Abstract
Objectives: This study aims to investigate the efficacy of thoracic endovascular aortic repair (TEVAR) for type B aortic dissection (TBAD) complicated by malperfusion.
Methods: This retrospective study included patients who underwent TEVAR for the treatment of TBAD complicated by malperfusion from June 1998 to June 2022 in four institutions. In addition to the common outcomes, including short- and medium-term mortality and morbidity, the preservation of each organ was investigated.
Results: A total of 23 patients were included in this analysis. The 30-day mortality was 4% (1/23) of the patients. The overall survival rate was 87% at 1 year. The preservation rate of each organ was 33% (4/12) for the visceral organs, 85% (17/20) for the kidneys, and 100% (18/18) for the legs. Fisher’s exact test showed a significant difference in the preservation rate between the viscera and the other organs (P = 0.018 vs. kidneys, P = 0.0025 vs. legs). It was shown that the survival rate of patients with visceral malperfusion was significantly lower than that of patients with non-visceral malperfusion (P = 0.006).
Conclusion: In terms of mortality, TEVAR showed satisfactory results. The preservation of visceral organs was still challenging even with TEVAR and adjunctive measures.
Keywords: type B aortic dissection, malperfusion, endovascular treatment, TEVAR
Introduction
Complicated type B aortic dissection (TBAD) is one of the catastrophic events that affect the descending aorta.1,2) Since Dake et al. reported their first study, thoracic endovascular aortic repair (TEVAR) has been established as the first-line treatment for complicated TBAD because of its superior outcome in the acute phase compared with open surgery in terms of operative mortality.3,4) Although many studies have supported the efficacy of TEVAR, most of them dealt with various types of complications altogether, including malperfusion, aortic rupture, intractable pain, and refractory hypertension.5–9) However, malperfusion is different from the other complications in terms of subsequent outcomes. Moreover, the mortality rate of patients with visceral malperfusion has been reported to be higher than that of patients without visceral malperfusion, and visceral malperfusion is considered a notably life-threatening condition among complicated TBAD patients.10) Therefore, it is important to discuss malperfusion separately and compare organ-specific preservation to provide a better clinical understanding. In this study, we investigated the efficacy of TEVAR in treating acute TBAD complicated by malperfusion. In addition to the common outcomes, we focused on the preservation of each organ compromised by aortic dissection.
Materials and Methods
Patient population
We retrospectively investigated patients with TBAD complicated by malperfusion who were treated at 4 institutions. Between June 1998 and June 2022, a total of 23 patients (20 men and 3 women) were included in the analysis. Malperfusion was diagnosed when the following symptoms, signs, and/or laboratory test data were observed: (1) visceral (abdominal pain, CT findings, and laboratory data), (2) renal (oliguria/anuria, CT findings, and elevated serum creatinine level), and (3) leg (leg pain and/or cyanosis and weakness or loss of leg pulsation).
Thirty-seven patients with TBAD complicated by malperfusion were identified between June 1998 and June 2022. Among them, 23 patients underwent TEVAR. Other treatment options were adopted according to the surgeon’s preference, anatomical limitations to TEVAR, and the patient’s general condition. Bypass surgery of the ischemic vessels was performed in 8 patients, bare-metal stent placement in ischemic branches in two, surgical aortic replacement in one, and conservative medical treatment in three.
This study was approved by the Clinical Research Ethics Review Committee of Mie University Hospital (No. 1717). Written informed consent was obtained from patients who were alive and followed up. Other patients and/or their families were given a chance to opt out by posting the explanation of the study on our institutional websites.
Preoperative CT evaluation
Contrast-enhanced CT was used to specify the obstructive patterns of the aortic branches in accordance with William’s definition, that is, dynamic obstruction in which the aortic true lumen is compressed by the false lumen, and static obstruction in which the aortic branches are stenosed by the dissection process propagating into them without reentry formation.11)
The diameters of the true and the false lumens of the descending thoracic aorta were measured at the levels of T4, T8, T12, and L3 before and after TEVAR to evaluate the remodeling of the aorta. They were measured along the perpendicular line to the intimal flap of the dissected aorta on axial CT images.
Endovascular procedures
TEVAR was performed using hand-made devices until 2008, which were fabricated with Z-stents and expanded polytetrafluoroethylene (Impra; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) or woven polyester (UBE, Japan Lifeline, Tokyo, Japan). Since 2009, manufacturer-made devices have been used. The treatment strategy was only to close the primary entry until 2008 because the length of the available devices was relatively short. Since 2009, it turned into both closing the primary entry tear and avoiding stent graft-induced intimal tears using longer devices as both ends of the device were positioned at the relatively straight site of the aorta. Composite bare aortic stents below the stent grafts were not used in any case. When sufficient blood flow into the aortic branches was not observed even after entry closure by TEVAR because of static obstruction, the bare-metal stent was added into the branch vessels. When sufficient blood flow was confirmed in the superior mesenteric artery after TEVAR with or without bare-metal stent placement, no intervention was added to the celiac artery even if it was occluded because the collateral flow from the superior mesenteric artery was expected to supply blood flow to the organs fed by the celiac artery. We also did not add intervention to the renal artery when the kidney supplied by it was judged as infarcted on the delayed phase of contrast-enhanced CT, and the contralateral kidney was judged as viable. The procedural endpoint was good, and rapid blood flow was observed in both the aortic true lumen and branches upon completion of angiography. We allowed minor type I endoleak on completion of angiography because the aim of TEVAR was not to obtain complete thrombosis of the false lumen but to relieve malperfusion.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median and interquartile range (IQR). The preservation rates of each organ and static obstruction rates of each compromised vessel were compared using Fisher’s exact test and corrected with the Bonferroni method. The diameters of the true and the false lumens before and after TEVAR were compared using Student’s t-test. Univariate logistic analysis was used to predict organ preservation. The survival rates were depicted by Kaplan–Meier analysis and were compared using the log-rank test. A P value of <0.05 was considered to be statistically significant. All data were analyzed using the R software package, version 3.6.1 (The R foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics and clinical presentation
The mean age was 62 ± 10, and none of the patients had connective tissue disease (Supplemental Table 1). Sixteen patients (70%) underwent TEVAR during the acute phase, while the other 7 patients (30%) underwent TEVAR during the subacute phase (Table 1). The average interval between the onset of aortic dissection and TEVAR was 6 days (IQR, 1–19). The interval was 0 days (IQR, 0–1) for visceral malperfusion, 12 days (IQR, 2–19) for renal, and 8 days (IQR, 0–22) for leg malperfusion, respectively.
Table 1 Dissection and treatment characteristics.
| Characteristics | n (%) |
|---|---|
| Chronicity of aortic dissection | |
| Acute | 16 (70%) |
| Subacute | 7 (30%) |
| Proximal extent of dissection | |
| Z2 | 4 (17%) |
| Z3 or below | 19 (83%) |
| Distal extent of dissection | |
| Infrarenal aorta | 6 (26%) |
| Iliac artery or below | 17 (74%) |
| Status of false lumen | |
| Patent | 15 (65%) |
| Partially thrombosed | 8 (35%) |
| Completely thrombosed | 0 (0%) |
| Device | |
| Handmade | 8 (35%) |
| Manufactured | 15 (65%) |
| Treatment length (median), cm | 15.0 (IQR: 10.0–19.0) |
IQR: interquartile range
Visceral, renal, and leg malperfusion were observed in 10 (10/23, 43%), 10 (10/23, 43%), and 13 (13/23, 57%) patients, respectively. Malperfusion was limited to one organ in 13 patients (13/23, 57%), while 2 or 3 organs were compromised in the other 10 patients (10/23, 43%). Visceral malperfusion was limited to the bowel in 7 patients and the pancreas and hepatobiliary system in one patient. In 2 patients, visceral malperfusion was observed in the bowel and hepatobiliary system. Therefore, malperfusion was observed in 12 viscera. As for renal malperfusion, 20 kidneys of 10 patients were judged as compromised. The preoperative serum creatinine value was 2.0 mg/dL (IQR: 1.4–4.1) in these patients. As for leg malperfusion, 5 patients had leg ischemia in both legs and 8 patients in one leg. Therefore, 18 legs were judged as compromised.
Procedural outcomes
In 8 patients, hand-made devices were used for TEVAR, whereas manufacturer-made devices were used in the other 15 patients (TAG or CTAG [W.L. Gore, Flagstaff, AZ, USA] in 12 patients, TX2 or TXD [Cook, Inc., Bloomington, IN, USA] in 3 patients. The median treatment length was 15 cm (IQR: 15–19). Composite bare aortic stents were not used in any patient. Bare-metal stents were placed in 7 branch vessels of 6 patients because of insufficient flow even after entry closure by the stent graft (the celiac artery, 1; the superior mesenteric artery, 5; and the external iliac artery, 1). Although a minor type Ia endoleak was observed in 2 patients, no additional procedures were performed because the recovery of the blood flow to the compromised organs was confirmed on completion of angiography.
Early outcomes
Thirty-day mortality was 4% (1/23) (Table 2). One patient with bowel ischemia underwent TEVAR and stenting to the superior mesenteric artery following arterial infusion of urokinase and showed relatively good blood flow in both the aortic true lumen and the superior mesenteric artery on completion of angiography. However, he underwent bowel resection because of bowel necrosis and died of multiple organ failure the next day. Thirty-day major adverse events were observed in 11 patients (48%). Bowel necrosis was observed in 5 patients, including the above-mentioned patient, and bowel resection was performed in all these patients. Hepatobiliary dysfunction was detected in 3 patients (ischemic cholecystitis, two; hepatic infarction, one), and 2 of them underwent cholecystectomy. Although 2 patients developed renal failure, they could avoid maintenance dialysis by temporary continuous hemodiafiltration. Stenting and balloon dilatation to the renal artery were added in 2 patients in whom renal artery stenosis remained after TEVAR. A patient with ischemic pancreatitis developed an intraperitoneal abscess and gallbladder perforation. He finally survived after cholecystectomy, gastrojejunostomy, and ileostomy.
Table 2 Early outcomes.
| Death within 30 days | 4% (1/23) |
| Cause of death | |
| Bowel necrosis | 1 |
| Major adverse events within 30 days | 48% (11/23) |
| Bowel necrosis | 5 |
| Ischemia cholecystitis | 2 |
| Hepatic infarction | 1 |
| Pancreatitis | 2 |
| Renal failure | 2 |
| Residual renal artery stenosis | 2 |
| Paraparesis | 2 |
| Hepatic infarction | 1 |
Favorable remodeling with increased true lumen diameter and decreased false lumen diameter was observed on post-TEVAR CT at any level of the descending thoracic aorta and the abdominal aorta (Supplemental Table 2).
Organ preservation and the obstructive pattern of its supplying artery
Organ preservation was obtained in 4 of 12 viscera (33%), 17 of 20 kidneys (85%), and 18 of 18 legs (100%). Fisher’s exact test, corrected with the Bonferroni method, showed a significant difference in the organ preservation rate between the viscera and legs and the viscera and kidneys (P = 0.0025 and P = 0.018, respectively). Open surgical intervention, including resection, was required in 7 viscera, which were judged as not preserved. Three kidneys developed infarction, and the serum creatinine level rose in 2 of 3 patients who had these kidneys, although hemodialysis was avoided. These kidneys were judged as infarcted on the diagnostic CT and no intervention, including bare stent placement, was added. Among the other 8 patients, postoperative renal function could be evaluated in 7 patients, and their serum creatinine levels improved from 3.8 (IQR: 1.9–4.6) to 1.2 (IQR: 0.9–1.8) (P = 0.11).
Table 3 shows the obstructive patterns of the arteries supplying compromised organs. Static obstruction, including the coexistence of both dynamic and static obstruction, was 83% (10/12) of the visceral arteries, 35% (7/20) of the renal arteries, and 50% (9/18) of the leg arteries. Static obstruction was significantly more frequent in the visceral arteries than in renal arteries (P = 0.035). Although dynamic obstruction was corrected after intervention in all patients, static obstruction remained in the 3 renal arteries, to which stenting was not added. As mentioned above, preservation was not obtained in 11 organs (8 viscera and 3 kidneys) in total. Static obstruction was related to all these organs. Although static obstruction was relieved in 8 arteries of these 11 organs following the intervention, organ preservation was not obtained.
Table 3 Obstructive patterns of compromised branch vessels and their outcomes.
| Obstructive patterns | Viscera (n) | Kidneys (n) | Legs (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Non-preserved | Pre | Post | Non-preserved | Pre | Post | Non-preserved | ||
| D | 2 | 0 | 0 | 13 | 0 | 0 | 9 | 0 | 0 | |
| DS | 4 | 0 | 4 | 7 | 0 | 0 | 8 | 0 | 0 | |
| S | 6 | 0 | 4 | 0 | 3* | 3 | 1 | 0 | 0 | |
| Total | 12 | 0 | 8 | 20 | 3 | 3 | 18 | 0 | 0 | |
*Among 7 patients with DS obstruction became S obstruction after TEVAR.
D: dynamic obstruction; DS: dynamic and static obstruction; S: static obstruction; TEVAR: thoracic endovascular aortic repair
Late outcomes
The median follow-up period was 25 months (IQR: 4–97). Five late deaths were recorded (Supplemental Table 3). Aorta-related mortality was 4.3% at 1 year (Supplemental Fig. 1). Two deaths among them were related to surgical aortic replacement: for the treatment of thoracoabdominal aortic aneurysm in one and both proximal and distal stent graft-induced new entry (SINE) in the other, respectively. One-year and 3-year survival rates were 87% and 83%, respectively (Supplemental Fig. 2). Late aorta-related morbidities developed in 9 patients (41%, 9/22) (Supplemental Table 3).
Late aorta-related event-free survival was 83% at 1 year (Supplemental Fig. 3). Except for type A aortic dissection and graft infection, invasive interventions were added to all morbidities. Of the 2 patients with type Ia endoleak, one was treated with TEVAR and the other with open surgery, respectively. Both 2 patients with abdominal aortic/iliac aneurysms were treated with endovascular aortic repair (EVAR), while one patient with thoracoabdominal aortic aneurysm was treated with open surgery. One patient with aortic rupture was treated with TEVAR. One patient with both proximal and distal SINEs was treated by open surgery. One patient with aortic valve stenosis was treated by open surgery. One patient with arterial occlusion of the lower limb was treated with thrombectomy and femoral-femoral bypass. Two patients with renal arterial stenosis were treated with percutaneous balloon angioplasty and stenting, respectively.
Predictive factors for failure of organ preservation
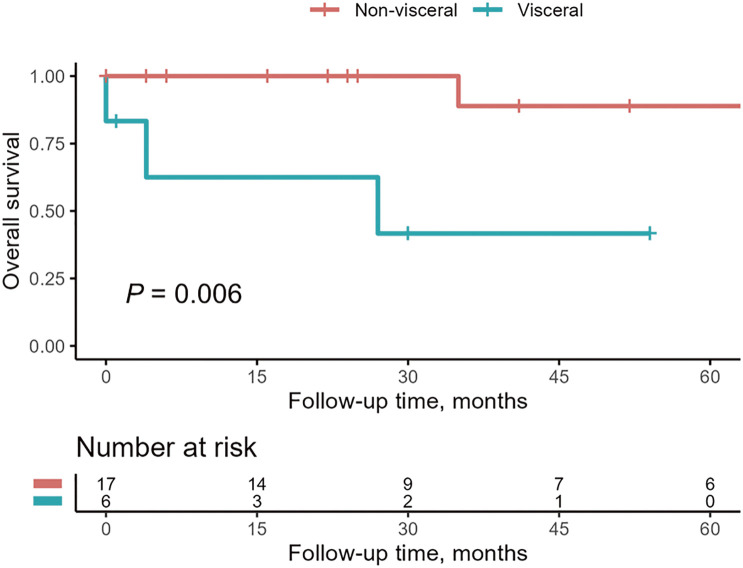
To investigate the predictive factors for organ preservation failure, variables were compared between organs without preservation and those with preservation using univariate logistic analysis (Table 4). Static obstruction was analyzed using the adjusted odds ratio (OR). Univariate analysis showed significant differences with visceral malperfusion (OR, 8.3; 95% confidence interval, 1.3–75.7; P = 0.04). The log-rank test showed a significant difference in survival rate between patients with visceral malperfusion and those without visceral malperfusion (P = 0.006) (Fig. 1).
Table 4 Univariate analysis for failure of organ preservation.
| Variables | Univariate analysis | |
|---|---|---|
| OR (95% CI) | P value | |
| Age >70 | 2.4 (0.34–17.5) | 0.37 |
| Male sex | 4.7 (0.22–104.5) | 0.32 |
| Hypertension | 0.10 (0.004–0.97) | 0.07 |
| Diabetes mellitus | 1.8 (0.06–48.2) | 0.74 |
| Hyperlipidemia | 0.25 (0.01–2.0) | 0.25 |
| Smoker | 0.40 (0.05–3.0) | 0.36 |
| COPD | 1.5 (0.22–9.8) | 0.67 |
| CAD | 2.3 (0.08–66.0) | 0.57 |
| Stroke | 2.3 (0.08–66.0) | 0.57 |
| CKD | 6.7 (0.31–140.9) | 0.22 |
| Viscera malperfusion | 8.3 (1.3–75.7) | 0.04 |
| Static obstruction | 15.0 (0.74–306.3) | 0.08 |
| Dynamic obstruction | 0.46 (0.04–4.6) | 0.49 |
| Acute dissection | 1.5 (0.23–12.9) | 0.68 |
COPD: chronic obstructive pulmonary disease; CAD: coronary artery disease; CKD: chronic kidney disease; OR: odds ratio
Fig. 1 Kaplan–Meier survival analysis of patients with visceral and non-visceral malperfusion. The 95% confidential intervals are not shown because of the standard error >10% at each time point.
Discussion
In-hospital mortality of patients with malperfusion, which was more than 50% decades ago, has reached less than 20% in the current TEVAR era.1,2,7,12,13) Indeed, this study showed an in-hospital mortality of only 4% and an overall survival rate of 87% at 1 year and 83% at 3 years. Similarly, the latest report describing the outcomes of patients with complicated TBAD who underwent TEVAR shows less than 8% overall mortality and 94% and 89% overall survival rates at 1 and 3 years, respectively.14) The application of TEVAR to aortic dissection in the past few years has provided a better understanding of its pathology, which has led to excellent clinical outcomes.
Among various organ compromises, visceral malperfusion has been considered the most life-threatening compared with renal or leg malperfusion.10,15) However, several studies concluded that overall mortality does not differ between patients with visceral malperfusion and those with non-visceral malperfusion.13,14) Since only one in-hospital death was observed in this study, no definitive conclusions can be derived in terms of short-term mortality stratified by the ischemic territory. However, a significant difference was observed between the survival rate of patients with visceral and non-visceral malperfusion in terms of the mid-term period.
Numerous studies have reported the mortality and morbidity following certain interventions, including endovascular procedures. However, no study has demonstrated the fate of each compromised organ, that is, whether the organs were preserved or not. This fact allowed us to investigate the preservation rate of each organ. In this series, the preservation rate of the viscera (33%, 4/12) was significantly lower than that of the non-visceral organs (kidneys, 85% [17/20]; legs, 100% [18/18]). Open surgical intervention, including bowel resection, was required in 8 viscera without preservation because of infarction. In addition, 3 kidneys became nonfunctioning because of infarction although permanent hemodialysis could be avoided in all these patients. This difference could be explained in several ways. One is the tolerability of each organ to ischemia. Generally, the legs can tolerate around 6 hours of warm ischemic time, while the viscera and kidneys can tolerate less than 2 hours.16–19) The other explanation is the different trend of the obstructive pattern for each branch vessel.
The concept of 2 branch vessel obstructive patterns, that is, dynamic and static obstruction, in aortic dissection has been widely recognized since Williams et al. advocated them in the late 1990s, and a thorough understanding of this concept has been essential for the treatment of TBAD complicated by malperfusion.11) However, a few studies have reported these patterns and their relationship with the clinical outcomes. Sobocinski et al.12) presented the obstructive mechanism involved in malperfusion in their study and claimed that it is unsystematically associated with clinical malperfusion syndrome. However, it is well known that TEVAR can be more effective in dynamic obstruction and potentially resolve it completely, as proven by Chung et al. two decades ago.20,21) The so-called PETTICOAT concept (composite TEVAR with the use of bare stents in the aortic true lumen) has been widespread for the treatment of complicated TBAD. Lombardi et al.22) reported that bowel ischemia was observed only in 1.4% and renal failure in 6.8% among 73 patients, including 57 patients with malperfusion. Although all patients in our study had a resolution of dynamic obstruction through entry closure by TEVAR, the adjunctive use of composite bare stents may promote aortic remodeling and contribute to better outcomes.
On the other hand, the preservation of organs compromised by static obstruction was not so straightforward. On the contrary to Sobocinski’s study, static obstruction was significantly more frequent in the visceral arteries (83%) than in the renal arteries (35%) (P = 0.035). In addition, only 2 of 10 viscera with static obstruction were preserved after TEVAR, while 4 of 7 kidneys and 9 of 9 legs with static obstruction were preserved, with 3 unpreserved kidneys having been abandoned beforehand. Considering these results, it should be crucial to emphasize static obstruction and visceral malperfusion to improve the outcomes in terms of organ preservation. Stenting is essential to the vessels with static obstruction because entry closure by TEVAR alone often leaves static obstruction uncorrected. Indeed, several studies have shown that stenting following entry closure by TEVAR is required to compromise vessels with static obstruction in more than 40% of patients.12,23) In our series, 6 patients (26%) underwent stenting to 7 vessels (celiac artery, one; superior mesenteric artery, five; and iliac artery, one) with static obstruction. However, only 2 organs supplied by stented vessels (superior mesenteric artery, one; iliac artery, one) were preserved, while the preservation of the other 5 organs was not obtained. As mentioned above, one possible explanation is that visceral organs are tolerable for a shorter warm ischemic time. Residual stenosis in the vascular bed of the stented vessel may be another explanation. It is not rare that stenosis in the branches of the stented vessels remains because the thrombosed false lumen in these branches does not quickly shrink. However, the stenosis of the proximal portion of the stented vessel certainly disappears. Thus, prolonged ischemia in the peripheral vascular bed may lead to final organ dysfunction.
Fortunately, this limitation may have several possible solutions. One of the most important solutions is prompt diagnosis and treatment. Norton et al.24) claimed that emergent endovascular evaluation, including angiography, which potentially identifies unsuspected vascular beds with malperfusion, should be preferable in patients with TBAD. They also claimed that favorable short- and long-term outcomes were achieved with upfront endovascular fenestration/stenting prior to open proximal aortic repair in type A aortic dissection and malperfusion syndrome.25)
The present study has several limitations. First, this study is a retrospective analysis. Therefore, available important data are considerably limited. Second, the number of cases was so small that it was difficult to reveal the risk factors for the failure of organ preservation with robust statistical power. It may be difficult to generalize the difficulty in visceral organ relief from the present results, but it was consistent with the previous reports. Third, due to the long period of the study, devices have changed significantly; that is, hand-made devices were used in the early period and manufacturer-made devices in the later period.
Conclusion
Our study showed that TEVAR can achieve satisfactory results in terms of mortality. However, the preservation of visceral organs was still challenging even with TEVAR and adjunctive measures. More efforts such as prompt diagnosis or strategy change should be mandatory to improve clinical outcomes.
Author Contributions
Study conception: NK
Data collection: NK, TT, TM, and KO
Manuscript preparation: HK and NK
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
Disclosure Statement
The authors have no conflict of interest.
Supplementary Materials
Patients’ characteristics
True and false lumen diameters before and after TEVAR
Late outcomes
Kaplan-Meier analysis of aorta-related death-free survival. The 95% confidential intervals are not shown because of the standard error > 10% at each time point.
Kaplan-Meier analysis of overall survival. The 95% confidential intervals are not shown because of the standard error > 10% at each time point.
Kaplan-Meier analysis of event-free survival. The 95% confidential intervals are not shown because of the standard error > 10% at each time point.
References
- 1).Cambria RP, Brewster DC, Gertler J, et al. Vascular complications associated with spontaneous aortic dissection. J Vasc Surg 1988; 7: 199–209. [PubMed] [Google Scholar]
- 2).Fann JI, Sarris GE, Mitchell RS, et al. Treatment of patients with aortic dissection presenting with peripheral vascular complications. Ann Surg 1990; 212: 705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Dake MD, Kato N, Mitchell RS, et al. Endovascular stent graft placement for the treatment of acute aortic dissection. N Engl J Med 1999; 340: 1546–52. [DOI] [PubMed] [Google Scholar]
- 4).Riambau V, Böckler D, Brunkwall J, et al. Editor’s Choice - Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2017; 53: 4–52. [DOI] [PubMed] [Google Scholar]
- 5).Wiedemann D, Ehrlich M, Amabile P, et al. Emergency endovascular stent grafting in acute complicated type B dissection. J Vasc Surg 2014; 60: 1204–8. [DOI] [PubMed] [Google Scholar]
- 6).Hanna JM, Andersen ND, Ganapathi AM, et al. Five-year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg 2014; 59: 96–106. [DOI] [PubMed] [Google Scholar]
- 7).Cambria RP, Conrad MF, Matsumoto AH, et al. Multicenter clinical trial of the conformable stent graft for the treatment of acute, complicated type B dissection. J Vasc Surg 2015; 62: 271–8. [DOI] [PubMed] [Google Scholar]
- 8).Stelzmueller ME, Nolz R, Mahr S, et al. Thoracic endovascular repair for acute complicated type B aortic dissections. J Vasc Surg 2019; 69: 318–26. [DOI] [PubMed] [Google Scholar]
- 9).Bavaria JE, Brinkman WT, Hughes GC, et al. Five-year outcomes of endovascular repair of complicated acute type B aortic dissections. J Thorac Cardiovasc Surg 2022; 163: 539–548.e2. [DOI] [PubMed] [Google Scholar]
- 10).Jonker FHW, Patel HJ, Upchurch GR, et al. Acute type B aortic dissection complicated by visceral ischemia. J Thorac Cardiovasc Surg 2015; 149: 1081–1086.e1. [DOI] [PubMed] [Google Scholar]
- 11).Williams DM, Lee DY, Hamilton BH, et al. The Dissected Aorta: part III. Anatomy and radiologic diagnosis of branch vessel compromise. Radiology 1997; 203: 37–44. [DOI] [PubMed] [Google Scholar]
- 12).Sobocinski J, Delloye M, Hongku K, et al. Malperfusions in acute type B aortic dissection—predictors of outcomes. Ann Vasc Surg 2019; 59: 119–26. [DOI] [PubMed] [Google Scholar]
- 13).Ryan C, Vargas L, Mastracci T, et al. Progress in management of malperfusion syndrome from type B dissections. J Vasc Surg 2013; 57: 1283–90; discussion, 1290. [DOI] [PubMed] [Google Scholar]
- 14).Iwakoshi S, Irie Y, Katada Y, et al. Comparison of outcomes and complications among patients with different indications of acute/subacute complicated stanford type B aortic dissection treated by TEVAR: Data from the JaPanese REtrospective multicenter stuDy of ThoracIc Endovascular Aortic Repair for Complicated Type B Aortic Dissection (J-Predictive Study). Cardiovasc Intervent Radiol 2022; 45: 290–7. [DOI] [PubMed] [Google Scholar]
- 15).Tolenaar JL, Froehlich W, Jonker FHW, et al. Predicting in-hospital mortality in acute type B aortic dissection evidence from international registry of acute aortic dissection. Circulation 2014; 130 Suppl 1: S45–50. [DOI] [PubMed] [Google Scholar]
- 16).Pederson WC. Replantation. Plast Reconstr Surg 2001; 107: 823–41. [DOI] [PubMed] [Google Scholar]
- 17).Kalisvaart M, Croome KP, Hernandez-Alejandro R, et al. Donor warm ischemia time in DCD liver transplantation-working group report from the ILTS DCD, liver preservation, and machine prefusion consensus conference. Transplantation 2021; 105: 1156–64. [DOI] [PubMed] [Google Scholar]
- 18).Schulak JA, Franklin WA, Stuart FP, et al. Effect of warm ischemia on segmental pancreas transplantation in the rat. Transplantation 1983; 35: 7–11. [DOI] [PubMed] [Google Scholar]
- 19).Thompson RH, Blute ML. At what point does warm ischemia cause permanent renal damage during partial nephrectomy? Eur Urol 2007; 52: 961–3. [DOI] [PubMed] [Google Scholar]
- 20).Chung JW, Elkins C, Sakai T, et al. True-lumen collapse in aortic dissection: part I. Evaluation of causative factors in phantoms with pulsatile flow. Radiology 2000; 214: 87–98. [DOI] [PubMed] [Google Scholar]
- 21).Chung JW, Elkins C, Sakai T, et al. True-lumen collapse in aortic dissection: part II. Evaluation of treatment methods in phantoms with pulsatile flow. Radiology 2000; 214: 99–106. [DOI] [PubMed] [Google Scholar]
- 22).Lombardi JV, Gleason TG, Panneton JM, et al. Five-year results of the STABLE II study for the endovascular treatment of complicated, acute type B aortic dissection with a composite device design. J Vasc Surg 2022; 76: 1189–97.e3. [DOI] [PubMed] [Google Scholar]
- 23).Eleshra A, Kölbel T, Panuccio G, et al. Endovascular therapy for nonischemic vs ischemic complicated acute type B aortic dissection. J Endovasc Ther 2020; 27: 145–52. [DOI] [PubMed] [Google Scholar]
- 24).Norton EL, Williams DM, Kim KM, et al. Management of acute type B aortic dissection with malperfusion via endovascular fenestration/stenting. J Thorac Cardiovasc Surg 2020; 160: 1151–1161.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Yang B, Norton EL, Rosati M, et al. Managing patients with acute type A aortic dissection and mesenteric malperfusion syndrome: A 20-year experience. J Thorac Cardiovasc Surg 2019; 158: 675–687.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patients’ characteristics
True and false lumen diameters before and after TEVAR
Late outcomes
Kaplan-Meier analysis of aorta-related death-free survival. The 95% confidential intervals are not shown because of the standard error > 10% at each time point.
Kaplan-Meier analysis of overall survival. The 95% confidential intervals are not shown because of the standard error > 10% at each time point.
Kaplan-Meier analysis of event-free survival. The 95% confidential intervals are not shown because of the standard error > 10% at each time point.