TO THE EDITOR:
Pediatric recipients of hematopoietic-cell transplants (HCT) are at high risk for influenza-related illness and death. They also have weaker humoral immune responses to influenza vaccination than healthy children, which suggests that alternative vaccine regimens are needed.1 Previous phase 1 studies showed that high-dose influenza vaccines are immunogenic in some high-risk populations without evident safety concerns, but data are lacking for pediatric recipients of HCT.2-4
We conducted a phase 2, multicenter, double-blind, randomized, controlled trial (Pediatric HCT Flu Study; ClinicalTrials.gov number, NCT02860039) that compared immunogenicity and safety between high-dose trivalent influenza vaccine (HD-TIV) and standard-dose quadrivalent influenza vaccine (SD-QIV) in children and adolescents 3 to 17 years of age who had received an allogeneic HCT 3 to 35 months earlier. The trial was conducted over three influenza seasons (2016 through 2019). The protocol (available with the full text of this letter at NEJM.org) was approved by the institutional review board at each site, and written informed consent was obtained from parents or legal guardians. Each participant received two vaccine doses, 28 to 42 days apart. Hemagglutination-inhibition (HAI) titers to all four vaccine-specific influenza antigens (A/H1N1, A/H3N2, B/Victoria, and B/Yamagata) were measured before each vaccine dose and 28 to 42 days after the second dose. The primary immunogenicity end point was the adjusted geometric mean ratio (GMR [HD-TIV vs. SD-QIV]) of HAI titers to influenza A antigens 28 to 42 days after the second dose. Solicited injection-site reactions and systemic adverse events were assessed for 7 days after each dose.
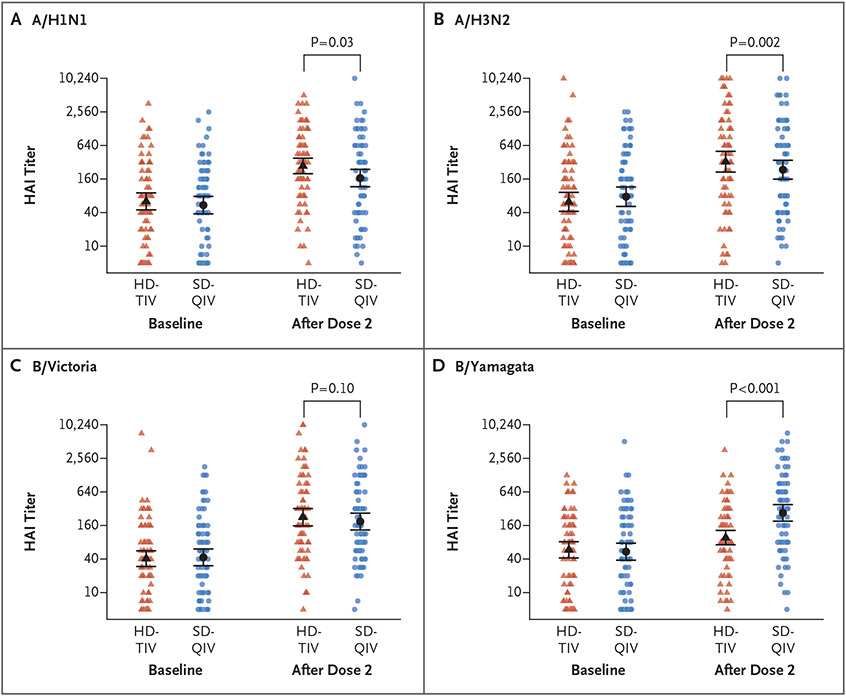
A total of 170 participants were randomly assigned to receive two vaccine doses of either HD-TIV (85 recipients) or SD-QIV (85 participants) (Fig. S1 in the Supplementary Appendix, available at NEJM.org). The median age of the participants was 10.9 years, 76 (45%) were female, 117 (69%) were White, 36 (21%) were Hispanic or Latino, and the median time from transplantation to enrollment was 7.8 months (Table S1). As compared with two doses of SD-QIV, two doses of HD-TIV were associated with significantly higher geometric mean titers to A/H1N1 (adjusted GMR, 1.65; 95% confidence interval [CI], 1.06 to 2.57; P = 0.03) and A/H3N2 (adjusted GMR, 2.11; 95% CI, 1.32 to 3.38; P = 0.002) and numerically higher titers to B/Victoria (adjusted GMR, 1.46; 95% CI, 0.93 to 2.31; P = 0.10) (Fig. 1 and Table S2) but lower titers to B/Yamagata (which is not included in HD-TIV). The HD-TIV group had a higher frequency of mild or moderate injection-site reactions after the second vaccine dose (Fig. S2). The frequency of any severe reaction was similar in the two groups: 7.5% with HD-TIV and 6.0% with SD-QIV after dose 1 and 7.6% with HD-TIV and 6.4% with SD-QIV after dose 2.
Figure 1. Hemagglutination-Inhibition Titers to Influenza Antigens.
Depicted are the raw data along with group- and time-specific estimates of the geometric mean titers for hemagglutination inhibition (HAI) along with 95% confidence intervals (indicated by I bars). P values are for the adjusted geometric mean ratios comparing high-dose trivalent influenza vaccine (HD-TIV) with standard-dose quadrivalent influenza vaccine (SD-QIV). B/Yamagata is not included in HD-TIV.
We found that two doses of HD-TIV resulted in higher antibody responses to influenza A antigens than two doses of SD-QIV in pediatric recipients of HCT. The overall safety profile was similar, with a slightly higher number of mild or moderate injection-site reactions after the second dose of HD-TIV than after the second dose of SD-QIV. Influenza is associated with substantial morbidity and mortality in this high-risk population. Improvement of vaccine strategies is critical, and the administration of two doses of high-dose inactivated influenza vaccine could be a practical strategy to increase immune responses.
Supplementary Material
THIS WEEK’S LETTERS.
Influenza Vaccine in Pediatric Recipients of Hematopoietic-Cell Transplants
Colonoscopy Screening and Colorectal Cancer Incidence and Mortality
Randomized Trial of a Bionic Pancreas in Type 1 Diabetes
Early Amino Acids in Extremely Preterm Infants and Neurodisability
Acknowledgments
Supported by a grant (U01 AI125135) from the National Institute of Allergy and Infectious Diseases. Sanofi donated vaccines and performed testing of hemagglutination inhibition.
Footnotes
The members of the Pediatric HCT Flu Study Group are listed in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Jennifer E. Schuster, Children’s Mercy Kansas City Kansas City, MO
Lubna Hamdan, Vanderbilt University Medical Center Nashville, TN
Daniel E. Dulek, Vanderbilt University Medical Center Nashville, TN
Carrie L. Kitko, Vanderbilt University Medical Center Nashville, TN
Einas Batarseh, Vanderbilt University Medical Center Nashville, TN
Zaid Haddadin, Vanderbilt University Medical Center Nashville, TN
Laura S. Stewart, Vanderbilt University Medical Center Nashville, TN
Anna Stahl, Vanderbilt University Medical Center Nashville, TN
Molly Potter, Vanderbilt University Medical Center Nashville, TN
Herdi Rahman, Vanderbilt University Medical Center Nashville, TN
Spyros A. Kalams, Vanderbilt University Medical Center Nashville, TN
Susan Coffin, Children’s Hospital of Philadelphia Philadelphia, PA
Monica I. Ardura D.O., Children’s Nationwide Children’s Hospital Columbus, OH
Rachel L. Wattier, University of California, San Francisco, Benioff Children’s Hospital–San Francisco San Francisco, CA
Gabriela Maron, St. Jude Children’s Research Hospital Memphis, TN
Claire E. Bocchini, Baylor College of Medicine Houston, TX
Elizabeth A. Moulton, Baylor College of Medicine Houston, TX
Michael Grimley, Cincinnati Children’s Hospital Medical Center Cincinnati, OH
Grant Paulsen, Cincinnati Children’s Hospital Medical Center Cincinnati, OH
Christopher J. Harrison, Children’s Mercy Kansas City Kansas City, MO
Jason Freedman, Children’s Hospital of Philadelphia Philadelphia, PA
Paul A. Carpenter, Seattle Children’s Research Institute Seattle, WA
Janet A. Englund, Seattle Children’s Research Institute Seattle, WA
Flor M. Munoz, Baylor College of Medicine Houston, TX
Lara Danziger-Isakov, Cincinnati Children’s Hospital Medical Center Cincinnati, OH
Andrew J. Spieker, Vanderbilt University Medical Center Nashville, TN
Natasha Halasa, Vanderbilt University Medical Center Nashville, TN
References
- 1.Ryan AL, Wadia UD, Jacoby P, et al. Immunogenicity of the inactivated influenza vaccine in children who have undergone allogeneic haematopoietic stem cell transplant. Bone Marrow Transplant 2020;55:773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McManus M, Frangoul H, McCullers JA, Wang L, O’Shea A, Halasa N. Safety of high dose trivalent inactivated influenza vaccine in pediatric patients with acute lymphoblastic leukemia. Pediatr Blood Cancer 2014;61:815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GiaQuinta S, Michaels MG, McCullers JA, et al. Randomized, double-blind comparison of standard-dose vs. high-dose trivalent inactivated influenza vaccine in pediatric solid organ transplant patients. Pediatr Transplant 2015;19:219–28. [DOI] [PubMed] [Google Scholar]
- 4.Halasa NB, Savani BN, Asokan I, et al. Randomized double-blind study of the safety and immunogenicity of standard-dose trivalent inactivated influenza vaccine versus high-dose trivalent inactivated influenza vaccine in adult hematopoietic stem cell transplantation patients. Biol Blood Marrow Transplant 2016;22:528–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.