Abstract
The human cytomegalovirus UL4 gene encodes a 48-kDa glycoprotein, expression of which is repressed at the translational level by a short upstream open reading frame (uORF2) within the UL4 transcript leader. Mutation of the uORF2 initiation codon in the viral genome eliminates ribosomal stalling at the uORF2 termination site, resulting in early and abundant gpUL4 protein synthesis. This mutation does not appear to affect viral replication kinetics in human fibroblasts. These results reveal that the unusual uORF2 inhibitory mechanism is a principal determinant of the abundance and timing of gpUL4 expression but is nonessential for replication in cell culture.
Viral gene expression during human cytomegalovirus (CMV) infection is regulated at multiple levels, including translation (14). Previous studies of gpUL4 (or gp48), the glycoprotein product of the UL4 gene (8), revealed that its expression is repressed by an unusual translational mechanism that depends on the nascent peptide product of the second of three short upstream open reading frames (uORF2) within the UL4 transcript leader (3, 4, 9, 10, 17). Results from transfection and cell-free translation assays support a model in which the peptide product of uORF2 inhibits translation termination at its own stop codon (3, 4, 6). As a result, ribosomes stall on the mRNA and block ribosomal access to the downstream UL4 cistron.
Clinical isolates of CMV have naturally occurring polymorphisms in uORF2 that change the predicted peptide sequence and, based on transfection assays, are predicted to reduce or eliminate the inhibitory effect of uORF2. In fact, the two such viral isolates analyzed thus far express considerably more gpUL4 protein than strains that have inhibitory uORF2 sequences, even though the levels of UL4 mRNA are similar (1). However, the conclusion that uORF2 controls gpUL4 expression during viral infection based on these observations is limited by the fact that clinical isolates differ at multiple genetic loci in addition to uORF2. Therefore, the present studies were designed to determine the role of uORF2 in controlling gpUL4 expression during infection using an engineered virus that contains precise mutations of the UL4 transcript leader but which is otherwise isogenic with wild-type CMV(Towne).
Construction of uORF2− CMV.
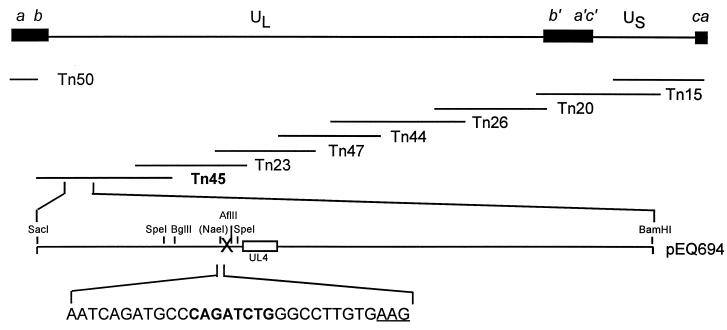
To assess the effect of uORF2 on gpUL4 expression when both are expressed from their natural positions within the viral genome, we constructed recombinant CMV in which the UL4 transcript leader was either wild-type or mutant (Fig. 1). The cosmids Tn15, Tn20, Tn23, Tn26, Tn44, Tn45, Tn47, and Tn50 (13) (kindly provided by G. Kemble, Aviron, Mountain View, Calif.) were digested with PacI and then cotransfected into diploid human foreskin fibroblasts (HF) by calcium phosphate coprecipitation (13) to generate a recombinant wild-type CMV (rTowne-1). Two independent mutant viruses that lack uORF2 (uORF2−), vEQ694-1 and vEQ694-2, were constructed using the same cosmid fragments except that Tn45 was digested with SpeI in addition to PacI and a SacI/BamHI fragment derived from plasmid pEQ694 was added to the mixture of fragments. pEQ694 was constructed by inserting an ∼9.6-kb SacI/BamHI fragment derived from Tn45 into pBS+ (Stratagene, Inc.). The uORF2 AUG codon in this plasmid was changed to AAG, thereby eliminating uORF2 (Fig. 1). In addition, eight nucleotides were inserted into the NaeI site in the UL4 transcript leader in pEQ694, creating a BglII site to facilitate the identification of these mutants. The insertion of this BglII linker did not alter uORF2 inhibitory activity in transient-transfection assays (data not shown). Viruses isolated from transfected cells were plaque purified three times prior to subsequent analyses.
FIG. 1.
Construction of recombinant wild-type and mutant CMV. The CMV genome containing unique long (UL) and unique short (US) regions flanked by repeats (ab, b′ a′c′, and ca) is depicted (top) along with the approximate positions of the cosmid fragments used in construction of the recombinant viruses (middle). The ∼9.6 kb SacI/BamHI insert present in pEQ694 (bottom) contains the UL4 gene and flanking regions. Relevant restriction sites are indicated. The pEQ694 sequence corresponding to the 5′ end of the UL4 mRNA contains a BglII linker insertion (bold) into a NaeI site and a mutation of the uORF2 AUG codon to AAG (underlined).
To confirm the genomic structures of the recombinant viruses, we analyzed viral DNA by Southern blot hybridization (Fig. 2). DNA purified from cells infected with CMV(Towne), rTowne-1, vEQ694-1, and vEQ694-2 cells was digested with HindIII alone or with HindIII and BglII, separated electrophoretically, and transferred to nitrocellulose paper. Ethidium bromide staining (Fig. 2A) and hybridization with a 32P-labeled probe corresponding to the whole CMV(Towne) genome (Fig. 2B) revealed similar patterns among all viruses, suggesting that no major deletions or rearrangements occurred during construction of the viruses. As expected, hybridization with a UL4-specific probe revealed the presence of the new BglII site in vEQ694-1 and vEQ694-2 [but not in CMV(Towne) or rTowne-1] ∼800 bp downstream from another BglII site in this region (Fig. 2C and D). We further confirmed the structures of the UL4 transcript leader region in rTowne-1, vEQ694-1, and vEQ694-2 using PCR and sequencing analyses (data not shown).
FIG. 2.
Analyses of wild-type and mutant CMV genomic structures. DNA purified from HF infected with the indicated viruses was digested with HindIII or with HindIII and BglII After electrophoretic separation, the ethidium bromide staining pattern was photographed (A) and samples were analyzed by Southern blot hybridization (B and C) using either a whole-virus probe (B) or a UL4 probe (C). (D) Diagram of the UL4 probe.
These results confirm that the transfection method yielded the expected viruses and that no gross alterations were evident in the recombinant viral genomes. It is possible that mutations elsewhere in the genome that would not be detected by these assays were generated during the transfection process. However, the identity of results (see below) using two viruses (vEQ694-1 and vEQ694-2) obtained from two independent transfections minimizes the possibility that any effects were due to mutations other than those intentionally introduced into the UL4 leader.
Abundant early expression of gpUL4 in uORF2−-CMV-infected cells.
Using the wild-type and recombinant viruses, we evaluated the effects of the mutations on gpUL4 expression. HF were infected with CMV(Towne), rTowne-1, vEQ694-1, or vEQ694-2 at a multiplicity of infection of 3. Cell extracts prepared by sodium dodecyl sulfate lysis every 12 h until 96 h postinfection (p.i.) were analyzed for gpUL4 accumulation by immunoblot assays using gpUL4 polyclonal rabbit serum (1). While little or no gpUL4 was detectable in CMV(Towne)- or rTowne-1-infected cells even at late times, abundant gpUL4 was detectable at 12 h p.i. in vEQ694-1- and vEQ694-2-infected cells (Fig. 3A). The level of accumulated gpUL4 increased by 24 h p.i. and then remained relatively constant in abundance through 96 h p.i. in these cells. The basis for the slight changes in electrophoretic mobility of gpUL4 during infection is unknown, but they may be due to variation in posttranslational modification of this heavily glycosylated protein (8).
FIG. 3.
Kinetics of UL4 gene expression by wild-type and mutant viruses. Extracts of HF were prepared at various times after infection with the indicated viruses. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis, gpUL4 (A) and ppUL44 (C) were detected by immunoblot analysis using rabbit anti-gpUL4 serum or a mouse monoclonal antibody, respectively. (B) RNA samples harvested from HF infected at the same time were analyzed by Northern blot hybridization, using probes specific for UL4 or β-actin. Since all the RNA samples were analyzed on one Northern blot for each probe, the mock-infected-cell lane, shown only in the CMV(Towne) panel, is the control for all panels.
Additional analyses supported the conclusion that the abundant early expression of gpUL4 following infection with vEQ694-1 and vEQ694-2 resulted from derepressed translation. First, Northern blot hybridizations revealed that UL4 mRNA levels in cells infected with vEQ694-1 or vEQ694-2 were no greater than those in cells infected with CMV(Towne) or rTowne-1 (Fig. 3B). Thus, differences in transcript accumulation could not explain differences in gpUL4 expression. To exclude the possibility that there was a generalized reduction in protein synthesis in cells infected with CMV(Towne) or rTowne-1, we measured expression of the viral protein ppUL44 (ICP36). Immunoblot assays using monoclonal antibody 10D8 (Virusys Corporation, North Berwick, Maine) revealed similar levels of ppUL44 protein in extracts from cells infected with each virus (Fig. 3C).
Mutation of uORF2 eliminates ribosomal stalling on the UL4 mRNA.
In previous studies, we utilized the toeprint (or reverse transcription inhibition) assay to detect ribosomal stalling on the UL4 mRNA. This assay is similar to a primer extension reaction but is performed in a crude translation extract such that premature termination occurs when the reverse transcriptase encounters a barrier, such as a ribosome, on the mRNA (11). Previous results demonstrated that cell-free translation of mRNAs containing wild-type but not mutant uORF2 sequences caused ribosomes to stall at the uORF2 termination codon (3, 6). Ribosomal stalling was also detected in CMV(Towne)-infected cells (3).
If the high-level expression of gpUL4 in cells infected with uORF2− viruses is a result of the elimination of ribosomal stalling at the uORF2 termination site, then ribosomes should no longer be detected at this position on the UL4 mRNA. To test this prediction, we performed toeprint assays (3) using cytoplasmic extracts of cells infected with rTowne-1 and vEQ694-1. Briefly, cytoplasmic fractions from mock-infected and CMV-infected cells were prepared by trypsinization of the cells followed by Dounce homogenization and ultracentrifugation (100,000 × g). After the samples had been heated to 55°C for 5 min, a 32P-labeled primer was annealed to the 3′ end of the gpUL4 transcript leader and was extended with reverse transcriptase (Superscript II; Gibco BRL). After phenol-chloroform extraction, the reaction products were separated on 6% denaturing polyacrylamide gels and visualized by autoradiography. A band corresponding to ribosomes stalled at the end of uORF2 was detected in rTowne-1- but not in vEQ694-1-infected cells (Fig. 4). In other experiments, the toeprint band corresponding to the uORF2 termination site was also present in cells infected with CMV(Towne) but not with vEQ694-2 (data not shown). These results reveal that mutation of uORF2 eliminates ribosomal stalling at the uORF2 termination site.
FIG. 4.
Mutation of the uORF2 AUG codon eliminates ribosomal stalling at the uORF2 termination site. Extracts of mock-infected HF or HF infected with rTowne-1 or vEQ694-1 were analyzed by toeprint assay using a primer that anneals to the 3′ end of the UL4 transcript leader as described previously (3).
uORF2− CMV has the same growth kinetics as wild-type virus.
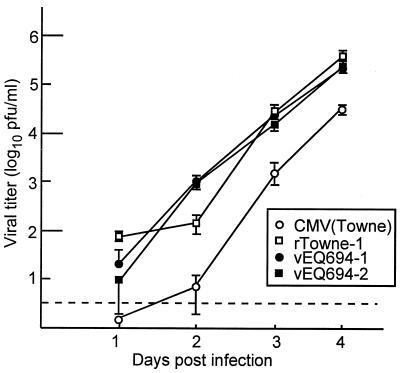
Our ability to isolate mutant viruses that lack uORF2 and overexpress gpUL4 demonstrates that the uORF2 regulatory mechanism is not essential for viral growth in cell culture. To assess the consequences of the uORF2 mutation for viral growth more carefully, we compared viral production after infection of HF with our wild-type and mutant viruses. As shown in Fig. 5, vEQ694-1, vEQ694-2, and rTowne-1 all produced similar titers of extracellular virus while CMV(Towne) produced slightly less virus at each time point. Although we have not investigated the basis for differences in production between CMV(Towne) and the other viruses, these results might reflect unrecognized genetic differences between CMV(Towne) and viruses that were generated from the cosmids even though the cosmids were derived from an isolate of CMV(Towne) (13). Regardless, these results suggest that the uORF2 inhibitory mechanism does not have a discernible impact on CMV replication kinetics in HF.
FIG. 5.
Kinetics of viral replication. After infection of triplicate dishes of HF with the indicated viruses (multiplicity of infection = 3), the cell medium was collected for determination of titers, and fresh medium was added every 24 h. CMV titers (means ± standard deviations) were determined by plaque assay. Values below the dotted line represent titers of less than ∼3 PFU/ml.
Discussion.
These studies demonstrate that the unusual translational mechanism mediated by uORF2, previously characterized using transient transfection, retrovirus-mediated gene transduction, and cell-free translation assays (1, 3–7, 9, 17), also functions to repress expression of the authentic UL4 gene contained in its natural context in the viral genome. Previous studies also identified polymorphisms within uORF2 that affect the inhibitory function of uORF2 in naturally occurring clinical isolates of CMV (1), suggesting that uORF2 is a principal determinant of gpUL4 expression. However, because clinical isolates are genetically heterogeneous, polymorphisms other than those within uORF2 could also be responsible for differences in gpUL4 expression among isolates. Therefore, this study of wild-type and uORF2 mutants in an isogenic background was necessary to establish the role of uORF2 in governing gpUL4 expression during viral infection. Our comparisons of wild-type and mutant viruses reveal that mutation of the uORF2 AUG codon in the viral genome prevents ribosomes from stalling on the gpUL4 transcript leader and results in early and abundant gpUL4 synthesis.
These results do not enable us to conclude whether the inhibitory effect of the wild-type uORF2 is modulated during infection. The gpUL4 detected at low levels at late times in cells infected with CMV(Towne) may result from constitutive low-level translation of the UL4 mRNA, resulting in gradual accumulation of the gpUL4 protein. Alternatively, the inhibitory effect of uORF2 may be circumvented late in infection. If the uORF2 inhibitory mechanism is regulated, the derepressed state apparently does not completely overcome the effect of the uORF since the level of gpUL4 expression in wild-type-infected cells never approaches that seen even at early times in cells infected with a uORF2− mutant virus (Fig. 3). Other uORFs that also act in a nascent-peptide-sequence-dependent manner, such as those present in the mammalian S-adenosylmethionine decarboxylase and Neurospora crassa arg-2 genes, are known to be conditional inhibitors of downstream translation (reviewed in references 10 and 15). For example, ribosomal stalling at the termination site of the uORF in arg-2 occurs only when arginine is abundant (19). Although ribosomal stalling at the uORF2 termination site is detectable at early and late times after infection (3), the stalling event is transient, at least in cell-free translation systems (6). Thus, further studies of duration of ribosomal stalling at various times after infection as well as of the rate of gpUL4 protein synthesis may be useful for determining whether uORF2 acts in a regulated or constitutive manner.
The present studies clearly show that uORF2 and its repressive effects on gpUL4 are not required for viral replication in cell culture. This conclusion is bolstered by the previous identification of low-passage-number clinical isolates of CMV derived from bone marrow transplant recipients that overexpress gpUL4, likely as a consequence of naturally occurring polymorphisms within uORF2 (1). As well, a recent analysis of uORF2 polymorphisms among AIDS patients identified one case in which a uORF2 variant predicted to express a high level of gpUL4 was the only genotype detected in multiple tissue samples, suggesting that this variant was in fact replicating in the patient (2). Together these studies suggest that the uORF2 inhibitory mechanism and the resulting repression of gpUL4 expression are not required for viral growth either in cell culture or in infected patients. The UL4 gene itself is also not essential for CMV growth in cell culture (12, 16, 18, 20). Thus, the role of gpUL4 and of the uORF2 inhibitory mechanism remain enigmatic.
The fact that gpUL4 is a unique protein, homologues of which have not been detected even in other members of the Betaherpesvirinae subfamily, limits our ability to determine why its expression is repressed in most isolates or what cofactor(s) might regulate its expression. One possibility is that inhibition of expression of gpUL4 confers a selective advantage on the virus at some site of infection or in some patients. For example, if gpUL4 is a target of the immune response (8), then limiting its expression may aid the virus in evading the immune system, especially in immunocompetent hosts. However, this speculation does not explain why such an unusual peptide-mediated ribosomal stalling mechanism has evolved to down-regulate gpUL4 expression in most viral isolates. A better understanding of the mechanism and regulation of translation termination may aid in elucidating the significance of the uORF2 regulatory strategy.
Acknowledgments
We thank George Kemble (Aviron) for providing the CMV(Towne) cosmids and Jianhong Cao and Sohail Jarrahian (Fred Hutchinson Cancer Research Center) for technical assistance and advice. We also thank the Biotechnology, Biocomputing and Image Analysis Resources of the Fred Hutchinson Cancer Research Center for technical assistance.
This work was supported by Public Health Service grant AI-26672 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Alderete J P, Jarrahian S, Geballe A P. Translational effects of mutations and polymorphisms in a repressive upstream open reading frame of the human cytomegalovirus UL4 gene. J Virol. 1999;73:8330–8337. doi: 10.1128/jvi.73.10.8330-8337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar M, Shannon-Lowe C, Geballe A P. Differentiation of human cytomegalovirus genotypes in immunocompromised patients on the basis of UL4 gene polymorphisms. J Infect Dis. 2001;183:218–225. doi: 10.1086/317939. [DOI] [PubMed] [Google Scholar]
- 3.Cao J, Geballe A P. Coding sequence-dependent ribosomal arrest at termination of translation. Mol Cell Biol. 1996;16:603–608. doi: 10.1128/mcb.16.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao J, Geballe A P. Inhibition of nascent-peptide release at translation termination. Mol Cell Biol. 1996;16:7109–7114. doi: 10.1128/mcb.16.12.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao J, Geballe A P. Mutational analysis of the translational signal in the human cytomegalovirus gpUL4 (gp48) transcript leader by retroviral infection. Virology. 1994;205:151–160. doi: 10.1006/viro.1994.1630. [DOI] [PubMed] [Google Scholar]
- 6.Cao J, Geballe A P. Ribosomal release without peptidyl tRNA hydrolysis at translation termination in a eukaryotic system. RNA. 1998;4:181–188. [PMC free article] [PubMed] [Google Scholar]
- 7.Cao J, Geballe A P. Translational inhibition by a human cytomegalovirus upstream open reading frame despite inefficient utilization of its AUG codon. J Virol. 1995;69:1030–1036. doi: 10.1128/jvi.69.2.1030-1036.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang C P, Vesole D H, Nelson J, Oldstone M B, Stinski M F. Identification and expression of a human cytomegalovirus early glycoprotein. J Virol. 1989;63:3330–3337. doi: 10.1128/jvi.63.8.3330-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degnin C R, Schleiss M R, Cao J, Geballe A P. Translational inhibition mediated by a short upstream open reading frame in the human cytomegalovirus gpUL4 (gp48) transcript. J Virol. 1993;67:5514–5521. doi: 10.1128/jvi.67.9.5514-5521.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geballe A P, Sachs M S. Translational control by upstream open reading frames. In: Hershey W B, Matthews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2000. pp. 595–614. [Google Scholar]
- 11.Hartz D, McPheeters D S, Traut R, Gold L. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- 12.Hobom U, Brune W, Messerle M, Hahn G, Koszinowski U H. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J Virol. 2000;74:7720–7729. doi: 10.1128/jvi.74.17.7720-7729.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemble G, Duke G, Winter R, Spaete R. Defined large-scale alterations of the human cytomegalovirus genome constructed by cotransfection of overlapping cosmids. J Virol. 1996;70:2044–2048. doi: 10.1128/jvi.70.3.2044-2048.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mocarski E S. Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2447–2492. [Google Scholar]
- 15.Morris D R, Geballe A P. Upstream open reading frames as regulators of mRNA translation. Mol Cell Biol. 2000;20:8635–8642. doi: 10.1128/mcb.20.23.8635-8642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ripalti A, Mocarski E S. The products of human cytomegalovirus genes UL1-UL7, including gp48, are dispensable for growth in cell culture. In: Landini M P, editor. Progress in cytomegalovirus research. New York, N.Y: Elsevier Science Publishers; 1991. pp. 57–62. [Google Scholar]
- 17.Schleiss M R, Degnin C R, Geballe A P. Translational control of human cytomegalovirus gp48 expression. J Virol. 1991;65:6782–6789. doi: 10.1128/jvi.65.12.6782-6789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takekoshi M, Maeda-Takekoshi F, Ihara S, Sakuma S, Watanabe Y. Site-specific stable insertion into the human cytomegalovirus genome of a foreign gene under control of the SV40 promoter. Gene. 1991;101:209–213. doi: 10.1016/0378-1119(91)90413-6. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Sachs M S. Ribosome stalling is responsible for arginine-specific translational attenuation in Neurospora crassa. Mol Cell Biol. 1997;17:4904–4913. doi: 10.1128/mcb.17.9.4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff D, Jahn G, Plachter B. Generation and effective enrichment of selectable human cytomegalovirus mutants using site-directed insertion of the neo gene. Gene. 1993;130:167–173. doi: 10.1016/0378-1119(93)90416-z. [DOI] [PubMed] [Google Scholar]