Abstract
Objective
The increasing utilization of various molecular tests for diagnosing and selecting treatments for patients with malignancies has led to a rising trend in both the frequency of biopsies and the required tissue volume. We aimed to compare the safety of outpatient ultrasound (US)-guided percutaneous liver biopsy (PLB) between the coaxial method with needle track plugging (NTP) and the conventional method.
Materials and Methods
This single-center, prospective, randomized controlled study was conducted from October 2022 to May 2023. Patients referred for US-guided PLB with target liver lesions measuring ≥1 cm and requiring ≥3 tissue cores were enrolled. Patients with severe coagulopathy or a substantial volume of ascites were excluded. Patients were randomly assigned to undergo PLB using either the coaxial method with NTP or the conventional method, in a 1:1 ratio, and were subsequently discharged after 2 hours. The primary endpoint was the presence of a patent track sign, defined as a linear color flow along the biopsy track on Doppler US, as an indication of bleeding. The secondary endpoints included clinically significant bleeding, delayed bleeding after discharge, and diagnostic yield. The incidences of these endpoints were compared between the two methods.
Results
A total of 107 patients completed the study protocol. Patent track signs were observed significantly less frequently in the coaxial method with NTP group than in the conventional method group: 16.7% (9/54) vs. 35.8% (19/53; P = 0.042). Clinically significant bleeding and delayed bleeding did not occur in either group, and both methods achieved a high diagnostic yield: 94.4% (51/54) vs. 98.1% (52/53; P = 0.624).
Conclusion
Compared with the conventional method, the coaxial method with NTP may potentially be safer, with a reduced risk of overall bleeding complications after PLB when retrieving ≥3 tissue cores. The coaxial method with NTP could be considered a viable option for acquiring multiple liver tissues on an outpatient basis.
Keywords: Liver; Image-guided biopsy; Hemorrhage; Bleeding; Ultrasonography, Doppler; Prospective studies
INTRODUCTION
Bleeding is one of the most significant complications studiesfollowing ultrasound (US)-guided percutaneous liver biopsy (PLB). Various risk factors contributing to bleeding complications have been inconsistently reported, including low platelet levels, elevated prothrombin time (PT) (international normalized ratio [INR]), the needle size, the use of cutting needles, the number of liver capsule penetrations, the hypervascularity of the target lesion, and subcapsular location [1,2,3,4,5]. Definitions of postbiopsy bleeding also varied among previous studies; some researchers encompassed all bleeding events, from minor subclinical hemorrhages to clinically significant bleeds [6,7,8,9,10], while others limited their definition to only clinically significant cases [1,11,12,13,14,15,16]. Overall, most bleeding events resolve spontaneously. The overall incidence of bleeding is frequent when including minor subclinical hemorrhages, with reported rates ranging from 12%–20% [9,10,17,18,19]. If bleeding persists without spontaneous resolution, although rare, it may progress to a clinically significant status, which may be fatal. Reported rates of clinically significant bleeding—defined as bleeding requiring hospitalization, transfusion, embolization, or surgery—range between 0.5%–1.2% [20]. Doppler US can be used to detect bleeding, which may appear as a linear track of color flow along the biopsy track (commonly referred to as a patent track sign) [17,19]. When a patent track sign is present after a biopsy, direct pressure should be immediately applied to the puncture site until it disappears [19,21]. Although the patent track sign does not specify the degree of bleeding, its presence may suggest a higher risk of progression to a clinically significant status, with a sensitivity and specificity of 80% and 89%, respectively [17]. Prolonged bleeding lasting for >5 minutes further increases the likelihood of progressing to a clinically significant hemorrhage, with the positive predictive value increasing from 9% to 75% [17].
Since the advent of precision medicine, the number of US-guided PLBs for liver tumors has steadily increased [7]. Particularly in the oncology setting, many clinical trials advocate or necessitate the acquisition of two or more tissue cores, often at multiple timepoints throughout the trials [22]. Given the availability of various targeted agents and immunotherapies, there is a clinical need for repeated biopsies with multiple cores in patients with cancer to investigate the mechanisms of acquired resistance to anticancer therapy. Accordingly, the average number of biopsy procedures and liver capsule penetrations have also increased [23]. This trend may prompt concerns about an increased risk of post-PLB bleeding, as three or more liver capsule penetrations were reported to be associated with a higher risk of bleeding than two or fewer penetrations [4,18]. Whether PLB may remain an outpatient-based procedure, as recommended by guidelines [18], or should now shift to an inpatient basis is another important clinical question.
The use of coaxial needles may theoretically mitigate the risk of post-PLB bleeding, as it allows for the acquisition of multiple tissue cores through a single penetration of the liver capsule. However, bleeding rates were reported to be similar between the coaxial and conventional methods [15,24]. Some authors speculate that compared with the conventional method, the longer transhepatic dwell time of the larger coaxial sheath may have offset the effects of single penetration [2]. Meanwhile, the coaxial method may provide another advantage in that it allows for subsequent needle track plugging (NTP) after biopsy. This approach has demonstrated its potential for higher safety since its initial report in 1984 by successfully decreasing the risk of bleeding [25,26,27,28,29,30,31,32,33]. However, the safety of the coaxial method with NTP has not been prospectively compared with that of the conventional method. Hence, this prospective, randomized controlled study aimed to compare the safety of outpatient US-guided PLB using the coaxial method with NTP versus the conventional method.
MATERIALS AND METHODS
This single center, prospective, randomized controlled study (ClinicalTrials.gov identifier: NCT05614973) was approved by the Institutional Review Board of our institution (IRB No. 4-2022-0211) and conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent.
Enrollment Criteria and Randomization
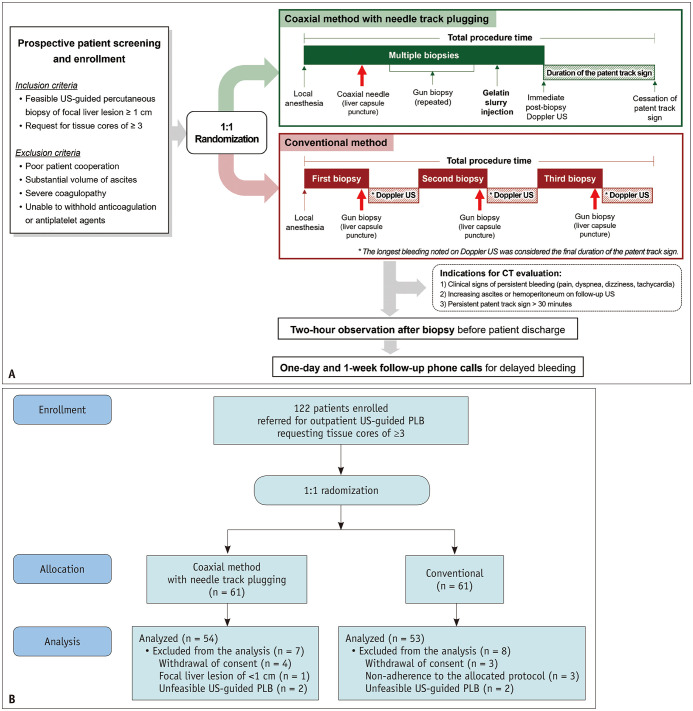
Adult patients referred for US-guided PLB of focal liver lesions requiring the acquisition of ≥3 tissue cores were screened (Fig. 1A). Patients 1) with a target liver lesion size ≥1 cm and 2) whose routine laboratory values fulfilled the Society of Interventional Radiology consensus guidelines (for patients without underlying liver disease: platelet count ≥50000/µL and PT/INR ≤1.8; for patients with chronic liver disease or cirrhosis: platelet count ≥30000/µL, PT/INR ≤2.5, and fibrinogen levels >100 mg/dL) were included in the study [34]. Patients who were contraindicated for PLB, including those with intellectual disabilities or severe psychiatric disorders; those who demonstrated poor cooperation; those with a substantial volume of ascites, severe coagulopathy, extrahepatic bile duct obstruction, active hepatobiliary inflammation, or clinical suspicion or confirmed amyloidosis; and those with an inability to withhold anticoagulation or antiplatelet agents, were excluded [18,34].
Fig. 1. Patient screening, enrollment, and study protocol. A: Patients with a focal liver lesion >1 cm, deemed feasible for US-guided PLB, and requiring three or more tissue cores were enrolled in the study. All enrolled patients were randomly allocated to one of two biopsy methods: the coaxial method with needle track plugging, which involves a single liver capsule penetration; or the conventional method, which requires three or more liver capsule penetrations. After the biopsy, Doppler US was performed to assess for track bleeding through the presence of a patent track sign, defined as a linear track of color flow along the biopsy track. Patients were discharged after a 2-hour observation period. Follow-up phone calls were made both 1 day and 1 week after the procedure to assess for any signs of delayed bleeding. B: A total of 122 patients were prospectively enrolled and randomized. After the exclusion of patients who withdrew their consent (n = 7), had a focal liver lesion of <1 cm measured on preprocedural US (n = 1), were deemed unfeasible for US-guided biopsy (n = 4), or did not adhere to the allocated protocol (n = 3), the remaining 54 and 53 patients were finally enrolled into the coaxial method with needle track plugging group and the conventional method group, respectively. US = ultrasound, PLB = percutaneous liver biopsy.
All eligible patients were randomly assigned to either the coaxial method with NTP group or the conventional method group using a computer-generated permuted block randomization procedure at a 1:1 ratio, without any stratification factors.
US-Guided PLB
All examinations were performed using standard US equipment and probes (EPIQ 5 scanner, C5-1 and C9-2 probes; Philips, Bothell, WA, USA). Preprocedural US was performed to evaluate the feasibility of PLBs. A subcapsular location was operationally defined as a scenario wherein the intended biopsy route failed to include the normal liver parenchyma. All procedures were performed by two radiologists (S.-S.K. and J.K.Y, with 7 and 5 years of experience in performing US-guided PLB, respectively) using the free-hand technique. An automatic (Marquee™; Bard Biopsy Systems, Tempe, AZ, USA) or semiautomatic (Mission™; Bard Biopsy Systems) 18-gauge biopsy gun was used according to the operator’s preference. In the coaxial method with NTP group, a 17-gauge coaxial needle (TruGuide™; Bard Biopsy Systems) was used. A gelatin slurry was prepared by mixing 2000–4000 µm gelatin particles (EGgel S PLUS; ENGAIN Co., Ltd, Seongnam, South Korea) with 15 cc of normal saline.
In cases of multiple liver lesions, the target lesion was selected at the discretion of the operator. In the coaxial method with NTP group, the coaxial needle was introduced into the liver, followed by the insertion of the biopsy gun through the outer sheath. Biopsies were repeatedly performed through the indwelling coaxial sheath as deemed necessary. After conducting the NTP procedure by injecting the gelatin slurry (≤5 cc) through the coaxial sheath, bleeding was evaluated using Doppler US. The assessment focused on identifying a patent track sign, characterized by a linear color flow along the needle track [17]. In the conventional method group, tissue cores were acquired through multiple penetrations of the liver capsule, each followed by an evaluation for the presence of a patent track sign [17]. For the evaluation of the patent track sign, only conventional color Doppler US was used, without employing power Doppler or microvascular US techniques. The color gain setting was individually adjusted to the highest level without creating background noise. The pulse repetition frequency was fixed at 1320 Hz, and the wall filter was consistently set to ‘low.’ When bleeding occurred multiple times during the conventional method, only the longest episode was recorded as the final duration of the patent track sign. The duration of the patent track sign and total procedure time (from local anesthesia to cessation of the final patent track sign) were documented (Fig. 1A). Additionally, the number of requested and acquired tissue cores was recorded.
Postbiopsy Management
All patients were discharged after a 2-hour observation and follow-up US evaluation (Fig. 1A). The potential biopsy-related symptoms, including pain, dyspnea, dizziness, and tachycardia, were recorded multiple times during the observation period to assess whether they followed an alleviating or aggravating course. The location and severity of pain, assessed using a numeric rating scale, were also recorded. When the patent track sign was present, the biopsy site was manually compressed. Indications for contrast-enhanced CT included: 1) clinical signs of ongoing bleeding or hypovolemic shock (persisting or aggravating biopsy-related symptoms, new-onset lower abdominal pain, or tachycardia), 2) continuously increasing ascites or hemoperitoneum detected on the follow-up US, and 3) a patent track sign persisting for >30 minutes. Patients were contacted both 1 day and 1 week after discharge to assess for any signs of delayed bleeding.
Endpoint
The primary endpoint was the patent track sign as an indication of biopsy track bleeding. The secondary endpoints included 1) the duration of the patent track sign, 2) clinically significant bleeding requiring hospitalization, transfusion, embolization, or surgery, 3) the total procedure time, 4) other biopsy-related symptoms such as pain, dyspnea, dizziness, and tachycardia, and 5) the diagnostic yield.
Statistical Analysis
This study was powered based on the results of previous studies. Given that the reported postbiopsy bleeding rate using the conventional method ranges from 12%–20% [9,10,17,18], we hypothesized that the expected incidence of patent track signs could reach up to 20% with three or more liver capsule penetrations. Conversely, based on the reported postbiopsy bleeding rate of 5.2% with the coaxial method [8], we hypothesized that the incidence of patent track signs could be <5% when NTP was performed in conjunction with this method. With a statistical power of 0.8 and an α value of 0.1, the enrollment size was calculated as 116 patients. Assuming a dropout rate of 5%, the final study sample was determined to be 122 patients. All analyses were performed on a per-protocol basis, as the aim of this study was more aligned with exploratory or hypothesis-generating purposes, rather than confirmatory purposes. Therefore, patients exhibiting nonadherence to the allocated biopsy method were excluded.
All statistical analyses were performed using R version 4.3.1 (The R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were compared using the Student’s t-test or Mann–Whitney U-test. Categorical variables were compared using the χ2 test. As a supplementary analysis, univariable and multivariable logistic regression analyses were performed to evaluate the factors associated with the patent track sign. Variables that were considered significant in the univariable analysis and crucial coagulation factors were included in the multivariable analysis. A P-value <0.05 (two-sided) was considered statistically significant.
RESULTS
Participant Characteristics
Between October 2022 and May 2023, 122 patients were enrolled and randomized (n = 61 for each group, Fig. 1B). Among them, 15 patients—who withdrew their consent to participate (n = 7), with a focal liver lesion measuring <1 cm on preprocedural US (n = 1), with unfeasible US-guided PLB (n = 4), and who showed nonadherence to the allocated biopsy protocol (n = 3)—were excluded from the final analysis. The reasons for nonadherence are summarized in Supplementary Table 1. The remaining 107 patients (54 males and 53 females; mean age ± standard deviation: 61.6 ± 12.2 years) were included for analysis, with 54 patients in the coaxial method with NTP group and 53 patients in the conventional method group. No significant differences were observed regarding the clinical characteristics between the two groups, including platelet levels and the presence of chronic liver disease or liver cirrhosis (Table 1). None of the patients had an underlying chronic kidney disease. All patients were referred for PLB due to the suspicion of primary or metastatic hepatic malignancy.
Table 1. Clinical characteristics of all enrolled patients according to the biopsy method used.
| Coaxial method with needle track plugging (n = 54) | Conventional method (n = 53) | P | |||
|---|---|---|---|---|---|
| Age, yrs | 60.9 ± 14.3 | 62.2 ± 9.5 | 0.571 | ||
| Male | 27 (50.0) | 27 (50.9) | >0.999 | ||
| Body mass index, kg/m2 | 23.2 ± 3.6 | 23.3 ± 3.7 | 0.935 | ||
| Platelet, ×103/µL | 290.0 ± 149.6 | 247.2 ± 91.1 | 0.076 | ||
| Chronic liver disease or liver cirrhosis | 11 (20.4) | 8 (15.1) | 0.645 | ||
| Pathologic diagnosis | 0.506 | ||||
| Primary hepatic malignancy | 14 (25.9) | 14 (26.4) | |||
| Hepatocellular carcinoma | 6 (11.1) | 5 (9.4) | |||
| Intrahepatic cholangiocarcinoma | 8 (14.8) | 7 (13.2) | |||
| Poorly differentiated carcinoma | 0 (0.0) | 1 (1.9) | |||
| Sarcomatoid carcinoma | 0 (0.0) | 1 (1.9) | |||
| Metastasis | 35 (64.8) | 37 (69.8) | |||
| Gallbladder and bile duct | 9 (16.7) | 1 (1.9) | |||
| Breast | 7 (12.9) | 8 (15.1) | |||
| Cervix | 0 (0.0) | 1 (1.9) | |||
| Colorectal | 3 (5.5) | 1 (1.9) | |||
| Lung | 4 (7.4) | 6 (11.3) | |||
| Malignant melanoma | 0 (0.0) | 1 (1.9) | |||
| Pancreas | 8 (14.8) | 15 (28.3) | |||
| Stomach | 2 (3.7) | 3 (5.6) | |||
| Thymus | 1 (1.9) | 0 (0.0) | |||
| Unknown primary | 1 (1.9) | 1 (1.9) | |||
| Benign | 2 (3.7) | 1 (1.9) | |||
| Focal nodular hyperplasia | 1 (1.9) | 0 (0.0) | |||
| Hemangioma | 1 (1.9) | 0 (0.0) | |||
| Inflammatory pseudotumor | 0 (0.0) | 1 (1.9) | |||
| Non-diagnostic | 3 (5.5) | 1 (1.9) | |||
Data are presented as numbers (%) or mean ± standard deviation
Comparison Between the Coaxial Method With NTP and Conventional Method
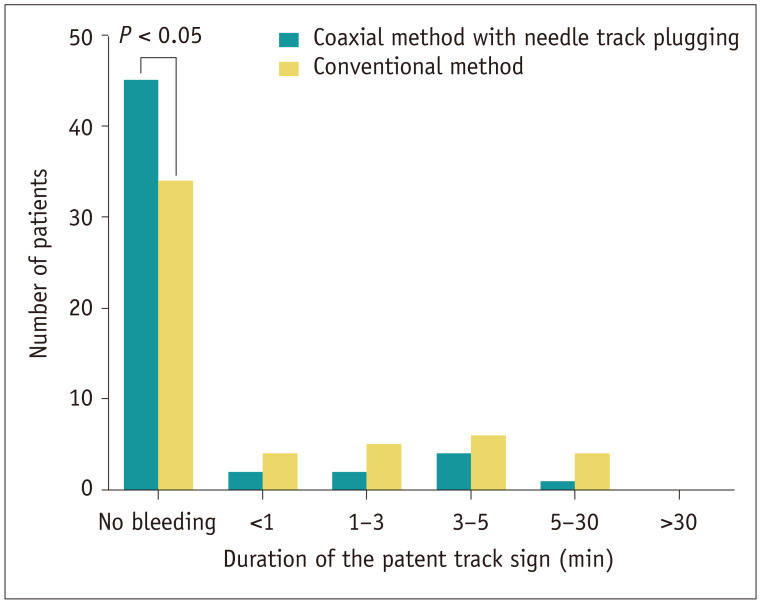
The patent track sign was significantly less frequently detected in the coaxial method with NTP group compared with the conventional method group (16.7% [9/54] vs. 35.8% [19/53]; P = 0.042; Table 2, Fig. 2). For patients with a positive patent track sign, the duration of the sign was shorter in the coaxial method with NTP group than in the conventional method group (Fig. 3). None of the patients showed clinical signs or US evidence of ongoing bleeding or hypovolemic shock requiring subsequent contrast-enhanced CT, or required hospitalization or further management. When contacted 1 day and 1 week after the biopsy, no patient reported any delayed-onset complications requiring hospitalization.
Table 2. Differences in procedure-related parameters according to the biopsy method used.
| Coaxial method with needle track plugging (n = 54) | Conventional method (n = 53) | P | |||
|---|---|---|---|---|---|
| Size, cm | 3.4 (2.4, 6.4) | 3.8 (2.0, 5.5) | 0.978 | ||
| Subcapsular location | 11 (20.4) | 9 (17.0) | 0.840 | ||
| Automatic biopsy gun | 52 (96.3) | 38 (71.7) | 0.001 | ||
| Number of liver capsule puncture | <0.001 | ||||
| 1 | 54 (100) | 1 (1.9) | |||
| 2 | 0 (0.0) | 1 (1.9) | |||
| 3 | 0 (0.0) | 39 (73.6) | |||
| 4 | 0 (0.0) | 11 (20.8) | |||
| 5 | 0 (0.0) | 1 (1.9) | |||
| Fulfillment of requested number of tissue cores | 54 (100) | 51 (96.2) | 0.818 | ||
| Total procedure time, minute | 6.1 ± 2.9 | 7.6 ± 4.5 | 0.053 | ||
| Patent track sign | 9 (16.7) | 19 (35.8) | 0.042 | ||
| Duration of the patent track sign, minute | 6.0 ± 3.1 | 7.0 ± 3.6 | 0.149 | ||
| Peri-procedural pain | 18 (33.3) | 25 (47.2) | 0.207 | ||
| Pain severity (numeric rating scale) | 0.0 (0.0, 2.0) | 0.0 (0.0, 5.0) | 0.076 | ||
| Most painful site | 0.382 | ||||
| Biopsy site | 9 (16.7) | 17 (32.1) | |||
| Other sites* | 9 (16.7) | 8 (15.1) | |||
| Diagnostic yield | 51 (94.4) | 52 (98.1) | 0.624 | ||
Data are presented as numbers (%) or median (1st quartile, 3rd quartile), or mean ± standard deviation.
*Ipsilateral shoulder (n = 4), lower abdomen (n = 4), epigastric area (n = 7), left back (n = 1), and right flank (n = 1)
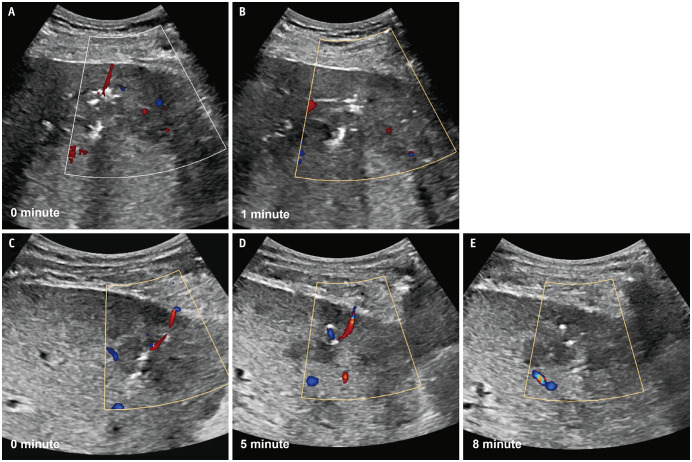
Fig. 2. Detection and cessation of track bleeding after percutaneous liver biopsy. A: A patent track sign, defined as a linear track of color flow along the biopsy track, was detected on Doppler US performed immediately after a US-guided percutaneous liver biopsy using the coaxial method with needle track plugging. B: The patent track sign disappeared within 1 minute. C: The patent track sign was observed on immediate postbiopsy Doppler US using the conventional method. D: The patent track sign persisted on 5-minute-delay Doppler US. E: The patent track sign disappeared in 8 minutes. US = ultrasound.
Fig. 3. Duration of the patent track sign according to the biopsy method. Patent track signs were observed significantly less frequently in the coaxial method with needle track plugging group than the conventional method group. When the patent track sign was present, its duration was shorter in the coaxial method with needle track plugging group than the conventional method group. All patent track signs spontaneously resolved within 30 minutes.
The coaxial method with NTP demonstrated a shorter total procedure time than the conventional method, without statistical significance (6.1 minutes vs. 7.6 minutes; P = 0.053; Table 2). Periprocedural dizziness (1.9% vs. 0.0%; P > 0.999) and pain (33.3% vs. 47.2%; P = 0.207) and postprocedural ascites/hemoperitoneum on US (3.7% vs. 1.9%; P = 0.974) were not significantly different between the two methods. Both methods achieved a high diagnostic yield (94.4% vs. 98.1%; P = 0.624).
Factors Associated With the Patent Track Sign
In the univariable analysis, the coaxial method with NTP was significantly associated with a lower risk of the patent track sign (odds ratio [OR]: 0.358, 95% confidence interval [CI]: 0.139–0.869; P = 0.027), while subcapsular location (OR: 2.928, 95% CI: 1.044–8.148; P = 0.038) significantly increased the risk of patent track sign (Table 3). Factors such as tumor size, the presence of underlying chronic liver disease or cirrhosis, a history of anticoagulation treatment, biopsy gun type, platelet level, and INR were not significantly associated with the patent track sign. In the subsequent multivariable analysis, which included significant factors identified in the univariable analysis alongside crucial hemostasis factors (platelet level and INR), the coaxial method with NTP was independently associated with a lower risk of the patent track sign (OR: 0.360, 95% CI: 0.131–0.923; P = 0.038), while subcapsular location again demonstrated an association with a higher risk of the patent track sign (OR: 3.149, 95% CI: 1.059–9.516; P = 0.038).
Table 3. Univariable and multivariable logistic regression analyses for patent track sign.
| Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Age, yrs | 0.972 | 0.938–1.007 | 0.113 | ||||
| Sex | |||||||
| Female | 1.000 | Reference | |||||
| Male | 1.183 | 0.499–2.807 | 0.702 | ||||
| Chronic liver disease or liver cirrhosis | 0.473 | 0.104–1.574 | 0.265 | ||||
| History of anticoagulation medication | 0.328 | 0.050–1.275 | 0.157 | ||||
| Platelet, ×103/µL | 0.996 | 0.991–1.001 | 0.086 | 0.996 | 0.991–1.001 | 0.147 | |
| PT (INR) | 1.670 | 0.007–292.561 | 0.848 | 4.302 | 0.008–2092.561 | 0.642 | |
| Tumor size, cm | 1.119 | 1.001–1.283 | 0.072 | ||||
| Subcapsular location | 2.928 | 1.044–8.148 | 0.038 | 3.149 | 1.059–9.516 | 0.038 | |
| Biopsy protocol | |||||||
| Conventional method | 1.000 | Reference | 1.000 | Reference | |||
| Coaxial method with needle track plugging | 0.358 | 0.139–0.869 | 0.027 | 0.360 | 0.131–0.923 | 0.038 | |
| Biopsy gun type | |||||||
| Semi-automatic | 1.000 | Reference | |||||
| Automatic | 3.047 | 0.785–20.197 | 0.157 | ||||
Variables with a P-value < 0.05 on univariable analysis and crucial hemostasis factors (platelet and INR) were included in the multivariable analysis. During stepwise variable selection using the bidirectional elimination method, none of the four candidate variables were eliminated.
INR = international normalized ratio, OR = odds ratio, CI = confidence interval, PT = prothrombin time
DISCUSSION
To our knowledge, this is the first prospective, randomized study to compare the safety of US-guided PLB between the coaxial method with NTP and conventional method. Patent track signs were less frequently observed when using the coaxial method with NTP than conventional method when acquiring three or more tissue cores. The total procedure time, biopsy-related symptoms, and diagnostic yields were similar between the two methods. No patient experienced clinically significant or delayed bleeding after discharge, which supports the safety of an outpatient basis. Both the biopsy method and subcapsular target lesion location were independently associated with the risk of postbiopsy bleeding.
The patent track sign was significantly less commonly observed in the coaxial method with NTP group than the conventional method group (16.7% vs. 35.8%; P = 0.042). Although the patent track sign is known to resolve spontaneously in most cases, its significance should not be underestimated, for three reasons. First, the patent track sign has been reported to be significantly associated with progression to clinically significant bleeding [17]. Second, the patent track sign might discourage the operator from completing the acquisition of the requested number of tissue cores due to concerns about potential evolution into clinically significant bleeding. It should be noted that among the 19 patients in our study who exhibited the patent track sign after conventional biopsy, operators failed to retrieve the full set of requested tissue cores from two patients (2/19, 10.5%). Thus, even minor bleeding during or after PLB using the conventional method may result in insufficient tissue samples and ultimately prevent patients from receiving appropriate treatments or participating in clinical trials. Third, unlike indirect signs—such as perihepatic hematoma or hemoperitoneum, which remain for some time even if bleeding has stopped—the patent track sign provides a real-time assessment of whether bleeding is ongoing or not. This may help the operator make decisions regarding the safety of discharging patients in the case of outpatient procedures [17,19]. Therefore, considering the expanding application of precision medicine within contemporary medical practices, we advocate for the coaxial method with NTP as a more suitable approach [22].
No patient in either group experienced clinically significant or delayed bleeding after discharge. Although the reported rate of clinically significant bleeding complications after PLB ranges from approximately 0.5%–1.2% [3,7,13,15,16,20], this rate reduced to 0% when using the coaxial method with NTP [28,31,32,33]. We speculate that the absence of clinically significant bleeding, even in the conventional method group, might be attributed to the small sample size. Meanwhile, all observed patent track signs spontaneously resolved within 30 minutes of observation, without progressing to clinically significant bleeding complications. This finding suggests that a 30-minute observation period might be sufficient to exclude potentially significant bleeding, as opposed to the previously suggested 1-hour observation period after US-guided PLB [9]. Furthermore, no patients developed delayed bleeding complications after discharge. While potentially fatal, delayed bleeding complications are known to occur much less frequently than immediate postbiopsy bleeding [35,36,37,38]. Although the exact mechanism for delayed bleeding after PLB remains unclear, the most likely cause is premature clot dissolution at the biopsy site related to impaired liver function and hyperfibrinolysis [18,39]. Our inclusion criteria, which required a fibrinogen level of >100 mg/dL for patients with chronic liver disease or cirrhosis as recommended by the Society of Interventional Radiology consensus guidelines [34], may have aided in excluding patients at a high risk for delayed bleeding. Therefore, we carefully suggest that PLB may remain an outpatient procedure—even when retrieving three or more tissue cores—with strict application of patient selection criteria and the use of the coaxial method with NTP.
Complications specifically related to the NTP procedure itself were rarely reported. We identified a case report where the biliary migration of the injected gelatin slurry led to acalculous cholecystitis in a pediatric patient [40]. In our study, US findings following gelatin slurry injection varied among patients; yet, none showed complications directly related to the NTP procedure (Supplementary Fig. 1). Furthermore, no technical failure was encountered following the application of the coaxial method with NTP and gelatin slurry injection, consistent with the results of previous studies that have documented a high technical success rate for this approach [31,33]. The procedure time for the coaxial method with NTP was comparable to that of the conventional method. In summary, our findings indicate that the coaxial method with NTP does not present any disadvantages over the conventional method.
Our results demonstrate that a subcapsular target lesion location was significantly associated with a higher risk of the patent track sign. The tamponade effect of the intervening liver parenchyma included in the biopsy track can reduce the risk of bleeding. Although a retrospective study reported no significant differences in the frequencies of moderate and severe bleeding between the biopsies of subcapsular and nonsubcapsular masses [3], it did not report or compare the rates of mild bleeding. Since the patent track sign encompasses all degrees of biopsy track bleeding, our results do not contradict those of the previous study. Meanwhile, low platelet levels were associated with the risk of postbiopsy bleeding only when the conventional method was used. We speculate that the impact of low platelet levels might have been mitigated by the external tamponade effect from NTP.
This study has several limitations. First, the coaxial method with NTP was not directly compared with the coaxial method without NTP; instead, it was compared with the conventional method. Our study aimed to explore the advantages of the coaxial method with NTP regarding safety and effectiveness compared with the most prevalent technique, the conventional method. Our focus was not solely on verifying the effects of the additional NTP procedure itself. Second, the biopsy was performed by two expert operators from the same institution. Third, the operators were not blinded to the patients’ coagulation indices, medical history, or comorbidities. Last, the primary endpoint of this study was the patent track sign, not clinically significant bleeding. Clinically significant bleeding is so rare that using it as a primary endpoint would require an impractically large sample size.
In conclusion, compared with the conventional method, the coaxial method in conjunction with NTP resulted in a lower rate of the patent track sign, suggesting a potentially reduced risk of bleeding complications after US-guided PLB when retrieving three or more tissue cores. In the era of precision medicine, the coaxial method with NTP could be considered a viable option for acquiring multiple liver tissues on an outpatient basis.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Choong-kun Lee, Seung-seob Kim.
- Data curation: Ja Kyung Yoon, Hongjeong Yoon, Seung-seob Kim.
- Formal analysis: Ja Kyung Yoon.
- Funding acquisition: Choong-kun Lee, Seung-seob Kim.
- Investigation: Ja Kyung Yoon, Choong-kun Lee, Seung-seob Kim.
- Methodology: Choong-kun Lee.
- Project administration: Seung-seob Kim.
- Resources: Ja Kyung Yoon, Hongjeong Yoon, Hye Jin Choi, Seung-seob Kim.
- Supervision: Seung-seob Kim.
- Validation: Ja Kyung Yoon, Hye Jin Choi.
- Visualization: Ja Kyung Yoon, Choong-kun Lee.
- Writing—original draft: Ja Kyung Yoon.
- Writing—review & editing: Ja Kyung Yoon, Choong-kun Lee, Seung-seob Kim.
Funding Statement: This study was funded by ENGAIN Co., Ltd.
Availability of Data and Material
Data generated or analyzed during the study are available from the corresponding author by request.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2024.0536.
References
- 1.Jing H, Yi Z, He E, Xu R, Shi X, Li L, et al. Evaluation of risk factors for bleeding after ultrasound-guided liver biopsy. Int J Gen Med. 2021;14:5563–5571. doi: 10.2147/IJGM.S328205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JW, Shin SS. Ultrasound-guided percutaneous core needle biopsy of abdominal viscera: tips to ensure safe and effective biopsy. Korean J Radiol. 2017;18:309–322. doi: 10.3348/kjr.2017.18.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potretzke TA, Saling LJ, Middleton WD, Robinson KA. Bleeding complications after percutaneous liver biopsy: do subcapsular lesions pose a higher risk? AJR Am J Roentgenol. 2018;211:204–210. doi: 10.2214/AJR.17.18726. [DOI] [PubMed] [Google Scholar]
- 4.Chi H, Hansen BE, Tang WY, Schouten JN, Sprengers D, Taimr P, et al. Multiple biopsy passes and the risk of complications of percutaneous liver biopsy. Eur J Gastroenterol Hepatol. 2017;29:36–41. doi: 10.1097/MEG.0000000000000731. [DOI] [PubMed] [Google Scholar]
- 5.Neuberger J, Patel J, Caldwell H, Davies S, Hebditch V, Hollywood C, et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut. 2020;69:1382–1403. doi: 10.1136/gutjnl-2020-321299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitchin DR, Del Rio AM, Woods M, Ludeman L, Hinshaw JL. Percutaneous liver biopsy and revised coagulation guidelines: a 9-year experience. Abdom Radiol (NY) 2018;43:1494–1501. doi: 10.1007/s00261-017-1319-9. [DOI] [PubMed] [Google Scholar]
- 7.Cui Z, Wright JD, Accordino MK, Buono D, Neugut AI, Hu JC, et al. Safety, utilization, and cost of image-guided percutaneous liver biopsy among cancer patients. Cancer Invest. 2016;34:189–196. doi: 10.3109/07357907.2016.1166232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao W, Cheng Z, Wang L, Zhao X, Li J, Zhou S. Analysis of risk factors of bleeding complications in percutaneous needle biopsy of liver occupying lesions. Int J Gen Med. 2021;14:2893–2899. doi: 10.2147/IJGM.S313407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Firpi RJ, Soldevila-Pico C, Abdelmalek MF, Morelli G, Judah J, Nelson DR. Short recovery time after percutaneous liver biopsy: should we change our current practices? Clin Gastroenterol Hepatol. 2005;3:926–929. doi: 10.1016/s1542-3565(05)00294-6. [DOI] [PubMed] [Google Scholar]
- 10.Minuk GY, Sutherland LR, Wiseman DA, MacDonald FR, Ding DL. Prospective study of the incidence of ultrasound-detected intrahepatic and subcapsular hematomas in patients randomized to 6 or 24 hours of bed rest after percutaneous liver biopsy. Gastroenterology. 1987;92:290–293. doi: 10.1016/0016-5085(87)90119-3. [DOI] [PubMed] [Google Scholar]
- 11.West J, Card TR. Reduced mortality rates following elective percutaneous liver biopsies. Gastroenterology. 2010;139:1230–1237. doi: 10.1053/j.gastro.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Howlett DC, Drinkwater KJ, Lawrence D, Barter S, Nicholson T. Findings of the UK national audit evaluating image-guided or image-assisted liver biopsy. Part II. Minor and major complications and procedure-related mortality. Radiology. 2013;266:226–235. doi: 10.1148/radiol.12120224. [DOI] [PubMed] [Google Scholar]
- 13.Atwell TD, Smith RL, Hesley GK, Callstrom MR, Schleck CD, Harmsen WS, et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am J Roentgenol. 2010;194:784–789. doi: 10.2214/AJR.08.2122. [DOI] [PubMed] [Google Scholar]
- 14.Terjung B, Lemnitzer I, Dumoulin FL, Effenberger W, Brackmann HH, Sauerbruch T, et al. Bleeding complications after percutaneous liver biopsy: an analysis of risk factors. Digestion. 2003;67:138–145. doi: 10.1159/000071293. [DOI] [PubMed] [Google Scholar]
- 15.Fotiadis N, De Paepe KN, Bonne L, Khan N, Riddell A, Turner N, et al. Comparison of a coaxial versus non-coaxial liver biopsy technique in an oncological setting: diagnostic yield, complications and seeding risk. Eur Radiol. 2020;30:6702–6708. doi: 10.1007/s00330-020-07038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyum JH, Atwell TD, Schmit GD, Poterucha JJ, Schleck CD, Harmsen WS, et al. Incidence and risk factors for adverse events related to image-guided liver biopsy. Mayo Clin Proc. 2016;91:329–335. doi: 10.1016/j.mayocp.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Kim KW, Kim MJ, Kim HC, Park SH, Kim SY, Park MS, et al. Value of “patent track” sign on Doppler sonography after percutaneous liver biopsy in detection of postbiopsy bleeding: a prospective study in 352 patients. AJR Am J Roentgenol. 2007;189:109–116. doi: 10.2214/AJR.07.2071. [DOI] [PubMed] [Google Scholar]
- 18.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 19.Longo JM, Bilbao JI, Barettino MD, Larrea JA, Pueyo J, Idoate F, et al. Percutaneous vascular and nonvascular puncture under US guidance: role of color Doppler imaging. Radiographics. 1994;14:959–972. doi: 10.1148/radiographics.14.5.7991826. [DOI] [PubMed] [Google Scholar]
- 20.Sheth RA, Baerlocher MO, Connolly BL, Dariushnia SR, Shyn PB, Vatsky S, et al. Society of interventional radiology quality improvement standards on percutaneous needle biopsy in adult and pediatric patients. J Vasc Interv Radiol. 2020;31:1840–1848. doi: 10.1016/j.jvir.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Dodd GD, 3rd, Esola CC, Memel DS, Ghiatas AA, Chintapalli KN, Paulson EK, et al. Sonography: the undiscovered jewel of interventional radiology. Radiographics. 1996;16:1271–1288. doi: 10.1148/radiographics.16.6.8946535. [DOI] [PubMed] [Google Scholar]
- 22.Overman MJ, Modak J, Kopetz S, Murthy R, Yao JC, Hicks ME, et al. Use of research biopsies in clinical trials: are risks and benefits adequately discussed? J Clin Oncol. 2013;31:17–22. doi: 10.1200/JCO.2012.43.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherukuri AR, Lubner MG, Zea R, Hinshaw JL, Lubner SJ, Matkowskyj KA, et al. Tissue sampling in the era of precision medicine: comparison of percutaneous biopsies performed for clinical trials or tumor genomics versus routine clinical care. Abdom Radiol (NY) 2019;44:2074–2080. doi: 10.1007/s00261-018-1702-1. [DOI] [PubMed] [Google Scholar]
- 24.Hatfield MK, Beres RA, Sane SS, Zaleski GX. Percutaneous imaging-guided solid organ core needle biopsy: coaxial versus noncoaxial method. AJR Am J Roentgenol. 2008;190:413–417. doi: 10.2214/AJR.07.2676. [DOI] [PubMed] [Google Scholar]
- 25.Riley SA, Irving HC, Axon ATR, Ellis WR, Lintott DJ, Losowsky MS. Percutaneous liver biopsy with plugging of needle track: a safe method for use in patients with impaired coagulation. Lancet. 1984;324:436. doi: 10.1016/s0140-6736(84)92910-6. [DOI] [PubMed] [Google Scholar]
- 26.Tobin MV, Gilmore IT. Plugged liver biopsy in patients with impaired coagulation. Dig Dis Sci. 1989;34:13–15. doi: 10.1007/BF01536147. [DOI] [PubMed] [Google Scholar]
- 27.Smith TP, McDermott VG, Ayoub DM, Suhocki PV, Stackhouse DJ. Percutaneous transhepatic liver biopsy with tract embolization. Radiology. 1996;198:769–774. doi: 10.1148/radiology.198.3.8628869. [DOI] [PubMed] [Google Scholar]
- 28.Fandrich CA, Davies RP, Hall PM. Small gauge gelfoam plug liver biopsy in high risk patients: safety and diagnostic value. Australas Radiol. 1996;40:230–234. doi: 10.1111/j.1440-1673.1996.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 29.Zins M, Vilgrain V, Gayno S, Rolland Y, Arrivé L, Denninger MH, et al. US-guided percutaneous liver biopsy with plugging of the needle track: a prospective study in 72 high-risk patients. Radiology. 1992;184:841–843. doi: 10.1148/radiology.184.3.1509076. [DOI] [PubMed] [Google Scholar]
- 30.Crummy AB, McDermott JC, Wojtowycz M. A technique for embolization of biopsy tracts. AJR Am J Roentgenol. 1989;153:67–68. doi: 10.2214/ajr.153.1.67. [DOI] [PubMed] [Google Scholar]
- 31.Kamphuisen PW, Wiersma TG, Mulder CJ, de Vries RA. Plugged-percutaneous liver biopsy in patients with impaired coagulation and ascites. Pathophysiol Haemost Thromb. 2002;32:190–193. doi: 10.1159/000070426. [DOI] [PubMed] [Google Scholar]
- 32.Kim SJ, Won JH, Kim YB, Wang HJ, Kim BW, Kim H, et al. Plugged percutaneous biopsy of the liver in living-donor liver transplantation recipients suspected to have graft rejection. Acta Radiol. 2017;58:771–777. doi: 10.1177/0284185116673121. [DOI] [PubMed] [Google Scholar]
- 33.Singhal S, M D P, Inuganti S, Botcha S, Deepashree DT, Uthappa MC. Percutaneous ultrasound-guided plugged liver biopsy - a single-centre experience. Pol J Radiol. 2021;86:e239–e245. doi: 10.5114/pjr.2021.105852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients Undergoing percutaneous image-guided interventions—part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30:1168–1184.e1. doi: 10.1016/j.jvir.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Huang JY, Lu Q, Liu JB. Delayed hepatic rupture post ultrasound-guided percutaneous liver biopsy: a case report. Medicine (Baltimore) 2018;97:e9955. doi: 10.1097/MD.0000000000009955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren FY, Piao XX, Jin AL. Delayed hemorrhage from hepatic artery after ultrasound-guided percutaneous liver biopsy: a case report. World J Gastroenterol. 2006;12:4273–4275. doi: 10.3748/wjg.v12.i26.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed A, Samuels SL, Keeffe EB, Cheung RC. Delayed fatal hemorrhage from pseudoaneurysm of the hepatic artery after percutaneous liver biopsy. Am J Gastroenterol. 2001;96:233–237. doi: 10.1111/j.1572-0241.2001.03482.x. [DOI] [PubMed] [Google Scholar]
- 38.Reichert CM, Weisenthal LM, Klein HG. Delayed hemorrhage after percutaneous liver biopsy. J Clin Gastroenterol. 1983;5:263–266. doi: 10.1097/00004836-198306000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, et al. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–1046. doi: 10.1002/hep.21303. [DOI] [PubMed] [Google Scholar]
- 40.Riddle C, Ahmed B, Doyle J, Connolly BL. Migration of Gelfoam to the gallbladder after liver biopsy. Pediatr Radiol. 2008;38:806–809. doi: 10.1007/s00247-008-0818-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated or analyzed during the study are available from the corresponding author by request.