Abstract
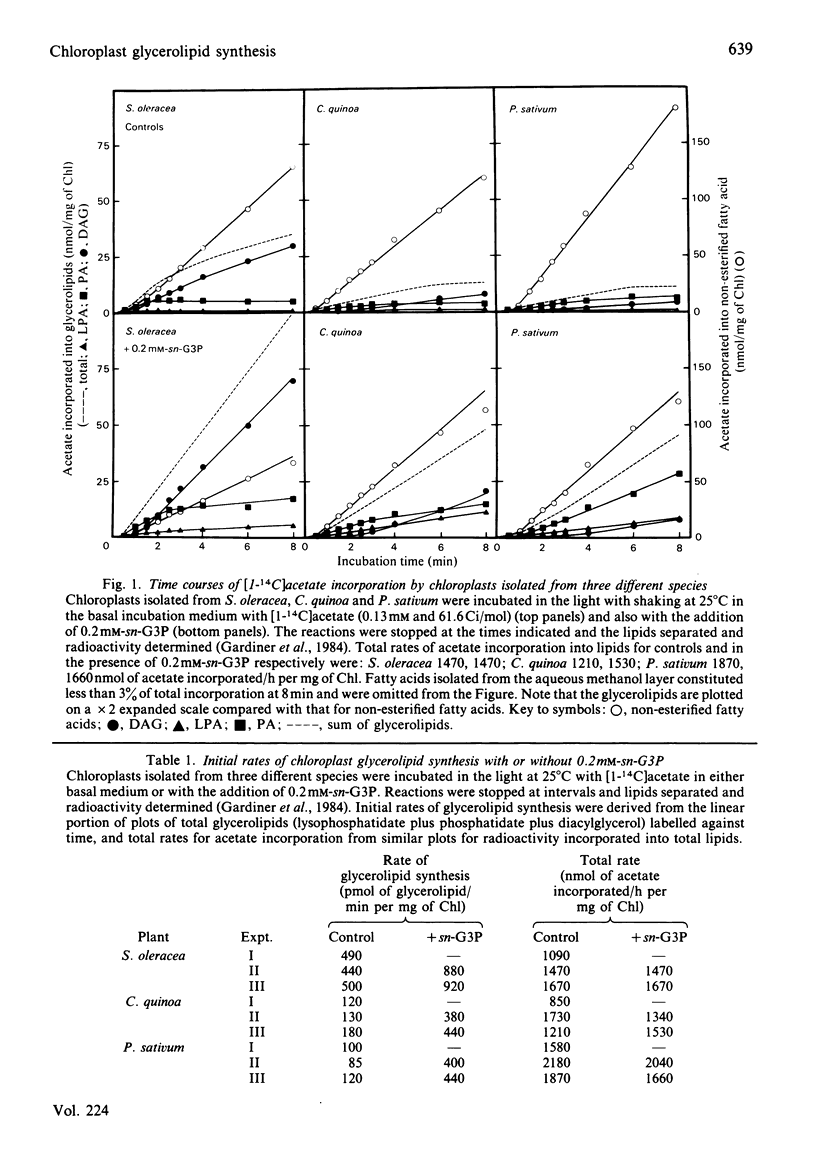
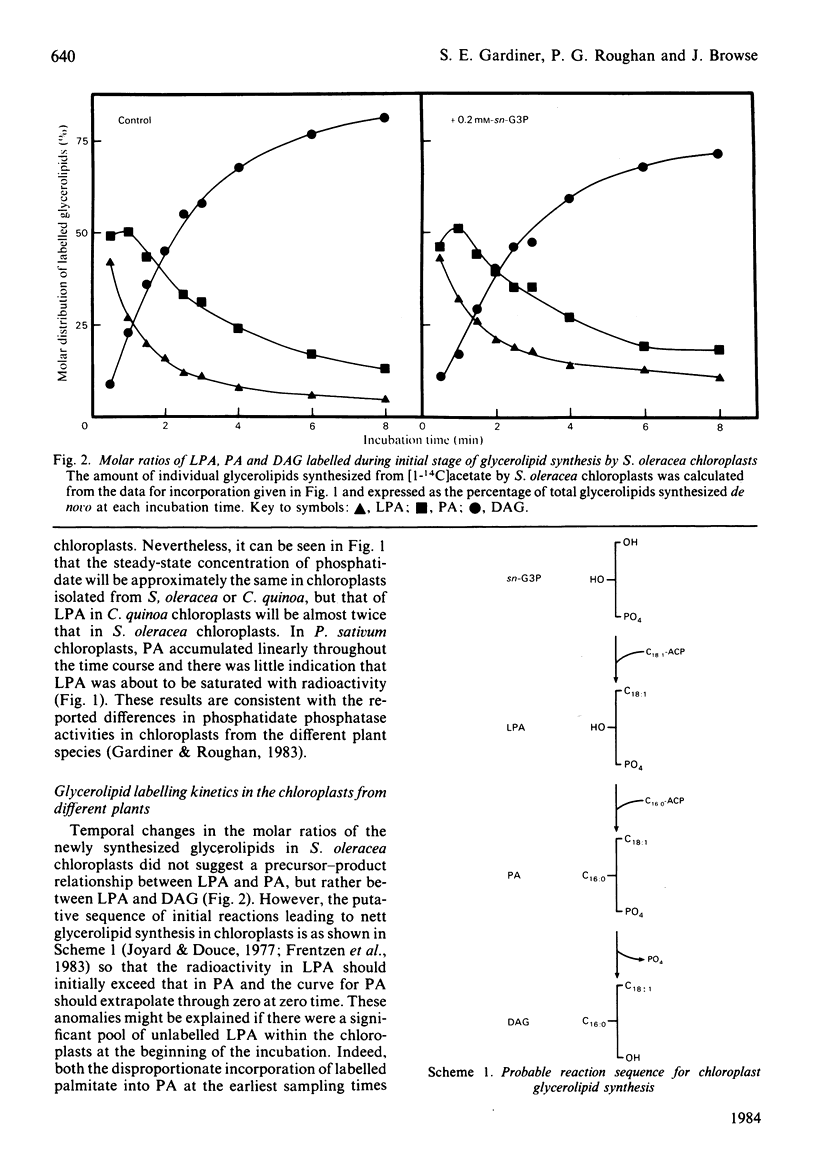
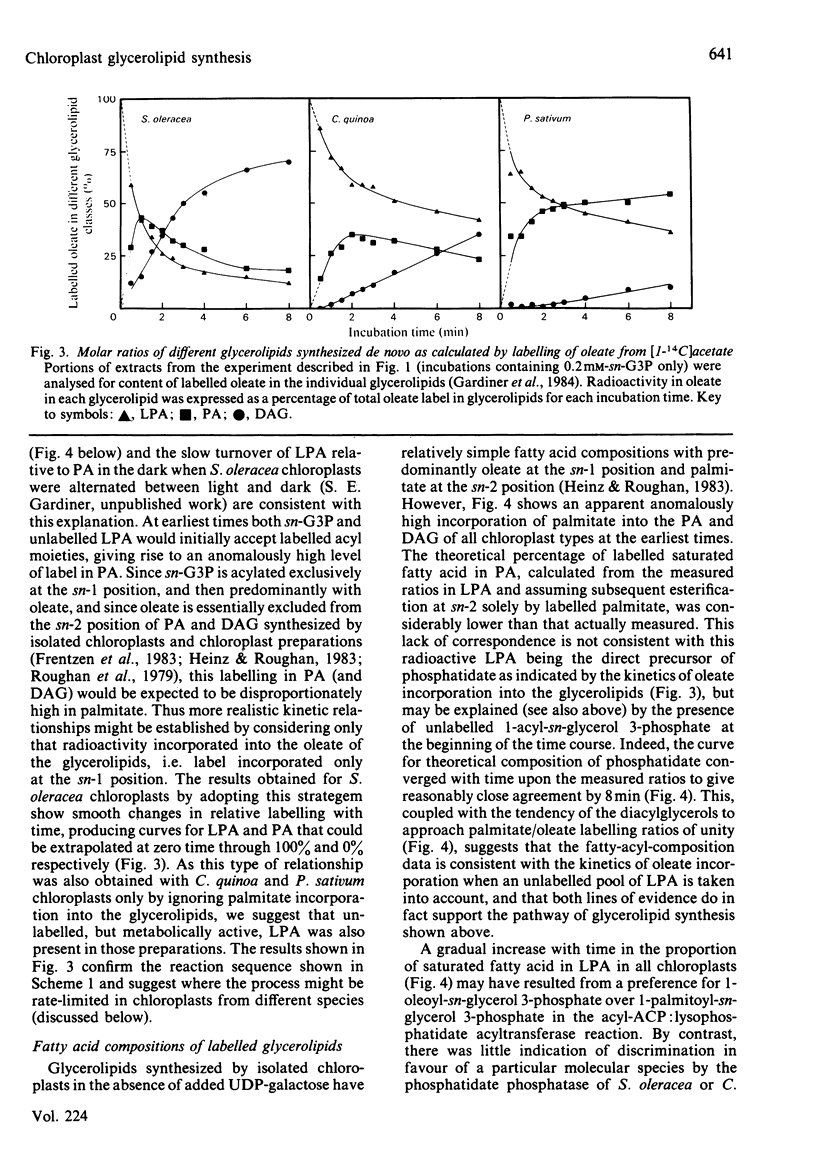
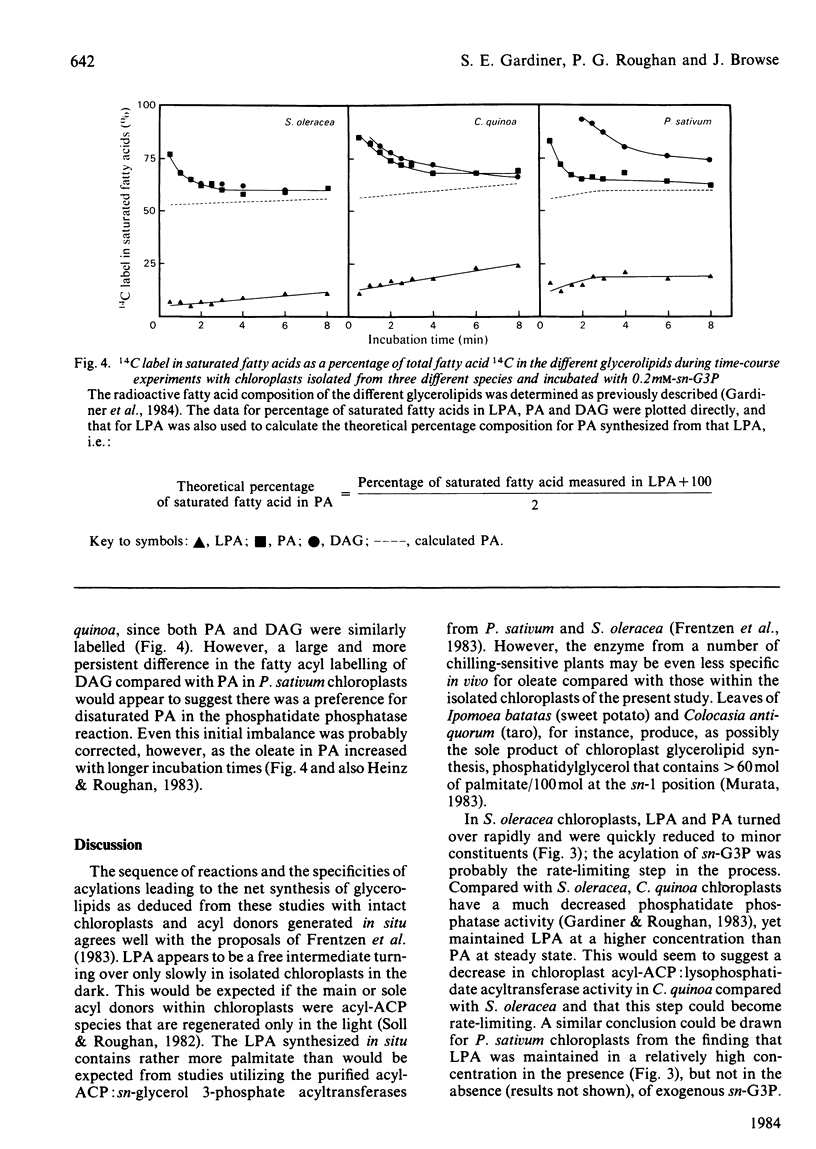
Glycerolipid synthesis was studied in intact chloroplasts isolated from three different plant species. The sequential acylation of sn-glycerol 3-phosphate and lysophosphatidate (1-acyl-sn-glycerol 3-phosphate) was confirmed by monitoring the incorporation of oleate synthesized in situ into lysophosphatidate, phosphatidate and diacylglycerol. Lysophosphatidate was not only readily detected in these experiments, but was also present in the chloroplasts at the beginning of the time courses. The rate of glycerolipid synthesis depended primarily on sn-glycerol 3-phosphate supply, and given adequate sn-glycerol 3-phosphate, the proportion of newly synthesized fatty acids diverted into glycerolipids appeared to be determined by differing acyltransferase activities in the chloroplasts isolated from different plant species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrams M., Heinz E. Positional Specificity and Fatty Acid Selectivity of Purified sn-Glycerol 3-Phosphate Acyltransferases from Chloroplasts. Plant Physiol. 1981 Sep;68(3):653–657. doi: 10.1104/pp.68.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Gardiner S. E., Heinz E., Roughan P. G. Rates and products of long-chain Fatty Acid synthesis from [1-C]acetate in chloroplasts isolated from leaves of 16:3 and 18:3 plants. Plant Physiol. 1984 Apr;74(4):890–896. doi: 10.1104/pp.74.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. E., Roughan P. G. Relationship between fatty-acyl composition of diacylgalactosylglycerol and turnover of chloroplast phosphatidate. Biochem J. 1983 Mar 15;210(3):949–952. doi: 10.1042/bj2100949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E., Roughan P. G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983 Jun;72(2):273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Douce R. Characterization of phosphatidate phosphohydrolase activity associated with chloroplast envelope membranes. FEBS Lett. 1979 Jun 1;102(1):147–150. doi: 10.1016/0014-5793(79)80947-3. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- McKee J. W., Hawke J. C. The incorporation of [14C]acetate into the constituent fatty acids of monogalactosyldiglyceride by isolated spinach chloroplasts. Arch Biochem Biophys. 1979 Oct 1;197(1):322–332. doi: 10.1016/0003-9861(79)90252-2. [DOI] [PubMed] [Google Scholar]
- Mudd J. B., Dezacks R. Synthesis of phosphatidylglycerol by chloroplasts from leaves of Spinacia oleracea L. (spinach). Arch Biochem Biophys. 1981 Jul;209(2):584–591. doi: 10.1016/0003-9861(81)90316-7. [DOI] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. The role of chloroplasts and microsomal fractions in polar-lipid synthesis from [1-14C]acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem J. 1980 Apr 15;188(1):17–24. doi: 10.1042/bj1880017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparace S. A., Mudd J. B. Phosphatidylglycerol synthesis in spinach chloroplasts: characterization of the newly synthesized molecule. Plant Physiol. 1982 Nov;70(5):1260–1264. doi: 10.1104/pp.70.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B., Beevers H. Phosphatidic Acid synthesis in castor bean endosperm. Plant Physiol. 1977 Mar;59(3):459–463. doi: 10.1104/pp.59.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz W., Stitt M., Heldt H. W. Enzymic determination of metabolites in the subcellular compartments of spinach protoplasts. Plant Physiol. 1980 Jul;66(1):187–193. doi: 10.1104/pp.66.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]


