Abstract
Spinal cord infarction (SCI) is a rare but serious complication of endovascular aortic repair (EVAR) for abdominal aortic aneurysms (AAA). It is difficult to predict, prevent, and treat and can cause significant impairment. We describe the case of a patient who experienced paraplegia and thermal pain dysfunction of the lower extremities shortly after EVAR for an infrarenal AAA. Immediately after confirming SCI, we initiated cerebrospinal fluid drainage, administered steroids, naloxone, and free radical scavengers, and maintained high blood pressure. However, the patient’s symptoms did not improve sufficiently. Since the possibility of a SCI exists, prompt treatment should be initiated.
Keywords: spinal cord infarction, infrarenal abdominal aortic aneurysm, endovascular aortic repair
Introduction
Spinal cord infarction (SCI) is a rare but serious complication of endovascular aortic repair (EVAR) for infrarenal abdominal aortic aneurysms (AAA). From 1994 to 2000, the incidence of SCI was reported as 0.21% in the Eurostar Registry1) and the Japanese Committee for Stent Graft Management reported it as 0.3% from 2006 to 2015.2) SCI after EVAR is difficult to predict and prevent, and the patient’s quality of life and activities of daily living are significantly impaired due to lower extremity paralysis and bladder and bowel dysfunction. Herein, we report a case of SCI immediately after elective EVAR.
Case Report
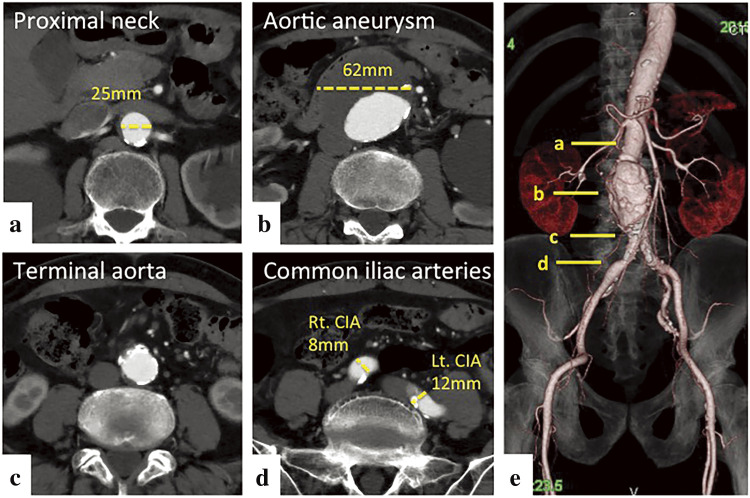
The patient was an 81-year-old male who had a history of emphysema and pneumothorax. An infrarenal AAA with a maximum diameter of 62 mm was identified at another hospital while admitted for intestinal paralysis. The patient was referred to our hospital for AAA treatment. The diameter of the AAA was 62 mm × 59 mm, the diameter of the proximal neck was 25 mm, and the length of the proximal landing zone was 19 mm. The diameter of the right common iliac artery (CIA) was 8 mm, and the length of the peripheral landing zone was 39 mm. The diameter of the left CIA was 12 mm, and the peripheral landing zone was 44 mm (Fig. 1). The AAA was anatomically suitable for an EVAR device, and we planned EVAR and coil embolization of the inferior mesenteric artery (IMA).
Fig. 1 Preoperative enhanced CT images. Axial images are shown in a–d. The solid lines in e indicate the corresponding positions in a–d. The proximal neck diameter of AAA is 25 mm (a). The maximum diameter of the AAA is 62 mm × 59 mm (b). The terminal aorta is not dilated (c and e). The diameter of the right CIA is 8 mm and the diameter of the left CIA is 12 mm (d). The AAA was anatomically suitable for an endovascular abdominal aortic repair device (e). AAA: abdominal aortic aneurysm; CIA: common iliac artery; CT: computed tomography.
EVAR procedure
Under general anesthesia, bilateral inguinal sections were obliquely incised to expose the bilateral common femoral arteries (CFAs). An 8 Fr sheath was inserted into the left CFA, and a 6 Fr sheath into the right CFA. For embolization of the IMA, we used two identical occlusion devices (4 mm × 12 cm Interlocking Detachable Coil Occlusion System; Boston Scientific, Marlborough, MA, USA). Embolization of the lumbar arteries was not performed because they were thin and narrow, and branched from the thick wall of the aortic aneurysm. The left and right CFA sheaths were replaced with 18 Fr and 12 Fr DrySeal Flex Introducer Sheaths (W. L. Gore & Associates, Newark, DE, USA), respectively, while the GORE Excluder AAA Endoprosthesis Trunk-Ipsilateral component (RLT281414; W. L. Gore & Associates, Newark, DE, USA) was deployed on the left and a contralateral leg component (PLC161000; W. L. Gore & Associates) was deployed on the right. A Coda LP balloon (Cook Medical, Bloomington, IN, USA) was used to expand the vascular prostheses. Endoleak was not detected on the final angiography. The sheaths were removed, the wounds were closed, and the procedure was considered uncomplicated. The surgical time was 1 hour 40 minutes. The amount of bleeding was very small, and no blood transfusion was performed during surgery. The patient was extubated in the operating room 15 minutes after surgery.
Postoperative course
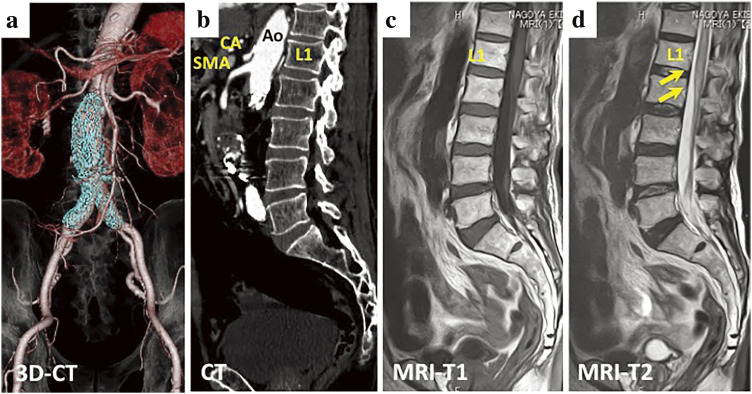
Immediately after surgery and extubation, paraplegia and thermal and pain sensation disorder of the lower extremities were confirmed. Considering that SCI had developed, we immediately administered 0.1 mg/h of naloxone, 1500 mg of methylprednisolone, and 30 mg of edaravone,3,4) cerebrospinal fluid drainage (CSFD) (3–10 cmH2O) was initiated 62 min after surgery.5,6) We continued with 0.1 mg/h of naloxone and 300 mg/h of methylprednisolone.6) The mean arterial pressure was maintained at ≥80 mmHg.7) Approximately 3 hours after surgery, the voluntary movement of the right knee recovered, but the left leg remained relaxed. Methylprednisolone administration was terminated on the second postoperative day, and 30 mg of edaravone every 12 hours was terminated on the third postoperative day. On the fourth postoperative day, CSFD was removed, and naloxone administration was terminated. The manual muscle strength test of the right lower extremity did not improve to a score of 3 or more, and tension of only the sartorius muscle was observed in the left lower extremity. Thermal pain sensation in the right lower leg, left femur, and lower leg remained impaired. The bladder and bowel dysfunctions did not improve. Postoperative magnetic resonance imaging (MRI) showed high signal intensity in the lumbar spinal cord below L1 in the T2-weighted images (Fig. 2). This finding indicated a peripheral SCI below L1. Although the artery of Adamkiewicz (AKA) could not be clearly identified on both pre- and postoperative contrast-enhanced computed tomography (CT), the lumbar and intercostal arteries proximal to the level of the renal arteries were patent on postoperative CT (Fig. 2). The bilateral internal iliac arteries were also patent on postoperative CT. Any types of endoleak were not detected on postoperative CT. Other than a pneumothorax on the 27th postoperative day, there were no additional complications. The patient was transferred to another hospital for further rehabilitation on the 90th postoperative day.
Fig. 2 Postoperative contrast-enhanced CT of the abdominal aorta (a and b) and MRI of the spinal cord (b and c). The abdominal stent graft (colored blue) is placed distal to the L2 level (a). The AKA could not be clearly identified, but the lumbar and intercostal arteries cranial to the level of the renal arteries were patent (b). Postoperative T2-weighted MRI revealed a high signal on the anterior spinal artery region of the spinal cord (d). SCI was diagnosed. AKA: artery of Adamkiewicz; SCI: spinal cord infarction; MRI: magnetic resonance imaging; CT: computed tomography; Ao: aorta; CA: celiac artery; SMA: superior mesenteric artery.
Discussion
The incidence of SCI after EVAR ranges from 0.21% to 0.64%.8) However, the reported incidence of SCI after open AAA repair ranges from 0.1% to 1.4%.7) Even though SCI occurs less often after EVAR than after open surgery, it remains a non-negligible complication. Possible causes of SCI after EVAR include embolization associated with intravascular manipulations of catheters and devices during surgery, as well as occlusion and interruption of the AKA and collateral circulation due to endograft placement. In EVAR, the endograft is usually placed distal to the renal arteries; therefore, the lumbar arteries and IMA below the renal arteries can be occluded during EVAR. Considering that renal arteries usually originate from the L1/L2 level, and the AKA branches from the L1/L2 level with 7.5% frequency and L3 with 0.8% frequency,9) the frequency of SCI after EVAR or open AAA surgery should be higher than reported. However, the incidence of SCI after open AAA surgery remains low. It is believed that the feeding arteries to the spinal cord depend on both the AKA and collateral circulation. Although AKA is considered the most important feeding artery for the spinal cord, the lumbar arteries, iliolumbar artery, medial and lateral sacral arteries, and IMA are also important collateral arteries of the lower spine.7) Since there are collateral arteries leading from the IMA to the superior rectal artery and middle sacral artery, ligation or embolization of the IMA may reduce blood flow in the lower spine if such collateral arteries are underdeveloped, AKA originates abnormally high, the blood flow from AKA is insufficient, or AKA branches from the L3 level. It was possible that the collateral blood flow in the lower spine was significantly reduced due to the embolization of IMA and lumber arteries in this case. Although there is no established treatment for lower-extremity paraplegia after EVAR, similar treatment to that of paraplegia after surgery for thoracoabdominal aortic aneurysm (TAAA) can be applied. The efficacy of the CSFD, maintaining high blood pressure, and the administration of steroids, naloxone, and free radical scavengers have been reported. Coselli et al. reported that perioperative CSFD could reduce the rate of paraplegia after the repair of the extent I and II TAAAs.5) Acher et al. also reported that CSFD significantly improved late-onset neurological deficits that occurred between postoperative days 1 and 14 after TAAA surgery.3) Bhama et al. reported that intravenous administration of solumedrol (5.4 mg/kg bolus and 30 mg/kg drip) and mannitol (100 mL of 25% solution bolus, followed by 50 mL 4 times daily) and maintaining mean arterial pressure ≥80–100 mmHg in addition to CSFD were effective.4) Acher et al. mentioned that the combined use of CSFD and naloxone offered significant protection from neurologic deficits in patients undergoing thoracic and thoracoabdominal aortic replacement.3) Even though we applied all of these protective treatments immediately after confirming SCI, the patient’s lower extremity paraplegia and bladder-rectal disorder did not improve sufficiently. Szilagyi et al. reported that complete recovery of lower extremity function from the SCI after open surgery for AAA was only 13% of the cases.10) Gloviczki et al. also reported that complete recovery of lower extremity function was rare in case of extensive spinal cord ischemia after open abdominal aortic surgery.7) In cases in which SCI occurs after abdominal aortic surgery, it is possible that collateral blood circulation in the lower spine is underdeveloped. Therefore, even if SCI after abdominal aortic surgery occurs due to decreased blood flow from the lumbar artery, median sacral artery, or IMA, lower extremity function may not be improved by CSFD and other treatments.
Conclusion
SCI after EVAR is a rare but serious complication that is difficult to prevent. Once it develops, recovery becomes difficult. Considering the possibility of SCI after EVAR, treatments, such as CSFD, can be started immediately if SCI is suspected.
Declarations
Funding
Not applicable.
Consent for publication
Informed consent for publication of case details was obtained from our patient.
Author contributions
Study conception: YK
Data collection: YK and FK
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
Disclosure statement
The authors declare that they have no competing interests.
Abbreviations
- AAA
abdominal aortic aneurysm
- AKA
the artery of Adamkiewicz
- CFA
common femoral artery
- CIA
common iliac artery
- CSFD
cerebrospinal fluid drainage
- CT
computed tomography
- EVAR
endovascular aortic repair
- IMA
inferior mesenteric artery
- MMT
manual muscle strength test
- MRI
magnetic resonance imaging
- SCI
spinal cord infarction
- TAAA
thoracoabdominal aortic aneurysm
References
- 1).Berg P, Kaufmann D, Merrewijk CJ, et al. Spinal cord ischaemia after stent-graft treatment for infra-renal abdominal aortic aneurysms. Analysis of the Eurostar Database. Eur J Vasc Endovasc Surg 2001; 22: 342–7. [DOI] [PubMed] [Google Scholar]
- 2).Hoshina K, Ishimaru S, Sasabuchi Y, et al. Outcomes of endovascular repair for abdominal aortic aneurysms. A nationwide study in Japan. Ann Surg 2019; 269: 564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Acher CW, Wynn MM, Hoch JR, et al. Cardiac function is a risk factor for paralysis in thoracoabdominal aortic replacement. J Vasc Surg 1998; 27: 821–30; discussion, 829–30. [DOI] [PubMed] [Google Scholar]
- 4).Bhama JK, Lin PH, Voloyiannis T, et al. Delayed neurologic deficit after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2003; 37: 690–2. [DOI] [PubMed] [Google Scholar]
- 5).Coselli JS, LeMaire SA, Köksoy C, et al. Cerebrospinal fluid drainage reduces paraplegia after thoracoabdominal aortic aneurysm repair: Results of a randomized clinical trial. J Vasc Surg 2002; 35: 631–9. [DOI] [PubMed] [Google Scholar]
- 6).Sugiura J, Oshima H, Abe T, et al. The efficacy and risk of cerebrospinal fluid drainage for thoracoabdominal aortic aneurysm repair: a retrospective observational comparison between drainage and non-drainage. Interact Cardiovasc Thorac Surg 2017; 24: 609–14. [DOI] [PubMed] [Google Scholar]
- 7).Gloviczki P, Cross SA, Stanson AW, et al. Ischemic injury to the spinal cord or lumbosacral plexus after aorto-iliac reconstruction. Am J Surg 1991; 162: 131–6. [DOI] [PubMed] [Google Scholar]
- 8).Maldonado TS, Rockman CB, Riles E, et al. Ischemic complications after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2004; 40: 703–9; discussion, 709–10. [DOI] [PubMed] [Google Scholar]
- 9).Melissano G, Bertoglio L, Civelli V, et al. Demonstration of the Adamkiewicz artery by multidetector computed tomography angiography analysed with the open-source software OsiriX. Eur J Vasc Endovasc Surg 2009; 37: 395–400. [DOI] [PubMed] [Google Scholar]
- 10).Szilagyi DE, Hageman JH, Smith RF, et al. Spinal cord damage in surgery of the abdominal aorta. Surgery 1978; 83: 38–56. [PubMed] [Google Scholar]