Abstract
A 53-year-old woman visited her district hospital complaining of right lower limb numbness 8 days after being diagnosed with COVID-19. She had been suffering diarrhea for 25 days before the hospital visit. Computed tomography showed multiple arterial and venous thromboses, and anticoagulation with a therapeutic dose of heparin was initiated. Acute aortic occlusion occurred on hospital day 5, and balloon thromboembolectomy was performed for revascularization of the lower limbs 9 hours after onset. Ulcerative colitis was diagnosed on postoperative day 7. With the anticoagulation and immunosuppression therapy, no thromboembolic event occurred postoperatively.
Keywords: acute aortic occlusion, COVID-19, ulcerative colitis
Introduction
Coronavirus disease 2019 (COVID-19) continues to spread globally with its variant mutations, carrying a high risk of coagulopathy evidenced by venous and arterial thromboses. Acute limb ischemia (ALI) has occurred as a rare but devastating complication of COVID-19. A meta-analysis of 15 studies from countries other than Japan showed the incidence of ALI to be 0.3% among 37217 patients hospitalized with COVID-19 and substantially higher than the reported 0.07% incidence of ALI among 455629 patients hospitalized with non-COVID-19 viral infection.1) In patients with COVID-19, thrombogenic risk is influenced by ethnic differences and distinct resource availability.2) A few cases of COVID-19 complicated by ALI have been reported from Japanese institutions.3) The CLOT-COVID Study, performed to analyze patients with COVID-19 hospitalized at 16 centers in Japan between April 2021 and September 2021 (n = 2894), showed only one patient with ALI, for an incidence of 0.035%.2)
Acute aortic occlusion (AAO) is a life-threatening cardiovascular emergency, and more than 80% of patients with AAO present with bilateral ALI.4) Although AAO occurring in patients with COVID-19 has been reported outside of Japan,5–7) its characteristics and treatment outcomes remain to be elucidated. We describe a case of AAO associated with COVID-19 infection and ulcerative colitis (UC).
Case Report
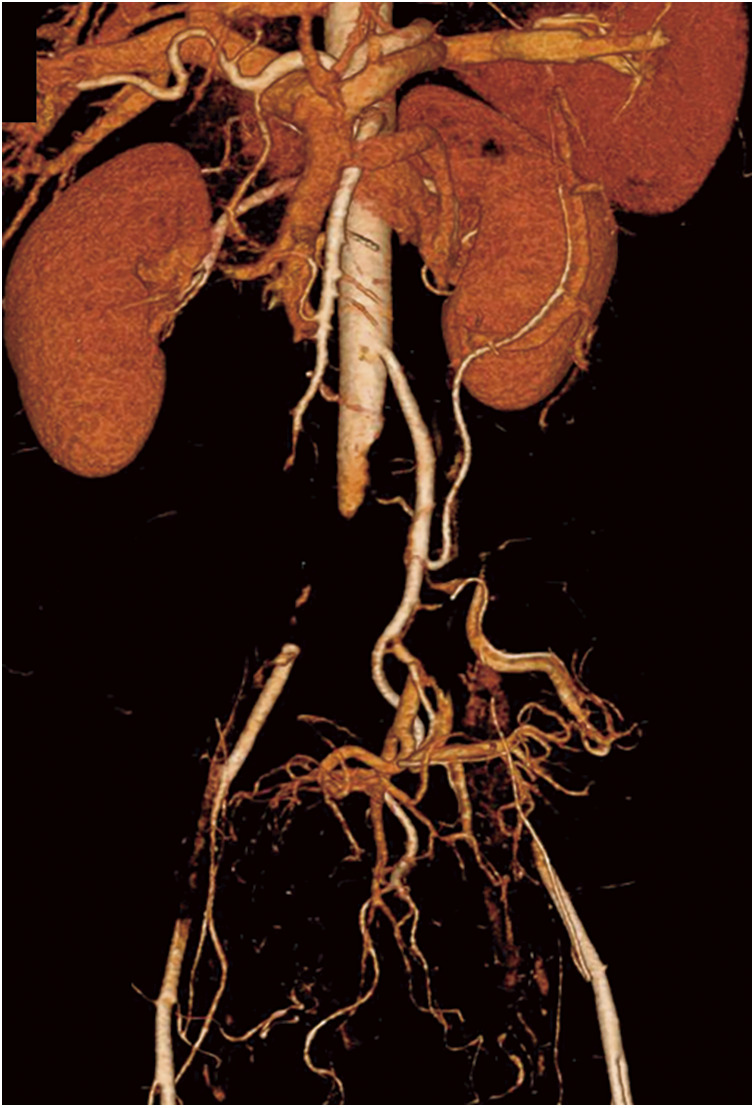
A 53-year-old woman with a history of myoma uteri visited the emergency department of her district hospital for numbness in her right lower leg. The patient had no smoking history. Computed tomography (CT) revealed a thrombus in the patient’s abdominal aorta and another in her right popliteal artery, with others seen in the right internal jugular vein, right brachiocephalic vein, and left inferior pulmonary artery (Supplemental Fig. 1; supplemental files are available online). There were no signs of pneumonia. However, the patient had been suffering intermittent diarrhea for 25 days prior to her visit to the hospital. She reported that her coworker had been diagnosed with COVID-19 13 days before her hospital visit, and 8 days before the hospital visit, she, having been feverish, tested positive for COVID-19 despite having been vaccinated against the disease twice. Multiple arteriovenous thromboses related to COVID-19 infection were diagnosed. The patient was then admitted, and anticoagulation therapy with therapeutic-dose heparin (20000–23000 units/day) was begun. On hospital day 5, she began to suffer bilateral lower limb pain and numbness. CT angiography revealed infrarenal aortic occlusion with extension into both external iliac arteries (Fig. 1). There was no arteriosclerotic lesion in the preoperative CT imaging data. AAO was diagnosed, and the patient was transferred to our hospital for emergency treatment 7 hours after symptom onset.
Fig. 1 Computed tomography angiography image showing infrarenal aortic occlusion with extension into both external iliac arteries.
On physical examination, the patient’s arterial blood pressure was 135/87 mmHg, and neither femoral artery was palpable. O2 saturation was 99% on room air. Both lower limbs were cyanotic and cold; the sensation was decreased. Motor function was reduced in the right lower limb. Preoperative laboratory tests showed a mildly elevated white blood cell count of 9080/μL and a C-reactive protein concentration of 4.2 mg/dL. Coagulation markers, including an international normalized ratio of prothrombin time of 1.3, activated partial thromboplastin time of 63.9 seconds, and D-dimer concentration of 5.8 μg/mL, were also increased. Antithrombin III was decreased (57%), and fibrinogen concentration was within the normal limits (292 mg/dL). The creatine kinase concentration was not increased (177 IU/dL). The patient was immediately taken to the operating room.
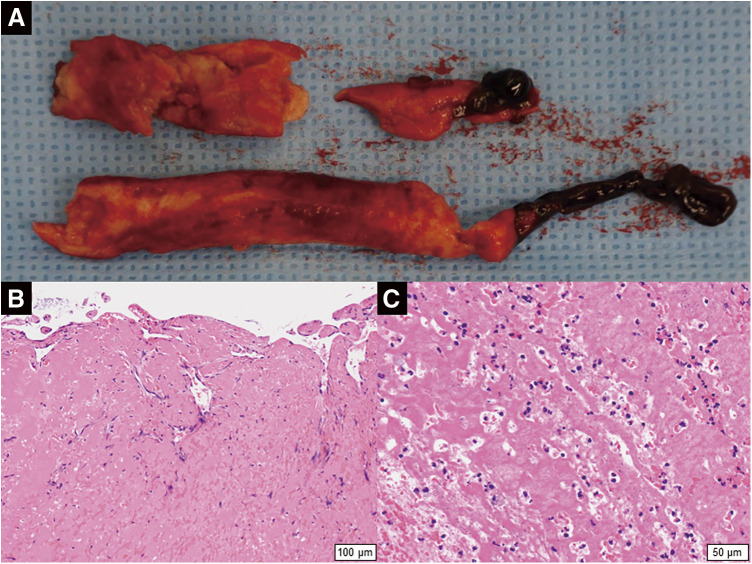
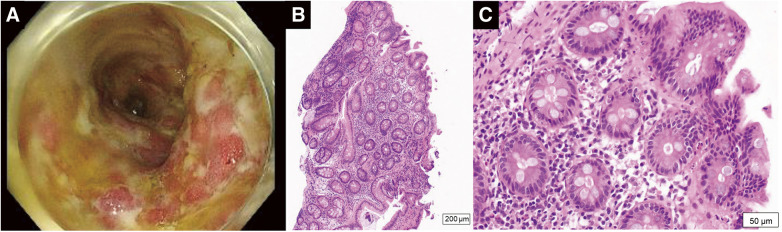
With the patient under general anesthesia, balloon thromboembolectomy was performed from both common femoral arteries. On each side, a 3-Fr balloon catheter was passed distally, and a massive thrombus was removed from the superficial femoral artery, leading to the restoration of retrograde blood flow. A 4-Fr balloon catheter was then inserted proximally toward the iliac arteries, and a massive thrombus located in the abdominal aorta extending into the iliac arteries was extracted. Intraoperative aortography showed restored lower limb perfusion 9 hours after symptom onset (Supplemental Fig. 2). Operation time was 272 minutes, total blood loss was 220 g, and there was no intraoperative post-reperfusion hyperkalemia. Components of the extracted aortic thrombus were white or dark red (Fig. 2A). Histologic examination revealed an organized thrombus with inflammatory cell infiltration (Figs. 2B and 2C). The case was not complicated by myonephropathic metabolic syndrome, with the creatine kinase concentration peaking at 5463 U/dL on postoperative day (POD) 1. Anticoagulation with therapeutic-dose heparin was resumed postoperatively, aimed at an activated partial thromboplastin time ranging between 60 and 80 seconds. The patient suffered gastrointestinal tract bleeding on PODs 4 and 5. Lower gastrointestinal endoscopy was performed on POD 7. The examination revealed erosions, ulcers, and spontaneous bleeding involving the entire colon (Fig. 3A). Histologic examination revealed inflammatory cell infiltration throughout the mucosa (Fig. 3B). Thus, severe pan-colic type UC was diagnosed. Prednisolone and mesalazine were started, and the UC symptoms improved. Laboratory tests performed on POD 1 showed an elevated proteinase 3-anti-neutrophil cytoplasmic antibody (PR3-ANCA) concentration of 3.8 IU/L and a von Willebrand factor level of 200%, but other coagulation biomarkers, including protein C, protein S, and lupus anticoagulant, were within normal limits. The anticoagulation therapy was converted from intravenous heparin to oral edoxaban on POD 8. The patient was discharged without sequelae on POD 33. She has been treated with edoxaban, and there has been no recurrence of thromboembolism for 18 months since her discharge. A postoperative CT scan performed 14 months after surgery showed the disappearance of multiple arteriovenous thrombi, except for a small thrombus in the right popliteal artery (Supplemental Fig. 3). Her ankle-brachial index 18 months after surgery was 0.99 for the left lower limb and 0.77 for the right lower limb. The patient was re-hospitalized for exacerbation of UC 14 months after hospital discharge. She is now treated with mesalazine and prednisolone for UC. The patient plans to continue edoxaban unless a significant bleeding event develops.
Fig. 2 Extracted abdominal aortic thrombus. (A) Macroscopic image. (B, C) Histological images (hematoxylin–eosin stain). (B) The high-power view (×100) shows an organized thrombus. (C) The high-power view (×200) shows inflammatory cell infiltration in the thrombus.
Fig. 3 Diagnostic evaluation of the patient’s ulcerative colitis. (A) Endoscopic imaging showed erosions, ulcers, and spontaneous bleeding in the entire colon. (B) Low-power view (×40) and (C) high-power view (×200) showed inflammatory cell infiltration throughout the mucosa.
Discussion
Several groups from countries other than Japan have reported cases of AAO in association with COVID-19 infection5–7); however, the incidence of AAO in hospitalized patients with COVID-19 has not been documented. To the best of our knowledge, the case we describe herein is the first case of AAO developing on a background of COVID-19 infection in Japan.
Several thrombogenic mechanisms have been implicated in thrombosis related to COVID-19 infection. These include cytokine storm, complement cascade, macrophage activation syndrome, platelet activation, antiphospholipid antibody syndrome, and renin–angiotensin system dysregulation.8) Such complex cascades may trigger venous and arterial thromboses via endothelial dysfunction.8) Our patient showed progressive arterial thromboembolism, leading to AAO, despite the administration of therapeutic-dose heparin. Thrombus formation has been reported to have an association with COVID-19 severity.2) However, our patient did not present with COVID-19 pneumonia. The von Willebrand factor elevation suggests that endothelial damage played a role in our patient’s multiple arteriovenous thromboses. The coagulopathy screening test also showed PR3-ANCA elevation. PR3-ANCA is often used to diagnose and evaluate the severity of UC.9) From the recent expert consensus meeting came a published statement that patients with inflammatory bowel disease have an increased risk of venous and arterial thrombotic events, with the risk of thrombosis being related to disease activity.10) Furthermore, a nationwide registry data study from the United States recently reported that patients with UC hospitalized with COVID-19 have a higher risk of deep vein thrombosis than COVID-19 patients hospitalized without UC.11) We think that our patient’s severe, untreated UC might have exacerbated her COVID-19-related coagulopathy. Another possible mechanism leading to the AAO is the defluxion of the attached thrombus in the infrarenal abdominal aorta during anticoagulation therapy. Histologic examination of the extracted thrombus revealed an organized thrombus with inflammatory cell infiltration, consistent with the notion that defluxion of an old thrombus in the abdominal aorta occluded the distal abdominal aorta.
AAO is a rare thrombotic complication of COVID-19. Therefore, patient volume, hospital to hospital, is limited. Caudron et al. reported their experience of 10 patients with COVID-19-related AAO (mean age, 65 years; sex, 90% male).5) According to their single-center study, representing the largest volume of patients, AAO developed in four critically ill patients treated in the intensive care unit, with a median interval of 5 days between the diagnosis of COVID-19 and the onset of AAO.5) Revascularization surgery was performed in 9 of their 10 patients using open (n = 6), endovascular (n = 2), or hybrid approach (n = 1). They reported a 30-day mortality rate of 30% (3/10). All postoperative deaths were due to respiratory failure.5) Kimyaghalam et al. searched the literature for cases of AAO related to COVID-19, including their case.6) A total of 9 articles covering 12 patients (mean age, 63 years; sex, 67% male) were identified, and 2 of the 12 had concomitant thromboembolic disease in vessels other than the abdominal aorta.6) Both studies showed that none of the 22 patients had a history of inflammatory bowel disease.5,6)
AAO is one of the most serious cardiovascular emergencies, and prompt revascularization surgery is mandatory to save lives. In a fairly recent Sweden-wide registry study (n = 715), the 1994–2014 incidence of AAO was found to be 3.8 per 1 million person-years, and operative outcomes were unfavorable, with an amputation rate of 8.6% and 30-day mortality rate of 19.9%.4) For optimal revascularization, the selection of the procedure should be individualized based on the general condition of the patient, concomitant chronic aortic occlusive disease, amount of thrombus, and underlying etiology of AAO. In cases of a large thrombus volume or the presence of chronic aortic occlusive disease, open repair or an extra-anatomical bypass procedure should be considered. Robaldo et al. reported an 89-year-old patient with COVID-19 in whom AAO developed in association with an 8-cm abdominal aortic aneurysm. Thrombus recanalization was achieved, followed by endovascular aortic repair.7) For high-risk patients with a concomitant abdominal aortic aneurysm, endovascular aortic repair may be a less invasive and feasible treatment approach.
Conclusion
We experienced a rare case of AAO occurring in association with COVID-19 infection and UC. Prompt revascularization achieved using balloon thromboembolectomy resolved the patient’s profound lower limb ischemia. The absence of pneumonia in this case leaves open the possibility that the patient’s severe untreated UC might have exacerbated coexisting COVID-19-associated coagulopathy. Patients with a combination of UC and COVID-19 infection should be carefully treated because of a high risk of thromboembolism.
Informed Consent
Informed consent was obtained from the patient regarding the publication of the manuscript.
Disclosure Statement
Yamaguchi serves as a consultant to Japan Lifeline Co., Ltd. Shiraishi obtained a research grant from Terumo Life Science Foundation and Bristol Myers Squibb K. K. The other authors have no conflict of interest to disclose.
Author Contributions
Study conception: NK and MN
Data collection: MY and NO
Investigation: MY, MN, and NO
Writing: MY and NK
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
Supplementary Materials
Computed tomography images obtained 5 days before the onset of acute aortic occlusion. Arrows indicate an arterial thrombus in the (A) abdominal aorta and (B) right popliteal artery, venous thrombi in the (C) right internal jugular vein and right brachiocephalic vein, and venous thrombus in the (D) left lower pulmonary artery.
Intraoperative angiograms. (A) Aortic angiography performed after balloon thromboembolectomy showed that most of the thrombus in the abdominal aorta extending into both external iliac arteries had been removed. Angiogram of the (B) right lower extremity and (C) left lower extremity. With balloon thromboembolectomy perfusion to both lower extremities was restored.
Computed tomography images obtained 14 months after surgery for acute aortic occlusion. (A) 3D imaging showing no residual thrombus in the abdominal aorta. (B) Venous thrombus in the right internal jugular vein and right brachiocephalic vein was disappeared. (C) A small residual thrombus (arrow) was present in the right popliteal artery.
References
- 1).Candeloro M, Schulman S. Arterial thrombotic events in hospitalized COVID-19 patients: a short review and meta-analysis. Semin Thromb Hemost 2023; 49: 47–54. [DOI] [PubMed] [Google Scholar]
- 2).Umetsu M, Kanamori H, Murakami K, et al. Clinical features comparing arterial thrombosis and venous thromboembolism in hospitalized patients with COVID-19: Result from the CLOT-COVID Study. Ann Vasc Dis 2023; 16: 115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Akita N, Osawa T, Todoroki H, et al. Acute limb ischemia in a COVID-19 patient: a case report. Ann Vasc Dis 2021; 14: 388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Grip O, Wanhainen A, Bjorck M. Acute aortic occlusion. Circulation 2019; 139: 292–4. [DOI] [PubMed] [Google Scholar]
- 5).Caudron C, Ben Abdallah I, Detriche G, et al. Aortic thrombosis as a dramatic vascular complication in COVID-19 disease. J Med Vasc 2022; 47: 169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Kimyaghalam A, Khan S, Bonilla H, et al. Abdominal aortic occlusion in the setting of Covid 19 infection: literature review and a case report. J Surg Case Rep 2023; 2023: rjad400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Robaldo A, Apostolou D, Persi F, et al. EVAR solution for acutely thrombosed abdominal aortic aneurysm in a patient with COVID-19. EJVES Vasc Forum 2022; 54: 41–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Hanff TC, Mohareb AM, Giri J, et al. Thrombosis in COVID-19. Am J Hematol 2020; 95: 1578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Takedatsu H, Mitsuyama K, Fukunaga S, et al. Diagnostic and clinical role of serum proteinase 3 antineutrophil cytoplasmic antibodies in inflammatory bowel disease. J Gastroenterol Hepatol 2018; 33: 1603–7. [DOI] [PubMed] [Google Scholar]
- 10).Olivera PA, Zuily S, Kotze PG, et al. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2021; 18: 857–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Aldiabat M, Alsakarneh S, Daniel T, et al. Inpatient outcomes of inflammatory bowel disease in hospitalized patients with COVID-19: analysis of a nationally representative sample. Proc Bayl Univ Med Cent 2024; 37: 239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Computed tomography images obtained 5 days before the onset of acute aortic occlusion. Arrows indicate an arterial thrombus in the (A) abdominal aorta and (B) right popliteal artery, venous thrombi in the (C) right internal jugular vein and right brachiocephalic vein, and venous thrombus in the (D) left lower pulmonary artery.
Intraoperative angiograms. (A) Aortic angiography performed after balloon thromboembolectomy showed that most of the thrombus in the abdominal aorta extending into both external iliac arteries had been removed. Angiogram of the (B) right lower extremity and (C) left lower extremity. With balloon thromboembolectomy perfusion to both lower extremities was restored.
Computed tomography images obtained 14 months after surgery for acute aortic occlusion. (A) 3D imaging showing no residual thrombus in the abdominal aorta. (B) Venous thrombus in the right internal jugular vein and right brachiocephalic vein was disappeared. (C) A small residual thrombus (arrow) was present in the right popliteal artery.