlled tissue loss, primarily driven by declining progesterone levels at the end of the menstrual cycle (reviewed in Critchley et al. 2020, Jabbour et al. 2006). This process has been characterized extensively in the endometrium of the rhesus macaque. Just prior to menstruation, the macaque endometrium undergoes a 25–75% regression in size, and specialized uterine arteries constrict to mitigate blood loss (Markee 1940). In the first phase of menstruation, progesterone withdrawal leads to a reversible upregulation of inflammatory mediators, such as interleukins, cytokines, and chemokines (Kelly et al. 2001, Slayden & Brenner 2006, Wang et al. 2013). In the second phase, degradation effectors, including matrix metalloproteinases, are upregulated and begin to break down the extracellular matrix (ECM) (Brenner et al. 1996, Marbaix et al. 1996, Rodgers et al. 1994).
Hysteroscopic observations and examinations of endometrial specimens from menstruating human uteri suggest that menstrual shedding occurs in a piecemeal manner (Figure 1b). Patches of intact, sloughing, and regenerating endometrium can all be found concurrently during menstruation, limiting the amount of exposed stroma at any given time (Ferenczy 1976; Garry et al. 2009, 2010; Nogales-Ortiz et al. 1978; Novak & Te Linde 1924). The newly exposed basalis harbors remnants of the glandular epithelial network and arteries (Ferenczy 1976, Ludwig & Spornitz 1991, Markee 1940, Nogales-Ortiz et al. 1978, Novak & Te Linde 1924, Tempest et al. 2020, Tempest et al. 2022, Yamaguchi et al. 2021). Recently denuded stretches of stroma are quickly covered with fibrin, cell debris, and blood cells (Ferenczy 1976, Garry et al. 2009, Ludwig & Metzger 1976, Ludwig & Spornitz 1991), and new surface epithelium is identifiable as early as menstrual cycle day one, with re-epithelialization complete by day five or six (Ferenczy 1976). While the residual glands are commonly attributed as the primary sources of new epithelium (see Section 3), they are not the only reservoirs of unshed epithelium during menstruation. Mature luminal epithelium is retained near the uppermost uterine wall (fundus), utero-cervical junction (isthmus), and utero-tubal junctions (cornua) of the uterine cavity and may re-epithelialize nearby regions (Ferenczy 1976).
Outside of primates, very few species have been found to menstruate. Among these are several species of bat, the elephant shrew, and the spiny mouse, which is the only rodent known to menstruate (Bellofiore et al. 2016, Rasweiler & Debonilla 1992, Van Der Horst 1954). Among many of these species, common features of menstruation include immune cell influx, artery remodeling, and focal tissue shedding (reviewed in Catalini & Fedder 2020). However, there are also numerous differences between species, including the process of tissue shedding itself. For example, in elephant shrews, shedding is compartmentalized to one region of the uterus, as opposed to affecting the entire endometrium (Carter 2018, Van Der Horst 1954).
2.2. Modeling Menstruation
Due to the technical limitations and ethical considerations associated with the study of naturally menstruating species, substantial work has been done to model hormone-driven endometrial shedding in lab mice (Mus musculus) (reviewed in Liu et al. 2020). Mouse and human uteri display key anatomical differences (Figure 1a,c). Whereas the human uterus is composed of a single cavity with glands throughout the whole endometrium, the mouse uterus consists of two tubular horns and the endometrial glands are distributed asymmetrically (Figure 1c). Glands reside along one side of the horn, which is termed the antimesometrial side as it is opposite a supportive ligament (mesometrium) that encloses the uterine artery supplying blood to the organ. Despite these differences, endometrial biology in the mouse shares many common features with humans. For instance, mice undergo a similar hormonal cycle to humans, termed an estrous cycle, which is broken into two estrogen-dominant stages (proestrus and estrus) and two progesterone-dominant stages (metestrus and diestrus). As in humans, the mouse endometrium undergoes substantial remodeling across the hormonal cycle (Wood et al. 2007). Thus, the mouse provides a tractable model for the mechanistic dissection of endometrial biology.
Efforts to model menstruation in mice derive from observations that human menstruation follows a spontaneous stromal differentiation process called decidualization. In mice, natural decidualization only occurs in response to embryo implantation, but it can be artificially induced in nonpregnant mice by combining estrogen and progesterone priming with physical or chemical manipulations of the uterine lining, such as oil injection (Finn & Pope 1984). Following hormone withdrawal, the artificially decidualized mouse endometrium recapitulates key aspects of primate menstruation, including vaginal bleeding, focal tissue shedding that preserves gland bases near the myometrial border, and the expression of menstruation-associated molecular markers (Figure 1d) (Brasted et al. 2003; Cousins et al. 2014; Finn & Pope 1984; Kaitu’u-Lino et al. 2007, 2010; Rudolph et al. 2012; Xu et al. 2013). To achieve precise hormonal control, mice may be ovariectomized and receive cyclic estrogen and progesterone injections and/or progesterone-releasing implants before the induction of decidualization (Brasted et al. 2003). Other models rely on the natural progesterone fluctuations associated with mating an intact female with a vasectomized male (this induces a physiological state called pseudopregnancy), which may be paired with progesterone signaling inhibitors or ovariectomy to control bleeding onset (Patterson & Pru 2013, Rudolph et al. 2012).
In addition to mouse models of induced menstruation, alternative approaches are emerging to accommodate the experimental manipulation of human endometrial tissue. In xenograft models, human endometrial tissue fragments or dissociated cells are transplanted under the kidney capsules of ovariectomized, immunodeficient mice, which are then treated with estrogen and progesterone to promote graft growth (reviewed in Kuokkanen et al. 2017). Additionally, technological breakthroughs in three-dimensional tissue culture models and bioengineering techniques have led to the development of modular cocultures integrating multiple endometrial compartments, including epithelium, stroma, and vasculature (Abbas et al. 2020, Ahn et al. 2021, Cheung et al. 2021, Jamaluddin et al. 2022, Young & Huh 2021). Efforts are ongoing to build increasingly sophisticated in vitro models of the human endometrium, particularly with the goal of modeling human embryo implantation (reviewed in Li et al. 2022). Such models also hold great promise for recapitulating other complex aspects of human uterine regeneration, including menstruation, in vitro.
2.3. Parturition in Humans
Gestation and parturition also result in the substantial remodeling of the endometrium, which is restored to its prepartum state through a process called uterine involution. Much of what is known about this process comes from samples obtained through ethically fraught sterilization procedures (Haig 1995). The postpartum uterus, like the postmenstrual uterus, exhibits nonuniform endometrial damage and regeneration (Figure 1e). The placental detachment site presents with a distinct pattern of damage and timelines of repair compared to the nonplacental surfaces (Anderson & Davis 1968, Williams 1931). Although the surface epithelium is lost from both regions, the placental detachment site sustains deeper damage that preserves fewer gland remnants than surrounding areas (Anderson & Davis 1968, Benirschke et al. 2012, Sharman 1953, Williams 1931). As in menstrual shedding, an irregular meshwork of fibrin and erythrocytes rapidly covers denuded areas (Ludwig 1971). In contrast to postmenstrual regeneration, uterine involution takes place over weeks rather than days. Within the first week, islands of luminal epithelium begin to emerge at nonplacental peripheries, where remnant gland density is highest. By the fourth week, these nonplacental regions are completely re-epithelialized, and the underlying endometrial structure is restored (Anderson & Davis 1968, Williams 1931). In contrast, endometrial irregularities can be observed at the placental detachment site macroscopically until at least 7 weeks postpartum and can still be identified microscopically beyond this point (Anderson & Davis 1968, Benirschke et al. 2012, Friedländer 1870, Williams 1931). Rounds of new tissue growth and subsequent exfoliation at the perimeter of the detachment site may contribute to the gradual restoration of the superficial endometrial architecture, but it is unclear whether this process returns the placental site to its pregravid functional state (Williams 1931).
2.4. Parturition in Common Rodent Models
Despite differences between rodent and human implantation and placentation (Aplin & Ruane 2017, Hemberger et al. 2020), the damage sustained during parturition appears to share some common features in both species. As in humans, mouse placental detachment sites exhibit substantial tissue damage, with the placenta detaching partway through the mesometrial decidua (Deno 1937). Placental detachment sites are gradually restored through epithelial downgrowth from peripheral regions, mirroring placental site recovery in humans (Brandon 1994, Deno 1937). However, architectural differences between rodents and humans may necessitate different reparative processes in the postpartum uterus. For example, in humans, the luminal epithelium is damaged throughout the uterine cavity, but a comparable extent of damage is unlikely in the mouse, as the endometrial epithelium is largely intact 1 day after parturition, with the exception of discontinuities at placental detachment sites (Strug et al. 2018) (Figure 1f). However, relatively little is known about uterine architecture on the day of parturition in rodents, and the degree of epithelial discontinuity remains an important avenue for future work.
Analyses of rodent models throughout pregnancy have revealed that alterations observed immediately postpartum not only reflect damage incurred during parturition but also encompass preceding remodeling that occurred during gestation. For example, the expansion of the decidua surrounding the embryo appears to displace the gland bases into the regions between implantation sites (Yuan et al. 2018). Whether these glands persist throughout gestation or are degraded is unclear. In addition, the luminal epithelium undergoes a variety of degradation and remodeling processes throughout pregnancy (Arora et al. 2016, Welsh & Enders 1983, Yuan et al. 2018). For example, a gap in the mesometrial luminal epithelium (termed the mesometrial aperture) forms to accommodate the placental vascular supply and persists up to parturition (Welsh & Enders 1983). Thus, postpartum repair requires the resolution of a subset of gestational alterations in addition to the acute tissue damage associated with parturition.
Although uterine involution in rodents reverses many of the remodeling events of the pregnant state, traces may remain. The gross morphology of postpartum uterine horns readily reveals regularly spaced, darkly pigmented, macrophage-rich foci, termed postpartum nodules—one for each pup in a litter (Figure 1f) (Brandon 1994, Deno 1937, Tal et al. 2021). These nodules are not composed of fibrous scar tissue but nonetheless denote areas of altered endometrial function in many mammals, as subsequent pregnancies primarily occur between postpartum nodules, and the tissue surrounding these features exhibits a decreased capacity for decidualization (Brandon 1990, 1994; Deno 1937). Notch signaling may promote uterine involution by repressing macrophage recruitment and thereby restricting nodule formation (Strug et al. 2018). Macrophages have also been noted to occasionally form conspicuous pigmented nodules in the human basalis after parturition, but little is known about their functional significance in uterine involution (Anderson & Davis 1968). Thus, although the endometrium regenerates after parturition, postpartum nodules highlight the possibility of persistent irregularities. Further studies are needed to fully understand the extent and duration of functional loss imparted at these sites.
3. PROPOSED MECHANISMS OF ENDOMETRIAL REGENERATION
Menstruation and parturition extensively disrupt both the epithelial and mesenchymal compartments of the endometrium, necessitating substantial cellular proliferation and differentiation to enable continued tissue function. In this section, we outline the cell types that have been proposed to repopulate the tissue (Figure 2). Much of this work has relied on lineage tracing experiments in mice, which frequently use a recombinase (e.g., Cre recombinase) to induce the heritable expression of a genetically encoded reporter in a cell of interest and all of its descendants. By assessing changes in the abundance, distributions, and identities of labeled cells over time, these methods reveal the contributions of specific cell lineages to tissues undergoing routine turnover and/or regeneration (reviewed in Hsu 2015). As lineage tracing is difficult to implement in humans, the capacity of isolated cells to self-renew and/or differentiate into multiple lineages in vitro has been used as a proxy for stemness. In addition, naturally occurring mutations have been used to deduce common cellular ancestry (clonality) between endometrial components, revealing, for example, that endometrial glands in humans, as in mice, are primarily monoclonal (Fu et al. 2020; Lipschutz et al. 1999; Moore et al. 2020; Tanaka et al. 2003; Tempest et al. 2020, 2022). Together, these approaches have revealed a diverse array of potential cellular sources that may contribute to tissue turnover in the absence of injury and/or repopulate endometrial compartments following menstruation and parturition.
Figure 2.
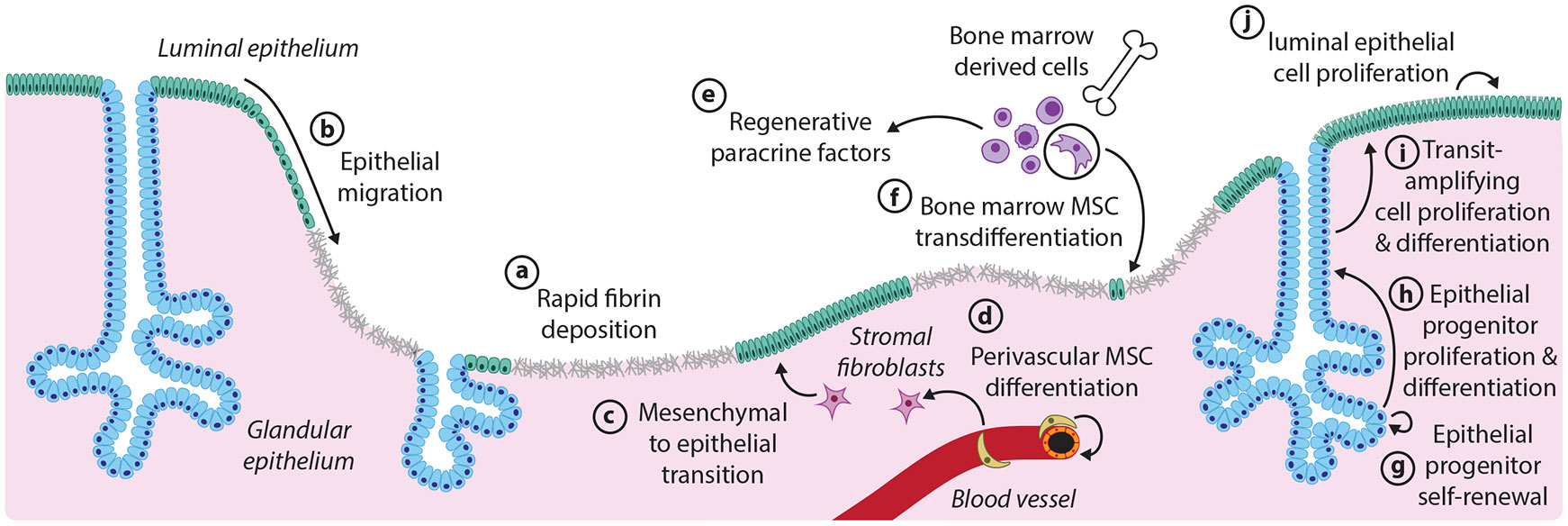
Proposed mechanisms of endometrial regeneration following physiological tissue damage. The mechanisms presented here derive from both human and mouse studies, although the endometrial architecture depicted is from the mouse. The relative timing and extent of the contributions of each mechanism remain to be elucidated. (a) Injured sites are rapidly covered with the fibrinous extracellular matrix. (b) Epithelial migration from nearby intact luminal epithelium (green) or glandular epithelium (blue) covers the denuded areas. (c) In the stromal compartment, fibroblasts (magenta) undergo a mesenchymal to epithelial transition to contribute to new luminal epithelium. (d) Perivascular MSCs differentiate to restore stromal fibroblasts. (e) Paracrine signaling factors from bone marrow-derived cells (purple), and other sources promote regenerative outcomes. (f) Bone marrow–derived MSCs (circled) transdifferentiate to make minor contributions to the endometrial epithelium. (g,h) Long-lived epithelial progenitors residing in the gland bases self-renew and give rise to transit-amplifying cells residing higher up in the gland. (i) Transit-amplifying cells proliferate and differentiate to form short-lived, luminal epithelial cells. (j) The local proliferation of luminal epithelial cells also sustains the surface epithelium. Abbreviation: MSC, mesenchymal stem cell.
3.1. Epithelial Progenitor Cells
Lineage tracing studies in mice have provided extensive evidence that epithelial cells that persist following injury play critical roles in repopulating the epithelium under various conditions (Fu et al. 2020, Jin 2019, Seishima et al. 2019, Syed et al. 2020). For instance, two studies performed with the pan-epithelial marker Pax8 showed that the proportion of labeled cells remained constant over the entire reproductive life span of the cycling mouse, as well as after recurrent pregnancies or following mechanical denudation, suggesting that the labeled epithelium is largely self-renewing in the face of a variety of injuries (Fu et al. 2020, Syed et al. 2020). This aligns with longstanding speculation in humans, where the conspicuous gland remnants dotting the denuded endometrium after menstruation and parturition have been proposed to supply new surface and glandular epithelium. Updated models reflecting the three-dimensional structure of human endometrial glands illustrate how the interconnected architecture of the gland plexus, which remains after tissue loss, could enable efficient re-epithelialization by multiple epithelial progenitor pools (Tempest et al. 2022). Both the proliferation of dedicated progenitors and migration of preexisting epithelial cells have been proposed as possible mechanisms for re-epithelialization (Cousins et al. 2014, Ferenczy 1976, Ludwig & Metzger 1976, Ludwig & Spornitz 1991, Markee 1940, Nogales-Ortiz et al. 1978, Novak & Te Linde 1924).
Consistent with the existence of endometrial epithelial progenitors, a subset of isolated human endometrial cells expressing the epithelial marker EpCAM exhibit high clonogenic, self-renewal, and proliferative capacities in vitro (Chan et al. 2004, Gargett et al. 2009, Schwab et al. 2005). Several molecular markers have been proposed to demarcate progenitor populations in humans, although a clear consensus has not been reached (Gil-Sanchis et al. 2013, Nguyen et al. 2017, Spooner et al. 2021, Tempest et al. 2018, Valentijn et al. 2013). The molecular markers, localization, and lineage potential of endometrial epithelial progenitors in mice have also been subjects of debate. Importantly, the contributions of different epithelial progenitor populations may change throughout the life span. For instance, while Lgr5 marks epithelial progenitors in neonatal mice, Lgr5-positive cells provide minimal contributions to the endometrial epithelium in adulthood (Seishima et al. 2019). Instead, emerging evidence in adult mice points to a model in which long-lived, potentially injury-responsive progenitors (marked with Axin2) reside at the gland bases and give rise to quickly dividing, transient populations that contribute to short-term epithelial maintenance, akin to the transit-amplifying cells in the intestine and skin (Kaitu’u-Lino et al. 2010, Syed et al. 2020) (Figure 2), although other models have been proposed (Jin 2019).
3.2. Lineage Plasticity in Resident Mesenchymal Populations
A growing body of literature suggests that the endometrium contains highly plastic mesenchymal populations capable of producing multiple cell types. Human endometrial SUSD2+ perivascular cells exhibit characteristics of mesenchymal stem cells (MSCs), particularly the ability to give rise to adipocytes, myocytes, osteocytes, and chondrocytes in vitro; these cells can also contribute to connective tissue in a xenograft model (Dimitrov et al. 2008, Gargett et al. 2009, Masuda et al. 2012, Schwab & Gargett 2007). Additionally, side population cells, a separate set of heterogeneous human endometrial cells identified by their ability to efflux Hoechst dye, can produce both hormone-responsive stroma and epithelium in vitro and when transplanted subrenally (Cervelló et al. 2010, 2011; Golebiewska et al. 2011; Kato et al. 2007; Tsuji et al. 2008). While it remains to be seen whether these cell types make significant contributions to the adult endometrium as a normal part of uterine physiology, their multilineage potential, at least in these experimental contexts, underscores the many cellular sources the human endometrium may have at its disposal during tissue repair.
Lineage tracing studies in the mouse have further implicated mesenchymal cells in regenerating the epithelium through a mesenchymal-to-epithelial transition (MET). However, debate about the contribution of this mechanism is ongoing. Many studies have taken advantage of Amhr2 gene expression in endometrial stromal cells, and therefore used mouse models in which Cre recombinase is expressed from the Amhr2 locus for lineage tracing. In contrast to the Pax8 lineage tracing described above, which suggests self-renewal of the epithelium (Fu et al. 2020, Syed et al. 2020), lineage tracing using Amhr2-Cre showed that labeled cells give rise to variable proportions of epithelial cells at various stages of the estrous cycle (Spooner-Harris et al. 2022) and following parturition (Huang et al. 2012, Patterson et al. 2013). However, recent work using two different mouse models revealed that widespread expression of Amhr2-Cre during early embryonic development may account for the labeled endometrial epithelium in the adult, rather than bona fide MET (Dickson et al. 2023). Similarly, the co-expression of epithelial and mesenchymal markers in the mouse embryonic endometrial epithelium may be a confounding factor in lineage tracing studies using mesenchymal Cre promoters that are active in the embryo (Ghosh et al. 2020). Nonetheless, evidence from an alternative approach using a different stromal cell promoter (Pdgfrα-CreERT2) and an inducible Cre, where recombination is restricted to adulthood, has provided further support of MET (Kirkwood et al. 2022). Using this approach in a mouse model of menstruation, the authors detected fibroblast-derived luminal epithelial cells after bleeding onset, bolstering earlier studies reporting MET in this model (Cousins et al. 2014, Patterson et al. 2013, Yin et al. 2019). In human menstruation, evidence for or against MET is technically challenging to acquire and therefore limited. A small number of studies have claimed that the lack of proliferation in the glandular epithelium during early menstruation, and the presence of growing epithelial islands free from remnant gland stumps, supports a role for MET in human epithelial regeneration (Baggish et al. 1967; Garry et al. 2009, 2010). Altogether, the conditions under which MET occurs in the endometrium are still under investigation, and how epithelialization through MET intersects with tissue restoration from preexisting epithelial populations remains to be determined.
3.3. Bone Marrow–Derived Mesenchymal Cells as Minor Contributors to the Regenerated Endometrium
Finally, it has been proposed that bone marrow–derived stem cells (BMDSCs), particularly MSCs, differentiate into endometrial tissue in humans and mice (Du & Taylor 2007, Du et al. 2012, Taylor 2004). However, bone marrow transplant studies have found that rates of bone marrow–derived cell engraftment are often low or negligible in the endometrium (Bratincsák et al. 2007, Du & Taylor 2007, Du et al. 2012, Morelli et al. 2013, Ong et al. 2018, Tal et al. 2016, Wolff et al. 2013), and these cells do not appear to expand within the human endometrium once established (Cervelló et al. 2012, Ikoma et al. 2009). These observations suggest that these cells are unlikely to constitute major endometrial progenitors under most circumstances. However, human and mouse bone marrow MSCs can be cultured to take on decidual phenotypes, which may indicate that bone marrow MSCs could play a transient role in pregnancy and/or involution (Aghajanova et al. 2010, Tal et al. 2019). Unfractionated bone marrow can also differentiate into short-lived murine endometrial epithelium during pregnancy before becoming senescent and dying in the days following parturition, although the functional significance of such a contribution remains unknown (Tal et al. 2021). Emerging evidence suggests that BMDSCs may not directly restore lost cellular populations long-term, but rather may provide paracrine factors that stimulate regenerative remodeling (Alawadhi et al. 2014, Cervelló et al. 2015).
4. NONREGENERATIVE HEALING OF THE UTERUS
Despite tremendous regeneration that occurs after most physiological disruptions of the endometrium, more than a century of clinical gynecological observations reveal numerous instances in which the endometrium fails to regenerate and instead develops fibrosis (Fritsch 1894, Johnson 1900) (Figure 3). In some cases, fibrotic lesions may span the uterine walls, forming intrauterine adhesions (IUAs), or synechiae, capable of partially or completely obstructing the uterine cavity (Deans & Abbott 2010). IUAs exhibit a wide range of histological and clinical manifestations (Foix et al. 1966, Sugimoto 1978). While some IUAs may go undiagnosed due to a lack of symptoms, those resulting in uterine cavity obstruction or loss of functional endometrium may contribute to pelvic pain, menstrual disruption, and fertility complications (Schenker & Margalioth 1982). IUAs manifesting in amenorrhea and infertility are characteristic of Asherman syndrome (AS), a condition with limited potential for treatment (Asherman 1948, Stamer 1946). The true incidence of IUAs remains unknown, but prevalence strongly correlates with gynecological dysfunction (March 2011b) and IUAs likely represent a significant and underappreciated health burden.
Figure 3.
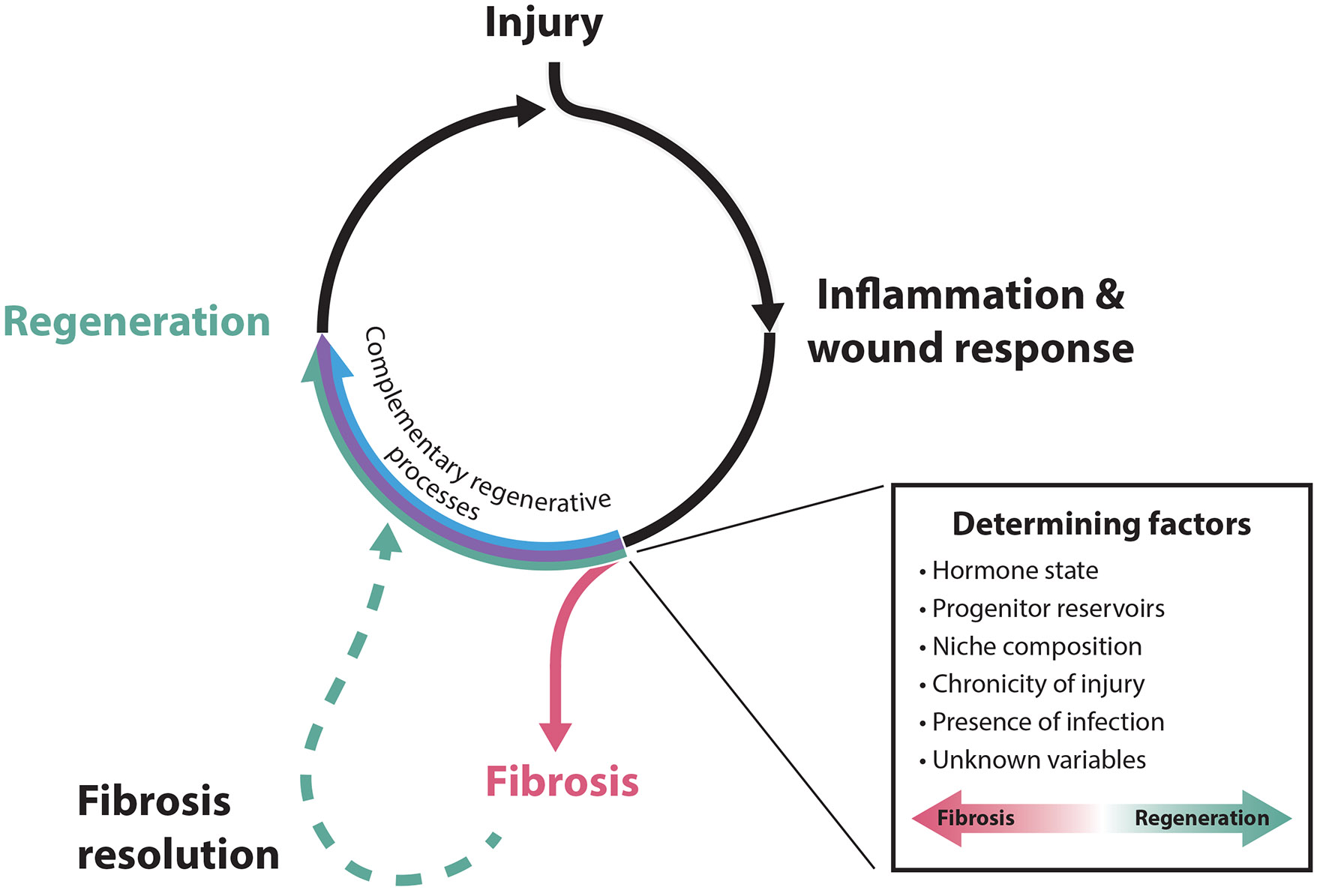
Wound healing outcomes in the endometrium. Both physiological and nonphysiological endometrial injuries induce an acute inflammatory/wound response, which can be resolved via complementary regenerative processes (outlined in Figure 2) to restore tissue architecture prior to subsequent injury. If coincident with underlying predisposing conditions, which may involve a number of known and unknown determining factors, this inflammation may, instead, progress to fibrosis. Without intervention, fibroses can be terminal or, at some frequency, undergo spontaneous reversion through poorly understood processes to restore functional endometrial tissues.
Injuries inflicted during medical interventions (iatrogenic injuries) are the most cited cause of IUA formation. The vast majority of cases are attributed to curettage of the endometrium for abortion or the removal of remnants of conception. However, infections and other procedures, including diagnostic curettage, myomectomy, cesarean section, and uterine artery embolization, also contribute (Asherman 1948, Hanstede et al. 2015, Schenker & Margalioth 1982). Moreover, interventions intended to induce fibrosis of the uterus (endometrial ablation) have been exploited since the late nineteenth century for the treatment of heavy menstrual bleeding (reviewed in Wortman 2017). Despite this storied clinical history, the basic mechanisms underlying nonregenerative healing in the uterus remain poorly understood.
4.1. Causal Factors of Uterine Fibrosis
Clinicians have long posited that physical trauma is the leading cause of IUAs, with the depth of injury playing an important role (Asherman 1948, Stamer 1946). In humans, this model has most compellingly been explored in the context of endometrial ablation, where the efficacy of ablation and extent of fibrosis can be examined following treatment. Early case studies of cauterization in the uterus indicated that extensive injury correlated with more dramatic compositional changes to the endometrium; following the most potent treatments, a complete loss of mucosa and, in some cases, uterine patency was observed (Johnson 1900).
In the context of incidental fibroses, the causative roles of physical trauma and injury depth are less clear. Human endometrium obtained from patients following curettage after abortion or within the immediate postpartum (puerperal) period often reveal myometrial components, with a higher incidence of IUAs, uterine atresia, and/or amenorrhea correlating with samples containing what the authors describe as “plentiful” myometrium, suggesting that deep tissue removal may contribute to fibrosis in iatrogenic contexts (Eriksen & Kaestel 1960, Hald 1949, Jensen & Stromme 1972). Additionally, evidence suggests that interventions in which the uterine cavity is opened, for example during some types of fibroid removal, may increase the potential for IUA formation (Capmas et al. 2018). However, it is important to note that correlational studies of uterine fibrosis rarely involve examination of the uterine cavity prior to medical interventions or other suspected inciting incidents. As a result, these studies preclude the identification of pre-existing factors that may predispose the uterus to fibrosis. Studies in which endometrial health metrics are obtained prior to curettage, for example in cases where the endometrium has been previously assessed as part of infertility treatment, will provide crucial opportunities to determine whether iatrogenic injury per se plays a causal role (Gilman et al. 2016).
In addition to iatrogenic trauma, female genital tuberculosis (FGTB) is now appreciated as an important risk factor for uterine fibroses (Netter et al. 1955, Sharma et al. 2008). Most cases of FGTB arise as a secondary manifestation of pulmonary tuberculosis, resulting in geographic variations in FGTB incidence paralleling those of tuberculosis infection (Schaefer 1976). FGTB is often discovered incidentally during investigation for infertility and/or amenorrhea, with lesions frequently accompanied by inflammation, extensive fibrotic tissue, and the loss of endometrium in advanced stages, despite an absence of iatrogenic injury (Bazaz-Malik et al. 1983, Schaefer 1976). Patterns of tissue destruction mirror the spread of tuberculosis infection, which often originates in the fallopian tubes and spreads to the uterus, suggesting a causal relationship between pathogenic damage and fibrosis (Schaefer 1976). Pelvic schistosomiasis has also been implicated in several cases of AS (Acosta Go & Ibrahim 2022, Krolikowski et al. 1995), suggesting that the role for infectious agents in uterine fibroses may extend beyond FGTB.
4.2. Factors Predisposing the Uterus to Fibrosis
Despite a clear role for injury in the formation of most IUAs, both clinical and animal studies suggest that injury alone is insufficient to induce fibrosis in most cases. For instance, signs of epithelial regeneration are evident within days of curettage of the nonpregnant human uterus (McLennan 1969). Similarly, nonhuman primates subjected to consecutive endometrial resections recovered a healthy endometrium capable of implantation and, in some cases, carrying viable offspring to term (Hartman 1945). A comparable resilience to traumatic injury has been observed in a variety of nonprimate laboratory models (Schenker et al. 1971, 1973a,b). Together, these observations suggest that injury must occur alongside other predisposing factors to result in fibrosis.
The pregnant and postpartum uterus appear to be particularly susceptible to IUA formation following trauma (Hanstede et al. 2015, Schenker & Margalioth 1982, Xiao et al. 2014). In one of the largest clinical studies of IUA incidence, researchers attributed 90.8% of 1,856 cases to trauma incurred during pregnancy or the puerperium. In contrast, only 3.7% of cases were attributed to comparable interventions independent of pregnancy (Schenker & Margalioth 1982). The uterus is particularly prone to fibrosis between postpartum weeks two and four (Bergman 1961, Eriksen & Kaestel 1960, Schenker & Margalioth 1982). Parturition coincides with dramatic changes in the hormonal milieu, including sudden decreases in estrogens and progesterone, which may contribute to the fibrotic outcome (Barkley et al. 1979, Lewis et al. 1987). Based on these observations, hormone supplementation has become a common peri-operative treatment for the prevention of IUA reformation after resection. However, the efficacy of this treatment remains debated (Farhi et al. 1993, Johary et al. 2014), and the precise contributions of hormones to endometrial regeneration are still being elucidated. Studies in which rhesus macaques and mice were depleted of sex hormones reported complete endometrial regeneration after menstruation and induced endometrial shedding, respectively (Hartman 1945, Kaitu’u-Lino et al. 2010), suggesting that sex hormones may be dispensable for regeneration in some contexts. In fact, progesterone may hinder regeneration in some cases, as human samples subjected to curettage during the secretory phase, which is progesterone-dominant, exhibit delayed endometrial regeneration (Johannisson et al. 1981, McLennan 1969). Similarly, in the mouse, erroneous healing is associated with injury to the diestrus uterus (Zhang et al. 2022). Further study of the role of hormones in endometrial regeneration after diverse injuries will provide valuable information to inform clinical practice.
Recurrent or chronic injury may also predispose uterine healing toward fibrosis. This is supported by the relatively high prevalence of IUAs reported in patients undergoing secondary curettage for incomplete or missed abortion (Westendorp et al. 1998). Several retrospective studies also show a higher prevalence and severity of IUAs in patients with a history of repeat miscarriage/abortion and curettage (Friedler et al. 1993, Römer 1994). However, elevated IUAs have been reported in patients with a history of pregnancy loss, irrespective of iatrogenic factors (Ventolini et al. 2004) and it is important to consider that pre-existing conditions that necessitate recurrent intervention (e.g. failure to release placental remnants) may also contribute or predispose the uterus to fibrosis.
The role of chronic infection in uterine fibrosis is controversial. While it is widely accepted that a subset of pathogens may induce IUAs, claims that most traumatic adhesions can be explained by injuries coinciding with endometritis (Rabau & David 1963) have been met with criticism due to inconsistent diagnostic criteria and mixed reports of the prevalence of endometritis in patients presenting with IUAs or AS (Jensen & Stromme 1972, Liu et al. 2019). Nevertheless, researchers have reported signs of chronic endometritis in 46% of patients presenting with moderate to severe IUAs (Liu et al. 2019) and histological signs of acute and subacute inflammation in the majority of puerperal curettings taken between postpartum weeks two and four (Smid & Bedö 1978). While it is unlikely that chronic infection contributes to most cases of IUAs, these findings highlight the broader possibility that inflammation may contribute to predisposing the uterus to fibrosis. Altogether, a survey of clinical studies on IUAs and AS suggests that most uterine fibroses likely arise from a confluence of an inciting injury alongside a predisposing state of the uterus.
4.3. Persistent Regenerative Potential in the Fibrotic Uterus
The endometrial response to iatrogenic injury mirrors that observed following menstruation, including moderate immune infiltration, the deposition of a provisional ECM, and the subsequent emergence of an epithelium from persisting progenitors (Johannisson et al. 1981, Wyss et al. 1996). In general, fibrosis is widely regarded as a consequence of the dysregulation of normal healing processes, as many of the effectors and pathways involved in inflammation and scarring play fundamental roles in regenerative wound healing (reviewed in Henderson et al. 2020, Nathan & Ding 2010). In the endometrium, the diversion of healing towards a fibrotic outcome is often attributed to the obliteration of the stem cell reservoir during injury. However, this model is complicated by the observation that diverse cell populations may contribute to re-epithelialization of the endometrium (see Section 3) and that epithelial compartments may persist even after extensive, catastrophic injury. This persistence is especially evident after endometrial ablation, where endometrial glands may be observed up to 30 months after treatment, with a penetrance of ~80% reported even in cases where ablation is considered clinically successful (Onoglu et al. 2007, Taskin et al. 2002).
The observation that the fibrotic uterus may retain the cell populations necessary for regeneration suggests that other factors promote nonregenerative outcomes. This point is further underlined by instances of the spontaneous remission of uterine fibroses, which indicate that fibrosis is not a consequence of a permanent, global loss of regenerative potential. This is best illustrated by late-onset endometrial ablation failure (LOEAF), a common outcome of ablation characterized by the continuation or restoration of menstruation >1 month after treatment (Wortman 2017). LOEAF is attributed to the persistence and/or regeneration of hormone-responsive endometrial tissues, which may arise from incomplete ablation in uterine regions with limited instrument access (Lisa et al. 1954, Turnbull et al. 1997) or from the persistence of basal glands. Intriguingly, the probability of LOEAF increases over time after treatment (Longinotti et al. 2008, Shavell et al. 2012), suggesting that fibrosed uterine tissues can retain the ability to regenerate and, remarkably, reverse the fibrotic course. In light of the data on uterine fibrosis and its reversion, it is tempting to speculate that the regeneration of the endometrium depends less on a singular mediator than on a tenuous balance between pathways underlying both regenerative and nonregenerative processes (Figure 3). In this regard, dissecting uterine regeneration requires understanding how changes associated with injury and fibrosis predisposition—injury timing, depth, and chronicity, among others—compromise or divert existing regenerative mechanisms.
4.4. Mechanisms of Uterine Fibrosis
The anatomy of the uterus ensures that deep tissue damage disproportionately impacts stromal and myometrial compartments, which contribute to the formation and maintenance of the niche in which progenitors reside. Clinical studies have revealed substantial alterations of uterine compartments in patients presenting with IUAs and AS, including increases in fibrotic tissue in the muscle (Yaffe et al. 1978) and stroma (Bergman 1961), reduced myometrial blood flow, and widespread vascular occlusion (Polishuk et al. 1977). Such changes may impact tissue mechanics and perfusion, which contribute to fibrotic disorders elsewhere in the body (Darby & Hewitson 2016, Van De Water et al. 2013). Furthermore, necrotizing granulomas and other immune cell infiltrates are frequently observed in postablation specimens (Ashworth et al. 1991, Silvernagel et al. 1997, Tresserra et al. 1999). Together, these observations highlight potential avenues by which the extensive injury and remodeling of the endometrium and adjacent compartments may impact cellular interactions to disrupt the balance of regenerative and nonregenerative healing processes.
Disentangling the complex interactions between endometrial progenitors and their niche factors requires experimental models of uterine fibroses. Attempts to develop such animal models using a variety of approaches associated with IUA formation in humans have had varying success, with few studies reporting the presence of bona fide IUAs or obstruction of the uterine cavity (Liang et al. 2022; Schenker & Polishuk 1972, 1973; Schenker et al. 1971, 1973a,b). Comparisons of existing experimental models are complicated in many cases by the use of different diagnostic criteria, ranging from functional measures, such as fecundity, to histological and molecular readouts. Challenges in developing uterine fibrosis models may further arise from interspecies variation related to fundamental differences in reproductive biology and/or the necessity to incorporate additional predisposing factors. Particularly compelling rabbit models of IUA and AS have arisen from dual injury approaches in which mechanical trauma is compounded with inflammatory [e.g., bacterial lipopolysaccharide (Liu et al. 2013)] or profibrotic stimuli [e.g., fibroblast- and collagen-enriched sponges (Schenker et al. 1975)], underscoring the potential role of predisposing physiological states in promoting uterine scarring.
Experiments in animal models have explored a wealth of molecular factors that contribute to uterine fibrosis (reviewed in Leung et al. 2020), many of which converge on signaling pathways underlying global injury responses, immune activation, fibroblast mobilization, and tissue remodeling. Among these, a number of effectors have been implicated in human IUAs and AS and, unsurprisingly, fibrosis in other organs. For instance, rabbit and rat models of induced IUAs exhibit elevated activation of TGF-β and NF-κB pathways, whch are also reported in human IUAs (Ning et al. 2018, Salma et al. 2016, Wang et al. 2017, Xue et al. 2015). NF-κB represents a family of transcription factors regulating critical pathways in immune signaling and inflammation (reviewed in Liu et al. 2017). TGF-β is well established as a driver of fibrosis in many organs, where its transcriptional activities contribute to an array of processes, including ECM accumulation and fibroblast recruitment, activation, and differentiation into myofibroblasts (reviewed in Budi et al. 2021, Rockey et al. 2015). Consistent with this, experimental hyperactivation of the TGF-β pathway in the mouse uterus promoted myofibroblast differentiation (Gao et al. 2015). The development and refinement of additional experimental models of uterine fibroses will play a critical role in facilitating further gain- and loss-of-function studies of these pathways.
Modern single-cell transcriptomics approaches offer additional opportunities to construct an unbiased portrait of nonregenerative healing in the uterus. For example, recent work compared single-cell expression profiles from a human endometrium atlas to those from patients with moderate and severe AS (Santamaria et al. 2022, Wang et al. 2020). This study reported profound changes in the composition and function of AS uteri, including unique epithelial and smooth muscle cell populations, a proinflammatory immune cell state, fibrotic stroma, and compromised vascularity. Moreover, the authors’ analyses of ligand-receptor pair expression suggested alterations to cell-cell communication in AS uteri, including a decrease in epithelial-stromal communication and an increase in autocrine stromal signaling and endothelial-immune communication. Such approaches hold great promise for both basic mechanistic and therapeutic discoveries in uterine biology.
4.5. Therapeutic Management of Uterine Fibrosis
Treatment of IUAs and AS primarily focuses on adhesiolysis, the resection of scar tissue under hysteroscopic visualization, often augmented with hormone treatment or the placement of physical barriers to prevent adhesion reformation (e.g. Foley catheter) (March 2011a). However, the efficacy of these interventions is variable, and adhesions may recur in as many as one-third to two-thirds of patients (reviewed in AAGL 2017). Nevertheless, the success of adhesiolysis in enhancing menstrual flow and fertility in some cases provides an additional tantalizing indication that latent regenerative potential may persist in a fibrotic uterus.
Experimental models of uterine injury have also been harnessed to explore new therapeutic prospects for preventing and treating IUAs, revealing a remarkable breadth of therapeutic strategies. These include a variety of biocompatible materials that may be transplanted into the injured uterus to serve as cellular scaffolds or enable the release of pro-regenerative factors (Jonkman et al. 1986, Li et al. 2011, Taveau et al. 2004, reviewed in Yin et al. 2023). Additionally, considerable effort has gone toward developing approaches to transplant putative stem/progenitor cells or enhance their homing to the target tissue (Sahin Ersoy et al. 2017). Bone marrow–based therapies have gained notable traction, with the transplantation of unfractionated bone marrow, a putative source of BMDSCs, improving fecundity in a mouse model of traumatic AS (Alawadhi et al. 2014). This improvement likely occurs through the modulation of the uterine niche, as opposed to substantial cellular repopulation, as human CD133+ BMDSCs transplanted into the same model engraft around endometrial vessels and promote glandular epithelial proliferation (Cervelló et al. 2015). BMDSC-based approaches have since been translated to phase I/II clinical trials for the treatment of AS and endometrial atrophy, and they partially reverse AS-associated phenotypes following treatment, as inferred by single-cell profiling (Santamaria et al. 2016, 2022). A variety of other cell-based therapies have been explored in similar models, including MSCs from adipose tissue, umbilical cord, or amniotic membranes (Gan et al. 2017, Kilic et al. 2014, Tang et al. 2016); uterine- or menstrual blood-derived cells (Hu et al. 2019, Liu et al. 2018); and human embryonic stem cell–derived endometrium-like cells (Song et al. 2015). All report some improvements in uterine regeneration and/or reproductive performance. The efficacy of these diverse interventions suggests that many strategies, ranging from resupplying component parts to niche priming with paracrine signals, may suffice to bias healing toward regenerative outcomes. Furthermore, this work offers hope for positive health and reproductive outcomes for diverse patients, as orthogonal therapeutic approaches may be necessary to address the poorly defined variability in uterine fibroses.
5. OUTLOOK
Efforts to synthesize information from basic science and medicine hold great promise for advancing our understanding of uterine biology. In summarizing how the endometrium responds to numerous injuries—menstruation, parturition, iatrogenic injury, and infection—this review underscores the plethora of factors that may determine regenerative versus fibrotic outcomes. These factors include the spatial heterogeneity of damage across the organ, which cell types survive the injury (both progenitors and their niche components), the extent and duration of inflammation, and the speed of repair processes. Each of these may be further modulated by the tissue state, including hormone levels or prior tissue history. Our inability to robustly predict the outcomes of many uterine injuries in a clinical setting or to understand pathological responses to physiological injuries, such as abnormal uterine bleeding, reveals that many important variables remain to be defined.
The true breadth of change to which the endometrium is exposed extends far beyond the conditions we have reviewed here. Endocrinological disorders, other disease states, and contraceptives provide additional variables that may modify endometrial architecture and regenerative capacity within the reproductive years. In addition, the reproductive life span, bounded by menarche and menopause, reflects only half of the total life span (Appiah et al. 2021, Martinez 2020, Stewart et al. 2022). Outside of the reproductive window, endometrial remodeling and regeneration also unfold in ways that remain exceedingly poorly understood. For example, some newborns exhibit a sparsely studied withdrawal bleed (neonatal uterine bleeding) upon removal from the hormone-rich in utero environment at parturition (reviewed in Benagiano et al. 2021). In addition, there is much to discover about the endometrium during childhood, perimenopause, and menopause, which may evoke different regenerative strategies and vulnerabilities compared to the reproductive years (Metcalf et al. 1981, Swain & Kulkarni 2021). While much remains unknown about the balance between regenerative and nonregenerative healing in the uterus, uncovering the secret to the remarkable resilience of this organ holds the potential to inform far-reaching fields, from wound healing and regeneration to inflammation and beyond.
ACKNOWLEDGMENTS
We apologize to those colleagues whose work we were unable to include due to space limitations. We thank Kate O’Neill, Pradeep Tanwar, Asgi Fazleabas, and members of the McKinley lab for discussions and feedback on the manuscript. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program to C.J.A. under grant number DGE 2140743. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. K.L.M. is a Freeman Hrabowski Scholar of the Howard Hughes Medical Institute and a New York Stem Cell Foundation (NYSCF) Robertson Investigator. We thank NYSCF and the National Institute of Child Health and Human Development (R00HD101021 and DP2HD111708) for supporting work in the McKinley lab.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- AAGL (Am. Assoc. Gynecol. Laparosc.). 2017. AAGL practice report: practice guidelines on intrauterine adhesions developed in collaboration with the European Society of Gynaecological Endoscopy (ESGE). J. Minim. Invasive Gynecol 24:695–705 [DOI] [PubMed] [Google Scholar]
- Abbas Y, Brunel LG, Hollinshead MS, Fernando RC, Gardner L, et al. 2020. Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus 10:20190079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta Go V-A, Ibrahim Y. 2022. Schistosomiasis induced Asherman’s syndrome in a patient undergoing assisted reproductive technology (ART): a case report and literature review. Fertil. Steril 118:e199 [Google Scholar]
- Aghajanova L, Horcajadas JA, Esteban FJ, Giudice LC. 2010. Bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast. Biol. Reprod 82:1076–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Yoon M-J, Hong S-H, Cha H, Lee D, et al. 2021. Three-dimensional microengineered vascularised endometrium-on-a-chip. Hum. Reprod 36:2720–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alawadhi F, Du H, Cakmak H, Taylor HS. 2014. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLOS ONE 9:e96662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WR, Davis J. 1968. Placental site involution. Am. J. Obstet. Gynecol 102:23–33 [DOI] [PubMed] [Google Scholar]
- Aplin JD, Ruane PT. 2017. Embryo–epithelium interactions during implantation at a glance. J. Cell Sci 130:15–22 [DOI] [PubMed] [Google Scholar]
- Appiah D, Nwabuo CC, Ebong IA, Wellons MF, Winters SJ. 2021. Trends in age at natural menopause and reproductive life span among US women, 1959–2018. JAMA 325:1328–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora R, Fries A, Oelerich K, Marchuk K, Sabeur K, et al. 2016. Insights from imaging the implanting embryo and the uterine environment in three dimensions. Development 143:4749–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherman JG. 1948. Amenorrhoea traumatica (atretica). BJOG 55:23–30 [DOI] [PubMed] [Google Scholar]
- Ashworth MT, Moss CI, Kenyon WE. 1991. Granulomatous endometritis following hysteroscopic resection of the endometrium. Histopathology 18:185–87 [DOI] [PubMed] [Google Scholar]
- Baggish MS, Pauerstein CJ, Woodruff JD. 1967. Role of stroma in regeneration of endometrial epithelium. Am. J. Obstet. Gynecol 99:459–65 [DOI] [PubMed] [Google Scholar]
- Barkley MS, Geschwind II, Bradford GE. 1979. The gestational pattern of estradiol, testosterone and progesterone secretion in selected strains of mice. Biol. Reprod 20:733–38 [DOI] [PubMed] [Google Scholar]
- Bazaz-Malik G, Maheshwari B, Lal N. 1983. Tuberculous endometritis: a clinicopathological study of 1000 cases. BJOG 90:84–86 [DOI] [PubMed] [Google Scholar]
- Bellofiore N, Ellery S, Mamrot J, Walker D, Temple-Smith P, Dickinson H. 2016. First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus). Am. J. Obstet. Gynecol 216:40.e1–11 [DOI] [PubMed] [Google Scholar]
- Benagiano G, Habiba M, Lippi D, Brosens IA. 2021. A history of neonatal uterine bleeding and its significance. Reprod. Med 2(4):171–84 [Google Scholar]
- Benirschke K, Burton GJ, Baergen RN. 2012. Pathology of the Human Placenta. Berlin, Ger.: Springer [Google Scholar]
- Bergman P. 1961. Traumatic intra-uterine lesions. Acta Obstet. Gynecol. Scand 40:1–39 [DOI] [PubMed] [Google Scholar]
- Brandon JM. 1990. Decidualization in the post-partum uterus of the mouse. J. Reprod. Fertil 88:151–58 [DOI] [PubMed] [Google Scholar]
- Brandon JM. 1994. Distribution of macrophages in the mouse uterus from one day to three months after parturition, as defined by the immunohistochemical localization of the macrophage-restricted antigens F4/80 and macrosialin. Anat. Rec 240:233–42 [DOI] [PubMed] [Google Scholar]
- Brasted M, White CA, Kennedy TG, Salamonsen LA. 2003. Mimicking the events of menstruation in the murine uterus. Biol. Reprod 69:1273–80 [DOI] [PubMed] [Google Scholar]
- Bratincsák A, Brownstein MJ, Cassiani-Ingoni R, Pastorino S, Szalayova I, et al. 2007. CD45-positive blood cells give rise to uterine epithelial cells in mice. Stem Cells 25:2820–26 [DOI] [PubMed] [Google Scholar]
- Brenner RM, Rudolph L, Matrisian L, Slayden OD. 1996. Non-human primate models: artificial menstrual cycles, endometrial matrix metalloproteinases and s.c. endometrial grafts. Hum. Reprod 11(Suppl. 2):150–64 [DOI] [PubMed] [Google Scholar]
- Brenner RM, Slayden OD. 2012. Molecular and functional aspects of menstruation in the macaque. Rev. Endocr. Metab. Disord 13:309–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budi EH, Schaub JR, Decaris M, Turner S, Derynck R. 2021. TGF-β as a driver of fibrosis: physiological roles and therapeutic opportunities. J. Pathol 254:358–73 [DOI] [PubMed] [Google Scholar]
- Capmas P, Pourcelot A-G, Fernandez H. 2018. Are synechiae a complication of laparotomic myomectomy? Reprod. Biomed. Online 36:450–54 [DOI] [PubMed] [Google Scholar]
- Carter AM. 2018. Classics revisited: C. J. van der Horst on pregnancy and menstruation in elephant shrews. Placenta 67:24–30 [DOI] [PubMed] [Google Scholar]
- Catalini L, Fedder J. 2020. Characteristics of the endometrium in menstruating species: lessons learned from the animal kingdom. Biol. Reprod 102:1160–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervelló I, Gil-Sanchis C, Mas A, Delgado-Rosas F, Martínez-Conejero JA, et al. 2010. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLOS ONE 5:e10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervelló I, Gil-Sanchis C, Mas A, Faus A, Sanz J, et al. 2012. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLOS ONE 7:e30260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervelló I, Gil-Sanchis C, Santamaría X, Cabanillas S, Díaz A, et al. 2015. Human CD133+ bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil. Steril 104:1552–60.e3 [DOI] [PubMed] [Google Scholar]
- Cervelló I, Mas A, Gil-Sanchis C, Peris L, Faus A, et al. 2011. Reconstruction of endometrium from human endometrial side population cell lines. PLOS ONE 6:e0021221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RWS, Schwab KE, Gargett CE. 2004. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod 70:1738–50 [DOI] [PubMed] [Google Scholar]
- Cheung VC, Peng C-Y, Marinić M, Sakabe NJ, Aneas I, et al. 2021. Pluripotent stem cell-derived endometrial stromal fibroblasts in a cyclic, hormone-responsive, coculture model of human decidua. Cell Rep. 35:109138. [DOI] [PubMed] [Google Scholar]
- Cousins FL, Murray A, Esnal A, Gibson DA, Critchley HOD, Saunders PTK. 2014. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLOS ONE 9:e86378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HOD, Maybin JA, Armstrong GM, Williams ARW. 2020. Physiology of the endometrium and regulation of menstruation. Physiol. Rev 100:1149–79 [DOI] [PubMed] [Google Scholar]
- Darby IA, Hewitson TD. 2016. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 365:553–62 [DOI] [PubMed] [Google Scholar]
- Deans R, Abbott J. 2010. Review of intrauterine adhesions. J. Minim. Invasive Gynecol 17:555–69 [DOI] [PubMed] [Google Scholar]
- Deno RA. 1937. Uterine macrophages in the mouse and their relation to involution. Am. J. Anat 60:433–71 [Google Scholar]
- Dickson MJ, Gruzdev A, DeMayo FJ. 2023. iCre recombinase expressed in the anti-Müllerian hormone receptor 2 gene causes global genetic modification in the mouse†. Biol. Reprod 108: 575–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov R, Timeva T, Kyurkchiev D, Stamenova M, Shterev A, et al. 2008. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction 135:551–58 [DOI] [PubMed] [Google Scholar]
- Du H, Naqvi H, Taylor HS. 2012. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev. 21:3324–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Taylor HS. 2007. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 25:2082–86 [DOI] [PubMed] [Google Scholar]
- Eriksen J, Kaestel C. 1960. The incidence of uterine atresia after post-partum curettage. A follow-up examination of 141 patients. Dan. Med. Bull 7:50–51 [PubMed] [Google Scholar]
- Farhi J, Bar-Hava I, Homburg R, Dicker D, Ben-Rafael Z. 1993. Induced regeneration of endometrium following curettage for abortion: a comparative study. Hum. Reprod 8:1143–44 [DOI] [PubMed] [Google Scholar]
- Ferenczy A. 1976. Studies on the cytodynamics of human endometrial regeneration. I. Scanning electron microscopy. Am. J. Obstet. Gynecol 124:64–74 [DOI] [PubMed] [Google Scholar]
- Finn CA, Pope M. 1984. Vascular and cellular changes in the decidualized endometrium of the ovariectomized mouse following cessation of hormone treatment: a possible model for menstruation. J. Endocrinol 100:295–300 [DOI] [PubMed] [Google Scholar]
- Foix A, Bruno RO, Davison T, Lema B. 1966. The pathology of postcurettage intrauterine adhesions. Am. J. Obstet. Gynecol 96:1027–33 [DOI] [PubMed] [Google Scholar]
- Friedländer C. 1870. Physiologisch-anatomische untersuchungen über den uterus. Leipzig, Ger.: Simmel [Google Scholar]
- Friedler S, Margalioth EJ, Kafka I, Yaffe H. 1993. Incidence of post-abortion intra-uterine adhesions evaluated by hysteroscopy—a prospective study. Hum. Reprod 8:442–44 [DOI] [PubMed] [Google Scholar]
- Fritsch H. 1894. Ein Fall von volligem Schwund ser Gebarmutterhohle nach Auskratzung. Centralblatt für Gynäkologie. 52:1337–1339 [Google Scholar]
- Fu D-J, De Micheli AJ, Bidarimath M, Ellenson LH, Cosgrove BD, et al. 2020. Cells expressing PAX8 are the main source of homeostatic regeneration of adult mouse endometrial epithelium and give rise to serous endometrial carcinoma. Dis. Models Mech 13:dmm047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Duan H, Xu Q, Tang Y-Q, Li J-J, et al. 2017. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy 19:603–16 [DOI] [PubMed] [Google Scholar]
- Gao Y, Duran S, Lydon JP, DeMayo FJ, Burghardt RC, et al. 2015. Constitutive activation of transforming growth factor beta receptor 1 in the mouse uterus impairs uterine morphology and function. Biol. Reprod 92:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alonso L, Handfield L-F, Roberts K, Nikolakopoulou K, Fernando RC, et al. 2021. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet 53:1698–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Zillwood RM, Nguyen HPT, Wu D. 2009. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol. Reprod 80:1136–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R, Hart R, Karthigasu KA, Burke C. 2009. A re-appraisal of the morphological changes within the endometrium during menstruation: a hysteroscopic, histological and scanning electron microscopic study. Hum. Reprod 24:1393–401 [DOI] [PubMed] [Google Scholar]
- Garry R, Hart R, Karthigasu KA, Burke C. 2010. Structural changes in endometrial basal glands during menstruation. BJOG 117:1175–85 [DOI] [PubMed] [Google Scholar]
- Ghosh A, Syed SM, Kumar M, Carpenter TJ, Teixeira JM, et al. 2020. In vivo cell fate tracing provides no evidence for mesenchymal to epithelial transition in adult fallopian tube and uterus. Cell Rep. 31:107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DA, Simitsidellis I, Collins F, Saunders PTK. 2020. Androgens, oestrogens and endometrium: a fine balance between perfection and pathology. J. Endocrinol 246:R75–93 [DOI] [PubMed] [Google Scholar]
- Gil-Sanchis C, Cervelló I, Mas A, Faus A, Pellicer A, Simón C. 2013. Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) as a putative human endometrial stem cell marker. Mol. Hum. Reprod 19:407–14 [DOI] [PubMed] [Google Scholar]
- Gilman AR, Dewar KM, Rhone SA, Fluker MR. 2016. Intrauterine adhesions following miscarriage: look and learn. J. Obstet. Gynaecol. Can 38:453–57 [DOI] [PubMed] [Google Scholar]
- Golebiewska A, Brons NHC, Bjerkvig R, Niclou SP. 2011. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell 8:136–47 [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, et al. 2001. Developmental biology of uterine glands. Biol. Reprod 65:1311–23 [DOI] [PubMed] [Google Scholar]
- Haig D, 1995. Whitridge Williams’ obstetrics. Am. J. Obstet. Gynecol 173:1351. [DOI] [PubMed] [Google Scholar]
- Hald H. 1949. On uterine atresia consequent to curettage. Acta Obstet. Gynecol. Scand 28:169–74 [DOI] [PubMed] [Google Scholar]
- Hanstede MMF, van der Meij E, Goedemans L, Emanuel MH. 2015. Results of centralized Asherman surgery, 2003–2013. Fertil. Steril 104:1561–68.e1 [DOI] [PubMed] [Google Scholar]
- Hartman CG. 1944. Regeneration of the monkey uterus after surgical removal of the endometrium and accidental endometriosis. West J. Surg. Obstet. Gynecol 52:87–102 [Google Scholar]
- Hemberger M, Hanna CW, Dean W. 2020. Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet 21:27–43 [DOI] [PubMed] [Google Scholar]
- Henderson NC, Rieder F, Wynn TA. 2020. Fibrosis: from mechanisms to medicines. Nature. 587:555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, Miller D, Brest A, et al. 2021. SEER cancer statistics review, 1975–2018. Rep., Natl. Cancer Inst., Bethesda, MD. https://seer.cancer.gov/csr/1975_2018/ [Google Scholar]
- Hsu YC. 2015. Theory and practice of lineage tracing. Stem Cells 33:3197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Song K, Zhang J, Zhang Y, Tan B-Z. 2019. Effects of menstrual blood-derived stem cells on endometrial injury repair. Mol. Med. Rep 19:813–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-C, Orvis GD, Wang Y, Behringer RR. 2012. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLOS ONE 7:e44285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma TMD, Kyo SMDP, Maida YMDP, Ozaki SP, Takakura MMDP, et al. 2009. Bone marrow–derived cells from male donors can compose endometrial glands in female transplant recipients. Am. J. Obstet. Gynecol 201:608.e1–8 [DOI] [PubMed] [Google Scholar]
- Isley MM. 2021. Postpartum care and long-term health considerations. In Gabbe’s Obstetrics: Normal and Problem Pregnancies, ed. Landon M, Galan H, Jauniaux E, Driscoll D, Berghella V, et al. , pp. 459–474. Philadelphia, PA: Elsevier. 8th ed. [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HOD. 2006. Endocrine regulation of menstruation. Endocr. Rev 27:17–46 [DOI] [PubMed] [Google Scholar]
- Jamaluddin MFB, Ghosh A, Ingle A, Mohammed R, Ali A, et al. 2022. Bovine and human endometrium-derived hydrogels support organoid culture from healthy and cancerous tissues. PNAS 119:e2208040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PA, Stromme WB. 1972. Amenorrhea secondary to puerperal curettage (Asherman’s syndrome). Am. J. Obstet. Gynecol 113:150–57 [DOI] [PubMed] [Google Scholar]
- Jin S. 2019. Bipotent stem cells support the cyclical regeneration of endometrial epithelium of the murine uterus. PNAS 116:6848–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannisson E, Fournier K, Riotton G. 1981. Regeneration of the human endometrium and presence of inflammatory cells following diagnostic curettage. Acta Obstet. Gynecol. Scand 60:451–57 [DOI] [PubMed] [Google Scholar]
- Johary J, Xue M, Zhu X, Xu D, Velu PP. 2014. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J. Minim. Invasive Gynecol 21:44–54 [DOI] [PubMed] [Google Scholar]
- Johnson FW. 1900. Steam in the treatment of chronic, hyperplastic, and senile endometritis, putrid abortion and puerperal sepsis. Boston Med. Surg. J 142:269–74 [Google Scholar]
- Jonkman MF, Kauer FM, Nieuwenhuis P, Molenaar I. 1986. Segmental uterine horn replacement in the rat using a biodegradable microporous synthetic tube. Artif. Organs 10:475–80 [DOI] [PubMed] [Google Scholar]
- Kaitu’u-Lino TJ, Morison NB, Salamonsen LA. 2007. Estrogen is not essential for full endometrial restoration after breakdown: lessons from a mouse model. Endocrinology 148:5105–11 [DOI] [PubMed] [Google Scholar]
- Kaitu’u-Lino TJ, Ye L, Gargett CE. 2010. Reepithelialization of the uterine surface arises from endometrial glands: evidence from a functional mouse model of breakdown and repair. Endocrinology 151:3386–95 [DOI] [PubMed] [Google Scholar]
- Kato K, Yoshimoto M, Kato K, Adachi S, Yamayoshi A, et al. 2007. Characterization of side-population cells in human normal endometrium. Hum. Reprod 22:1214–23 [DOI] [PubMed] [Google Scholar]
- Kelly RW, King AE, Critchley HO. 2001. Cytokine control in human endometrium. Reproduction 121:3–19 [DOI] [PubMed] [Google Scholar]
- Kilic S, Yuksel B, Pinarli F, Albayrak A, Boztok B, Delibasi T. 2014. Effect of stem cell application on Asherman syndrome, an experimental rat model. J. Assist. Reprod. Genet 31:975–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PM, Gibson DA, Shaw I, Dobie R, Kelepouri O, et al. 2022. Single cell RNA sequencing and lineage tracing confirm mesenchyme to epithelial transformation (MET) contributes to repair of the endometrium at menstruation. eLife 11:e77663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PM, Gibson DA, Smith JR, Wilson-Kanamori JR, Kelepouri O, et al. 2021. Single-cell RNA sequencing redefines the mesenchymal cell landscape of mouse endometrium. FASEB J. 35:e21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolikowski A, Janowski K, Larsen JVM. 1995. Asherman syndrome caused by schistosomiasis. Obstet. Gynecol 85:898–99 [DOI] [PubMed] [Google Scholar]
- Kuokkanen S, Zhu L, Pollard JW. 2017. Xenografted tissue models for the study of human endometrial biology. Differentiation. 98:62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung RK, Lin Y, Liu Y. 2020. Recent advances in understandings towards pathogenesis and treatment for intrauterine adhesion and disruptive insights from single-cell analysis. Reproductive Sciences. 28:1812–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PR, Galvin PM, Short RV. 1987. Salivary oestriol and progesterone concentrations in women during late pregnancy, parturition and the puerperium. J. Endocrinol 115:177–81 [DOI] [PubMed] [Google Scholar]
- Li X, Kodithuwakku SP, Chan RWS, Yeung WSB, Yao Y, et al. 2022. Three-dimensional culture models of human endometrium for studying trophoblast-endometrium interaction during implantation. Reprod. Biol. Endocrinol 20: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Sun H, Lin N, Hou X, Wang J, et al. 2011. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials 32:8172–81 [DOI] [PubMed] [Google Scholar]
- Liang S, Huang Y, Xia Y, Liang S, Wu Q, Zhi Z. 2022. Animal models in intrauterine adhesion research. J. Obstet. Gynaecol 42(8):3409–15 [DOI] [PubMed] [Google Scholar]
- Lipschutz JH, Fukami H, Yamamoto M, Tatematsu M, Sugimura Y, et al. 1999. Clonality of urogenital organs as determined by analysis of chimeric mice. Cells Tissues Organs 165:57–66 [DOI] [PubMed] [Google Scholar]
- Lisa JR, Gioia JD, Rubin IC. 1954. Observations on the interstitial portion of the fallopian tube. Surg. Gynecol. Obstet 99:159–69 [PubMed] [Google Scholar]
- Liu F, Zhu Z-J, Li P, He Y-L. 2013. Creation of a female rabbit model for intrauterine adhesions using mechanical and infectious injury. J. Surg. Res 183:296–303 [DOI] [PubMed] [Google Scholar]
- Liu L, Yang H, Guo Y, Yang G, Chen Y. 2019. The impact of chronic endometritis on endometrial fibrosis and reproductive prognosis in patients with moderate and severe intrauterine adhesions: a prospective cohort study. Fertil. Steril 111:1002–10.e2 [DOI] [PubMed] [Google Scholar]
- Liu T, Shi F, Ying Y, Chen Q, Tang Z, Lin H. 2020. Mouse model of menstruation: an indispensable tool to investigate the mechanisms of menstruation and gynaecological diseases. Mol. Med. Rep 22:4463–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun S-C. 2017. NF-κB signaling in inflammation. Signal Transduct. Target. Ther 2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tal R, Pluchino N, Mamillapalli R, Taylor HS. 2018. Systemic administration of bone marrow-derived cells leads to better uterine engraftment than use of uterine-derived cells or local injection. J. Cell. Mol. Med 22:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Doan QV, Blumenthal P, Dubois RW. 2007. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 10:183–94 [DOI] [PubMed] [Google Scholar]
- Longinotti MK, Jacobson GF, Hung Y-Y, Learman LA. 2008. Probability of hysterectomy after endometrial ablation. Obstet. Gynecol 112:1214–20 [DOI] [PubMed] [Google Scholar]
- Ludwig H. 1971. Surface structure of the human term placenta and of the uterine wall post partum in the screen scan electron microscope. Am. J. Obstet. Gynecol 111:328–44 [DOI] [PubMed] [Google Scholar]
- Ludwig H, Metzger H. 1976. The re-epithelization of endometrium after menstrual desquamation. Arch. Gynakol 221:51–60 [DOI] [PubMed] [Google Scholar]
- Ludwig H, Spornitz UM. 1991. Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Ann. N.Y. Acad. Sci 622:28–46 [DOI] [PubMed] [Google Scholar]
- Marbaix E, Kokorine I, Moulin P, Donnez J, Eeckhout Y, Courtoy PJ. 1996. Menstrual breakdown of human endometrium can be mimicked in vitro and is selectively and reversibly blocked by inhibitors of matrix metalloproteinases. PNAS 93:9120–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March CM. 2011a. Asherman’s syndrome. Semin. Reprod. Med 29:83–94 [DOI] [PubMed] [Google Scholar]
- March CM. 2011b. Management of Asherman’s syndrome. Reprod. Biomed. Online 23:63–76 [DOI] [PubMed] [Google Scholar]
- Markee JE. 1940. Menstruation in intraocular endometrial transplants in the rhesus monkey. Contrib. Embryol. Carnegie Inst. Wash 28:219–308 [Google Scholar]
- Martinez GM. 2020. Trends and patterns in menarche in the United States: 1995 through 2013–2017. Natl. Health Stat. Rep. 146, Natl. Cent. Health Stat., Hyattsville, MD: [PubMed] [Google Scholar]
- Masuda H, Anwar SS, Bühring H-J, Rao JR, Gargett CE. 2012. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 21:2201–14 [DOI] [PubMed] [Google Scholar]
- McLennan CE. 1969. Endometrial regeneration after curettage. Am. J. Obstet. Gynecol 104:185–94 [DOI] [PubMed] [Google Scholar]
- Metcalf MG, Donald RA, Livesey JH. 1981. Pituitary-ovarian function in normal women during the menopausal transition. Clin. Endocrinol 14:245–55 [DOI] [PubMed] [Google Scholar]
- Moore L, Leongamornlert D, Coorens THH, Sanders MA, Ellis P, et al. 2020. The mutational landscape of normal human endometrial epithelium. Nature 580:640–46 [DOI] [PubMed] [Google Scholar]
- Morelli SS, Rameshwar P, Goldsmith LT. 2013. Experimental evidence for bone marrow as a source of nonhematopoietic endometrial stromal and epithelial compartment cells in a murine model. Biol. Reprod 89:7. [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A. 2010. Nonresolving inflammation. Cell 140:871–882 [DOI] [PubMed] [Google Scholar]
- Netter A, Musset R, Lambert A, Salomon Y, Montbazet G. 1955. Symphyses endo-utérines tuberculeuses: un syndrome anatomo-clinique et radiologique caractéristique. Gynecol. Obstet 54:19–36 [PubMed] [Google Scholar]
- Nguyen HPT, Xiao L, Deane JA, Tan K-S, Cousins FL, et al. 2017. N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays. Hum. Reprod 32:2254–68 [DOI] [PubMed] [Google Scholar]
- Ning J, Zhang H, Yang H. 2018. MicroRNA-326 inhibits endometrial fibrosis by regulating TGF-β1/Smad3 pathway in intrauterine adhesions. Mol. Med. Rep 18:2286–92 [DOI] [PubMed] [Google Scholar]
- Nogales-Ortiz F, Puerta J, Nogales FF. 1978. The normal menstrual cycle: chronology and mechanism of endometrial desquamation. Obstet. Gynecol 51:259–64 [DOI] [PubMed] [Google Scholar]
- Novak E, Te Linde RW. 1924. The endometrium of the menstruating uterus. JAMA 83:900–6 [Google Scholar]
- Ong YR, Cousins FL, Yang X, Mushafi AAAA, Breault DT, et al. 2018. Bone marrow stem cells do not contribute to endometrial cell lineages in chimeric mouse models. Stem Cells 36:91–102 [DOI] [PubMed] [Google Scholar]
- Onoglu A, Taskin O, Inal M, Sadik S, Simsek M, et al. 2007. Comparison of the long-term histopathologic and morphologic changes after endometrial rollerball ablation and resection: a prospective randomized trial. J. Minim. Invasive Gynecol 14:39–42 [DOI] [PubMed] [Google Scholar]
- Patterson A, Pru J. 2013. Long-term label retaining cells localize to distinct regions within the female reproductive epithelium. Cell Cycle 12:2888–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AL, Zhang L, Arango NA, Teixeira J, Pru JK. 2013. Mesenchymal-to-epithelial transition contributes to endometrial regeneration following natural and artificial decidualization. Stem Cells Dev. 22:964–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polishuk WZ, Siew FP, Gordon R, Lebenshart P. 1977. Vascular changes in traumatic amenorrhea and hypomenorrhea. Int. J. Fertil 22:189–92 [PubMed] [Google Scholar]
- Rabau E, David A. 1963. Intrauterine adhesions, etiology, prevention, and treatment. Obstet. Gynecol.22:626–29 [PubMed] [Google Scholar]
- Rasweiler JJ, Debonilla H. 1992. Menstruation in short-tailed fruit bats (Carollia spp.). J. Reprod. Fertil 95:231–48 [DOI] [PubMed] [Google Scholar]
- Rockey DC, Bell PD, Hill JA. 2015. Fibrosis—a common pathway to organ injury and failure. N. Engl. J. Med 372:1138–49 [DOI] [PubMed] [Google Scholar]
- Rodgers WH, Matrisian LM, Giudice LC, Dsupin B, Cannon P, et al. 1994. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J. Clin. Investig 94:946–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer T 1994. Post-abortion-hysteroscopy–a method for early diagnosis of congenital and acquired intrauterine causes of abortions. Eur. J. Obstet. Gynecol. Reprod. Biol 57:171–73 [DOI] [PubMed] [Google Scholar]
- Rudolph M, Döcke W-D, Müller A, Menning A, Röse L, et al. 2012. Induction of overt menstruation in intact mice. PLOS ONE 7:e32922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin Ersoy G, Zolbin MM, Cosar E, Moridi I, Mamillapalli R, Taylor HS. 2017. CXCL12 promotes stem cell recruitment and uterine repair after injury in Asherman’s syndrome. Mol. Ther. Methods Clin. Dev 4:169–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salma U, Xue M, Ali Sheikh MS, Guan X, Xu B, et al. 2016. Role of transforming growth factor-β1 and smads signaling pathway in intrauterine adhesion. Mediat. Inflamm 2016:e4158287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, et al. 2016. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum. Reprod 31:1087–96 [DOI] [PubMed] [Google Scholar]
- Santamaria X, Roson B, Perez R, Venkatesan N, Gonzalez-Fernandez J, et al. 2022. Decoding the endometrial niche of Asherman’s Syndrome at single-cell resolution. medRxiv 2022.10.21.22281346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer G. 1976. Female genital tuberculosis. Clin. Obstet. Gynecol 19:223–39 [DOI] [PubMed] [Google Scholar]
- Schenker JG, Margalioth EJ. 1982. Intrauterine adhesions: an updated appraisal. Fertil. Steril 37:593–610 [DOI] [PubMed] [Google Scholar]
- Schenker JG, Nicosia SV, Polishuk WZ, Garcia CR. 1975. An in vitro fibroblast-enriched sponge preparation for induction of intrauterine adhesions. Isr. J. Med. Sci 11:849–51 [PubMed] [Google Scholar]
- Schenker JG, Polishuk WZ. 1972. Regeneration of rabbit endometrium after cryosurgery. Obstet. Gynecol 40:638–45 [PubMed] [Google Scholar]
- Schenker JG, Polishuk WZ. 1973. Regeneration of rabbit endometrium following intrauterine instillation of chemical agents. Gynecol. Investig 4(1):1–13 [DOI] [PubMed] [Google Scholar]
- Schenker JG, Polishuk WZ, Sacks MI. 1973a. Regeneration of the endometrium in rabbits after curettage. I. Pretreatment with estrogens. J. Reprod. Med 11:43–48 [PubMed] [Google Scholar]
- Schenker JG, Polishuk WZ, Sacks MI. 1973b. Regeneration of the endometrium in rabbits after curettage. II. Pretreatment with progesterone. J. Reprod. Med 11:49–57 [PubMed] [Google Scholar]
- Schenker JG, Sacks MI, Polishuk WZ. 1971. I. Regeneration of rabbit endometrium following curettage. Am. J. Obstet. Gynecol 111:970–78 [DOI] [PubMed] [Google Scholar]
- Schwab KE, Chan RWS, Gargett CE. 2005. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil. Steril 84:1124–30 [DOI] [PubMed] [Google Scholar]
- Schwab KE, Gargett CE. 2007. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod 22:2903–11 [DOI] [PubMed] [Google Scholar]
- Seishima R, Leung C, Yada S, Murad KBA, Tan LT, et al. 2019. Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nat. Commun 10:5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma JB, Roy KK, Pushparaj M, Gupta N, Jain SK, et al. 2008. Genital tuberculosis: an important cause of Asherman’s syndrome in India. Arch. Gynecol. Obstet 277:37–41 [DOI] [PubMed] [Google Scholar]
- Sharman A. 1953. Post-partum regeneration of the human endometrium. J. Anat 87:1–10 [PMC free article] [PubMed] [Google Scholar]
- Shavell VI, Diamond MP, Senter JP, Kruger ML, Johns DA. 2012. Hysterectomy subsequent to endometrial ablation. J. Minim. Invasive Gynecol 19:459–64 [DOI] [PubMed] [Google Scholar]
- Silvernagel SW, Harshbarger KE, Shevlin DW. 1997. Postoperative granulomas of the endometrium: histological features after endometrial ablation. Ann. Diagn. Pathol 1:82–90 [DOI] [PubMed] [Google Scholar]
- Slayden OD, Brenner RM. 2006. A critical period of progesterone withdrawal precedes menstruation in macaques. Reprod. Biol. Endocrinol 4(Suppl. 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid I, Bedö T. 1978. Curettage during puerperium and its late consequences. Zentralbl Gynakol 100:916–20 [PubMed] [Google Scholar]
- Song T, Zhao X, Sun H, Li Xa, Lin N, et al. 2015. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng. Part A 21:353–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner MK, Lenis YY, Watson R, Jaimes D, Patterson AL. 2021. The role of stem cells in uterine involution. Reproduction 161:R61–77 [DOI] [PubMed] [Google Scholar]
- Spooner-Harris M, Kerns K, Zigo M, Sutovsky P, Balboula A, Patterson AL. 2022. A re-appraisal of mesenchymal-epithelial transition (MET) in endometrial epithelial remodeling. Cell Tissue Res. 391:393–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer S. 1946. Partial and total atresia of the uterus after excochleation. Acta Obstet. Gynecol. Scand 26:263–97 [Google Scholar]
- Stewart CA, Stewart MD, Wang Y, Mullen RD, Kircher BK, et al. 2022. Chronic estrus disrupts uterine gland development and homeostasis. Endocrinology 163:bqac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strug MR, Su RW, Kim TH, Mauriello A, Ticconi C, et al. 2018. RBPJ mediates uterine repair in the mouse and is reduced in women with recurrent pregnancy loss. FASEB J. 32:2452–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto O. 1978. Diagnostic and therapeutic hysteroscopy for traumatic intrauterine adhesions. Am. J. Obstet. Gynecol 131:539–47 [DOI] [PubMed] [Google Scholar]
- Swain M, Kulkarni AD. 2021. Endometrium at menopause: the pathologist’s view. J. Midlife Health 12:310–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed SM, Kumar M, Ghosh A, Tomasetig F, Ali A, et al. 2020. Endometrial Axin2+ cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell 26:64–80.e13 [DOI] [PubMed] [Google Scholar]
- Tal R, Kisa J, Abuwala N, Kliman HJ, Shaikh S, et al. 2021. Bone marrow-derived progenitor cells contribute to remodeling of the postpartum uterus. Stem Cells 39:1489–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal R, Liu Y, Pluchino N, Shaikh S, Mamillapalli R, Taylor HS. 2016. A murine 5-fluorouracil-based submyeloablation model for the study of bone marrow-derived cell trafficking in reproduction. Endocrinology 157:3749–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal R, Shaikh S, Pallavi P, Tal A, López-Giráldez F, et al. 2019. Adult bone marrow progenitors become decidual cells and contribute to embryo implantation and pregnancy. PLOS Biol. 17:e3000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Kyo S, Kanaya T, Yatabe N, Nakamura M, et al. 2003. Evidence of the monoclonal composition of human endometrial epithelial glands and mosaic pattern of clonal distribution in luminal epithelium. Am. J. Pathol 163:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-Q, Gan L, Xu Q, Wang S, Li J-J, Duan H. 2016. Effects of human umbilical cord mesenchymal stem cells on intrauterine adhesions in a rat model. Int. J. Clin. Exp. Pathol 9:12119–29 [Google Scholar]
- Taskin O, Onoglu A, Inal M, Turan E, Sadik S, et al. 2002. Long-term histopathologic and morphologic changes after thermal endometrial ablation. J. Am. Assoc. Gynecol. Laparosc 9:186–90 [DOI] [PubMed] [Google Scholar]
- Taveau JW, Tartaglia M, Buchannan D, Smith B, Koenig G, et al. 2004. Regeneration of uterine horn using porcine small intestinal submucosa grafts in rabbits. J. Investig. Surg 17:81–92 [DOI] [PubMed] [Google Scholar]
- Taylor HS. 2004. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 292:81–85 [DOI] [PubMed] [Google Scholar]
- Tempest N, Hill CJ, Maclean A, Marston K, Powell SG, et al. 2022. Novel microarchitecture of human endometrial glands: implications in endometrial regeneration and pathologies. Hum. Reprod. Update 28:153–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest N, Jansen M, Baker AM, Hill CJ, Hale M, et al. 2020. Histological 3D reconstruction and in vivo lineage tracing of the human endometrium. J. Pathol 251:440–51 [DOI] [PubMed] [Google Scholar]
- Tempest N, Maclean A, Hapangama DK. 2018. Endometrial stem cell markers: current concepts and unresolved questions. Int. J. Mol. Sci 19:3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresserra F, Grases P, Ubeda A, Pascual MA, Grases PJ, Labastida R. 1999. Morphological changes in hysterectomies after endometrial ablation. Hum. Reprod 14:1473–77 [DOI] [PubMed] [Google Scholar]
- Tsuji S, Yoshimoto M, Takahashi K, Noda Y, Nakahata T, Heike T. 2008. Side population cells contribute to the genesis of human endometrium. Fertil. Steril 90:1528–37 [DOI] [PubMed] [Google Scholar]
- Turnbull LW, Jumaa A, Bowsley SJ, Dhawan S, Horsman A, Killick SR. 1997. Magnetic resonance imaging of the uterus after endometrial resection. BJOG 104:934–38 [DOI] [PubMed] [Google Scholar]
- Valentijn AJ, Palial K, Al-lamee H, Tempest N, Drury J, et al. 2013. SSEA-1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum. Reprod 28:2695–708 [DOI] [PubMed] [Google Scholar]
- Van De Water L, Varney S, Tomasek JJ. 2013. Mechanoregulation of the myofibroblast in wound contraction, scarring, and fibrosis: opportunities for new therapeutic intervention. Adv. Wound Care 2:122–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Horst CJ. 1954. Elephantulus going into anoestrus menstruation and abortion. Phil. Trans. R. Soc. B 238:27–61 [Google Scholar]
- Ventolini G, Zhang M, Gruber J. 2004. Hysteroscopy in the evaluation of patients with recurrent pregnancy loss: a cohort study in a primary care population. Surgical Endoscopy. 18:1782–1784 [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu X, He B, Li Y, Chen X, Wang J. 2013. A critical period of progesterone withdrawal precedes endometrial breakdown and shedding in mouse menstrual-like model. Human Reprod. 28:1670–78 [DOI] [PubMed] [Google Scholar]
- Wang W, Vilella F, Alama P, Moreno I, Mignardi M, et al. 2020. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med 26:1644–53 [DOI] [PubMed] [Google Scholar]
- Wang X, Ma N, Sun Q, Huang C, Liu Y, Luo X. 2017. Elevated NF-κB signaling in Asherman syndrome patients and animal models. OncoTargets Ther. 8:15399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh AO, Enders AC. 1983. Occlusion and reformation of the rat uterine lumen during pregnancy. Am. J. Anat 167:463–77 [DOI] [PubMed] [Google Scholar]
- Westendorp IC, Ankum WM, Mol BW, Vonk J. 1998. Prevalence of Asherman’s syndrome after secondary removal of placental remnants or a repeat curettage for incomplete abortion. Hum. Reprod 13:3347–50 [DOI] [PubMed] [Google Scholar]
- Williams JW. 1931. Regeneration of the uterine mucosa after delivery, with especial reference to the placental site. Am. J. Obstet. Gynecol 22:664–96 [PubMed] [Google Scholar]
- Winkler I, Tolkachov A, Lammers F, Lacour P, Schneider N, et al. 2022. The function and decline of the female reproductive tract at single-cell resolution. bioRxiv 2022.10.26.513823. 10.1101/2022.10.26.513823 [DOI] [Google Scholar]
- Wolff EF, Uchida N, Donahue RE, Metzger ME, Hsieh MM, et al. 2013. Peripheral blood stem cell transplants do not result in endometrial stromal engraftment. Fertil. Steril 99:526–32.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GA, Fata JE, Watson KLM, Khokha R. 2007. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction 133:1035–44 [DOI] [PubMed] [Google Scholar]
- Wortman M. 2017. Late-onset endometrial ablation failure. Case Rep. Women’s Health 15:11–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss P, Steiner R, Liaw LH, Wyss MT, Ghazarians A, et al. 1996. Uterus and endometrium: regeneration processes in rabbit endometrium: a photodynamic therapy model. Hum. Reprod 11:1992–97 [DOI] [PubMed] [Google Scholar]
- Xiao S, Wan Y, Xue M, Zeng X, Xiao F, et al. 2014. Etiology, treatment, and reproductive prognosis of women with moderate-to-severe intrauterine adhesions. Int. J. Gynaecol. Obstet 125:121–24 [DOI] [PubMed] [Google Scholar]
- Xu X, Chen X, Li Y, Cao H, Shi C, et al. 2013. Cyclooxygenase-2 regulated by the nuclear factor-κB pathway plays an important role in endometrial breakdown in a female mouse menstrual-like model. Endocrinology 154:2900–11 [DOI] [PubMed] [Google Scholar]
- Xue X, Chen Q, Zhao G, Zhao J-Y, Duan Z, Zheng P-S. 2015. The overexpression of TGF-β and CCN2 in intrauterine adhesions involves the NF-κB signaling pathway. PLOS ONE 10:e0146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe H, Ron M, Polishuk WZ. 1978. Amenorrhea, hypomenorrhea, and uterine fibrosis. Am. J. Obstet. Gynecol 130:599–601 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Yoshihara K, Suda K, Nakaoka H, Yachida N, et al. 2021. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience 24:102258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M, Zhou HJ, Lin C, Long L, Yang X, et al. 2019. CD34+ KLF4+ stromal stem cells contribute to endometrial regeneration and repair. Cell Rep. 27:2709–24.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Wang J, Cui W, Tong C. 2023. Advanced biomaterials for promoting endometrial regeneration. Adv. Healthcare Mater 2202490. [DOI] [PubMed] [Google Scholar]
- Young RE, Huh DD. 2021. Organ-on-a-chip technology for the study of the female reproductive system. Adv. Drug. Deliv. Rev 173:461–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Deng W, Cha J, Sun X, Borg J-P, Dey SK. 2018. Tridimensional visualization reveals direct communication between the embryo and glands critical for implantation. Nat. Commun 9:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ET, Wells KL, Steinmetz L, Baker JC. 2022. Uterine injury during diestrus leads to embryo spacing defects and perturbations in the COX pathway in subsequent pregnancies. bioRxiv 2022.03.15.484521. 10.1101/2022.03.15.484521 [DOI] [Google Scholar]
- Zondervan KT, Becker CM, Missmer SA. 2020. Endometriosis. N. Engl. J. Med 382:1244–56 [DOI] [PubMed] [Google Scholar]


