Abstract
This research aimed to examine the effect of daily intake of food containing Lactococcus lactis strain T21 (T21) on skin conditions and inflammation-related markers in healthy adults who experience itching because of dry skin and have an atopic predisposition. A randomized, double-blind, placebo-controlled parallel-group study was conducted on 44 subjects aged 20 to 64 years. Subjects were randomly assigned to receive a T21-containing food or placebo daily for 8 weeks. The hydration of stratum corneum, trans-epidermal water loss, skin brightness (L*), skin redness (a*), and quality of life (QOL) scores were evaluated. Moreover, SCCA2, Th1/Th2, peripheral blood eosinophil count, TGF-β1, TARC, total IgE, and LDH were measured as inflammation-related markers. The results showed that, compared with the placebo, food containing T21 reduced trans-epidermal water loss in the neck and increased neck skin brightness (L*) after 8 weeks of consumption. Furthermore, a stratified analysis in subjects with a history of atopy showed improvements in neck skin redness (a*) and skin-related QOL. No significant improvement in inflammation-related markers was observed. Intake of food containing T21 for 8 weeks in healthy adult with atopic predisposition was suggested to improve skin barrier function in the neck and brightness in the neck skin. Furthermore, the results also suggested that it had the effect of improving rough skin and reducing discomfort due to dryness in healthy adults with a history of atopy.
Keywords: Lactococcus lactis strain T21, lactic acid bacteria, skin condition, trans-epidermal water loss, quality of life, inflammation-related marker
INTRODUCTION
In recent years, the increase in the incidences of allergic diseases and their onset at a younger age have become social problems. Immunotherapy has shown promise as a curative therapy for allergic diseases; however, a cure has yet to be established. Meanwhile, improving dietary habits to prevent the onset of allergic diseases or alleviate the symptoms of those who develop them is one of the most powerful means of prevention and improvement. The intestinal flora is deeply involved in the onset of allergic diseases [1]; however, recent studies on the efficacy of heat-sterilized lactic acid bacteria and bifidobacteria, which directly exert biological regulatory effects, such as immunostimulation without involving the intestinal flora, have been reported. In particular, lactic acid bacteria, a representative probiotic, are known to exhibit beneficial effects in maintaining health, such as regulating intestinal function, improving sleep quality, and modulating immune function [2,3,4,5]; in addition to live bacteria, heat-sterilized bacteria are also used in various food forms. Lactococcus lactis strain T21 (T21) has a high capacity for promoting the production of interleukin-12 (IL-12), an immunomodulator that suppresses allergic reactions such as pollinosis [6]. Furthermore, symptoms of pollinosis and perennial rhinitis were found to be alleviated by the intake of food containing heat-sterilized T21 [7]. In this study, the effects of T21 on skin conditions and inflammation-related markers were evaluated in a randomized, double-blind, placebo-controlled parallel-group comparative study after 8 weeks of continuous intake of food containing this lactic acid bacteria, with the aim of verifying the efficacy of T21 against cutaneous allergic reactions.
MATERIALS AND METHODS
Subjects
The selected subjects were healthy males and females who met the selection criteria described below and did not violate the exclusion criteria. The subjects were briefed on the study prior to participation, understood and agreed with the purpose of the study, and gave their written consent. The following 4 points were established as the inclusion criteria: (1) Males or females aged between 20 and 64 years, (2) persons who experience itching associated with dry skin, (3) persons who had been judged by a physician as not requiring treatment for atopic dermatitis, and (4) persons with specific predisposing factors for atopy. The predisposing factors were (i) a history of bronchial asthma, allergic rhinitis, conjunctivitis, or atopic dermatitis or a blood relative (within the third degree of consanguinity) currently suffering from any of these conditions; (ii) a history of bronchial asthma, allergic rhinitis, conjunctivitis, atopic dermatitis and not currently receiving treatment, and (iii) a tendency to produce IgE antibodies. The following 20 items were established as the exclusion criteria: (1) persons who regularly consumed Lactobacillus-rich foods (such as yogurt, Lactobacillus drinks, cheese, kimchi, pickles), health foods, and pharmaceutical products for a minimum of three times a week; (2) persons who routinely consumed health supplements (such as supplements claiming allergy-improving effects) that may affect the test results; (3) persons who had experience with cosmetic treatments (such as Botox injections, hyaluronic acid injections, collagen injections, and photo facials) that would affect the measurement area; (4) persons who had undergone cosmetic medical treatment or hormone replacement therapy on the skin at the measurement site (including taking oral contraceptives (pills)) in the year prior to the screening tests; (5) persons who had undergone beauty treatments, scrubbing, or hair removal on the skin at the measurement area during the month prior to the screening tests or those who planned to perform these activities during the study period; (6) persons who planned to work outdoors for long periods of time, engage in leisure, exercise, or get a tan* from one month prior to the screening tests to the end of the study (*a short period of tanning within the scope of daily life was considered acceptable); (7) persons who had the habit of washing their bodies by applying abrasive materials to the measurement site, such as the use of nylon towels (however, towels made of a soft material were considered acceptable); (8) persons who regularly used bath salts (guide: at least one day a week) or were in the habit of bathing in hot springs at least one day a month; (9) persons who had scars, inflammation, or other diseases (such as atopic dermatitis and acne) on the skin at the measurement site that may affect the tests; (10) persons who felt that skin irritation affecting the skin test at the measurement site occurs around menstruation; (11) persons who worked day and night shifts or multiple night shifts during the study period; (12) persons who planned to travel abroad during the study period; (13) persons with serious diseases such as diabetes, brain disease, circulatory system disease, liver disease, renal disease, cardiac disease, or other diseases affecting the secretion of sex hormones or those with a history of such diseases; (14) persons who were at risk of developing allergy-like symptoms to the test food; (15) persons who had a disease for which he/she was receiving treatment that would affect the evaluation of this study or those who had a pre-existing serious disease that requires medication treatment; (16) persons whose physical measurements, physical examination values, and clinical laboratory values in the screening tests deviated significantly from the reference range; (17) persons who had participated in another clinical trial within one month prior to giving consent to participate in this study or were scheduled to participate in another clinical trial after giving consent to participate in this study; (18) persons who were pregnant or breastfeeding or planning to become pregnant or breastfeed during the study period; (19) persons who were deemed ineligible as subjects based on the results of their responses to a lifestyle questionnaire, and (20) persons deemed ineligible to be a subject by the principal investigator for other reasons.
Test foods
T21 was heat-sterilized and then compressed together with reduced maltose syrup, maltodextrins, powdered cellulose, calcium stearate, and fine-grained silicon dioxide to produce the T21-containing food. A tablet without T21 was used as placebo food. Subjects received two tablets (400 mg) per day of test food. One grain of the T21-containing food contained 25 mg of T21 powder. No differences in the nutrient composition of each test food that could be considered problematic was noted. L. lactis strain T21 is a plant-derived lactic acid bacterium. L. lactis is a safe species with a long history of being used in fermented milk and health foods [8]. The T21 used for the test food was cultured at 30°C for 18 hr.
Study design
This study was a randomized, double-blind, placebo-controlled, parallel-group study. An allocation manager not directly involved in the study created an allocation chart using a randomization table, and subjects were randomly assigned to the T21 group or placebo group accordingly. The allocation chart was sealed by the allocation manager and stored in a sealed envelope until the end of the study. Blinding was maintained for all parties except the test food allocation manager. In establishing the number of subjects, we referred to a similar interventional study [9] of fermented lactic acid bacteria-degrading substances for atopic dermatitis. Because the number of subjects per group in the reference study was 19 to 20, the number of subjects in this study was set to 22 subjects per group or 44 subjects in total, in anticipation of dropout cases. Before the intervention, candidate subjects were asked to complete a lifestyle questionnaire and screening tests, including a physical condition check (medical interview), physical examination, clinical laboratory tests (blood and urine), dermatologist’s diagnosis, confirmation of predisposition to atopy, quality of life (QOL) questionnaire, and infectious disease test. Subjects suitable for the purpose of the study were selected and assigned from the results of the screening tests of candidate subjects who met the selection criteria and did not violate the exclusion criteria. After conducting inflammation-related marker measurements and skin measurements as a pre-consumption examination, subjects were asked to begin ingesting the test food and keeping a daily diary during the period of consumption. At weeks 4 and 8 of test food intake, the subjects visited the hospital and completed a physical condition checkup, physical examination, clinical laboratory tests, QOL questionnaire, and inflammation-related marker and skin measurements.
Efficacy assessment
To evaluate the efficacy of the test foods, skin measurements and QOL questionnaires were used as primary endpoints, and inflammation-related markers were used as secondary endpoints. The primary endpoints, i.e., skin measurements, were the stratum corneum water content, trans-epidermal water loss (TEWL), skin brightness (L*), and redness (a*). Subjects were acclimatized to a constant temperature and humidity room (20°C ± 1°C, 50 ± 5% humidity) for a minimum of 20 min with the measurement site exposed after washing. Skin measurements were then obtained at 4 locations: cheek, neck, upper arm, and shin. The stratum corneum moisture content was measured using a Corneometer® CM825 (Courage + Khazaka electronic GmbH). TEWL was measured using a Tewameter® TM300 (Courage + Khazaka electronic GmbH) to measure transpiration (g/hm2). Skin brightness (L*) and redness (a*) were measured using a CM-2600d (Konica Minolta Japan, Inc., Tokyo, Japan), with subjects in a lateral position. The SKINDEX16 [10], Dermatology Life Quality Index (DLQI) Japan version [11, 12], and VAS questionnaires on skin condition were used for the subjective evaluation of skin condition, and the MOS 36-Item Short-Form Health Survey (SF-36) [13, 14] was used for the subjective evaluation of health condition. Known biomarkers of atopic dermatitis include squamous cell carcinoma antigen 2 (SCCA2), a serine protease inhibitor family molecule produced by epithelial cells upon IL-4 or IL-13 stimulation; Th1/Th2, which indicates the ratio of Th1 cells involved in cellular immunity to Th2 cells involved in humoral immunity among helper T (Th) cells; peripheral blood eosinophil count, which is an effector of allergic inflammation; transforming growth factor-β1 (TGF-β1), which has anti-inflammatory effects and various functions; thymus and activation-regulated chemokine (TARC), a ligand for the chemokine receptor CCR4 expressed on Th2 cells; total IgE, which is associated with an immediate allergic response, and lactate dehydrogenase (LDH) that is released because of cell injuries. Therefore, these items were measured and evaluated as inflammation-related markers, i.e., the secondary endpoints. The subjects for the efficacy analysis were those who completed the prescribed study schedule and all study details, and those who met the following exclusion criteria for analysis were excluded: (1) persons with a test food intake rate of less than 80%; (2) persons who had exhibited conspicuous behavior that undermined the reliability of test results, such as missing diary entries; (3) persons who were found after inclusion in the study to have met the exclusion criteria or who were unable to comply with the restrictions during the study period; and (4) any other person for whom a clear, appropriate reason for them to be excluded existed.
Safety assessment
The principal investigator treated all unfavorable or unintended injuries or signs of such injuries or illnesses that occurred in the subjects during the intake period as adverse events. In addition, the following laboratory tests were performed. Hematological tests included measurement of the white blood cell count, red blood cell count, hemoglobin (Hb), hematocrit (Ht), and the platelet count (PLT). Blood biochemical tests measured total protein (TP), albumin (Alb), total bilirubin (TB), direct bilirubin (D-B), indirect bilirubin (I-B), alkaline phosphatase (ALP), aspartate aminotransferase (AST (GOT)), alanine aminotransferase (ALT (GPT)), lactate dehydrogenase (LDH), gamma-glutamyl transpeptidase (γ-GT), total cholesterol (TC), triglycerides (TG), HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), urea nitrogen (UN), creatinine (Cr), uric acid, Na, K, Cl, and blood glucose (GLU). Urinalysis measured proteins, glucose, and occult blood response.
Whether or not the test results of individual subjects were abnormal fluctuations that could be classified as adverse events were judged by the principal investigator based on the reference values established by the study site and referring to the criteria for abnormal fluctuations established by the Japanese Society of Chemotherapy [15], CTCAE v5.0-JCOG, and the judgment classification of the Japanese Society of Ningen Dock (revised April 1, 2020).
Ethics
The study was conducted after the Medical Station Clinic Ethics Committee (IRB No. 20000022) reviewed and approved the scientific and ethical appropriateness of conducting the study (approval date: October 28, 2021). This trial was registered in UMIN-CRT as UMIN000046242.
The study was conducted in the spirit of the Declaration of Helsinki (revised in October 2013), through observance of the Ethical Guidelines for Life Science and Medical Research involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology/Ministry of Health, Labour and Welfare/Ministry of Economy, Trade and Industry Notification No. 1, March 23, 2021), Guidance on Ethical Guidelines for Life Science and Medical Research involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology/Ministry of Health, Labour and Welfare/Ministry of Economy, Trade and Industry, April 16, 2021), and the Act on the Protection of Personal Information (Act No. 57, May 30, 2003), as well as strict adherence to the approved research plan.
Study implementation
The study was commissioned to a third-party organization, EP Mediate Co., Ltd., and was conducted at the Fukushima Health Care Center and DRC Clinic from October 2021 to August 2022.
Statistical analysis
Even values that appeared to be outliers in test and measurement values were used for evaluation. Test values and changes from baseline values at each time point before and after intake for comparisons of the characteristics of each subject group are shown as means ± standard deviation or standard error (gender is shown as the number of subjects). The χ2 test was used for intergroup comparisons of gender. A two-sample t-test was used for intergroup comparisons of changes from baseline levels at each post-intake time point for stratum corneum water content, TEWL, skin brightness (L*), and redness (a*), as well as for the SF-36, VAS questionnaire, and various inflammation-related markers. One-sample t-tests were used for intragroup comparisons of the changes from baseline in measurements at each post-intake time point, and Mann–Whitney’s U test was used to compare the changes from baseline at each post-intake time point for SKINDEX16 and DLQI. Furthermore, in each group, the amount of change from before intake at each time point after intake was evaluated using Wilcoxon’s signed rank test. Atopic dermatitis causes dry skin due to a decrease in skin barrier function and moisturizing factors. This condition leads to itchy skin because of nonspecific irritation and facilitates the entry of various allergens, which may induce dermatitis [16]. Moreover, in cases with a history of atopic symptoms, even seemingly normal skin is histologically prone to repeated inflammation due to residual inflammatory cells, which can be triggered by external or internal factors [17, 18]. Therefore, we conducted a subgroup analysis focusing on the amount of change from before intake at 8 weeks for a group of subjects with a history of atopic dermatitis themselves or among blood relatives, as they were considered to be more likely to develop dry skin. The same statistical methods as above were used for intergroup and intragroup comparisons in the subgroup analysis. Excel for Microsoft 365 (Microsoft, Redmond, WA, USA) and IBM SPSS Statistics version 26 (IBM, Armonk, NY, USA) were used as statistical analysis software. The significance level for the test was 5% on both sides.
RESULTS
Subjects
The process from subject selection to analysis is shown in a flowchart of the study in Fig. 1. A screening test was conducted on 93 subjects who provided written consent to participate in the study. Based on the results of the screening test, 44 subjects (22 in the T21 group and 22 in the placebo group) who met the inclusion criteria and did not violate the exclusion criteria were selected as eligible subjects for the study and underwent before intake tests. Although several subjects had systolic blood pressure and laboratory values outside the reference range, all were reviewed by the principal investigator on a subject-by-subject basis and deemed acceptable for study participation before being included in the study. All 44 subjects started their intake of the test foods; however, one subject in the T21 group dropped out of the study of his own free will, and one subject was withdrawn from the study by the principal investigator because of a deviation from the research plan. A total of 42 subjects completed the prescribed study schedule and study content. After the treatment of the subjects for analysis was reviewed before opening the allocation chart, two subjects (placebo group) were excluded from the analysis because their intake rate of the test food was below 80%, and 40 subjects (20 in the T21 group and 20 in the placebo group) were included in the efficacy analysis. The demographics of the subjects in the efficacy analysis are shown in Table 1. There were no significant differences between groups in any of the items. As for safety, all 44 subjects were included in the adverse event analysis, and 42 subjects were included in the measurements and laboratory value analyses.
Fig. 1.
Flow chart showing the trial design.
Table 1. Baseline characteristics of the subjects.
| Item | Placebo group (n=20) | T21 group (n=20) | p-value* |
|---|---|---|---|
| Age (years) | 45.7 ± 9.8 | 43.4 ± 11.5 | 0.50 |
| Gender (number of people) | Male: 9/Female: 11 | Male: 7/Female: 13 | 0.75 |
| Height (cm) | 162.57 ± 9.25 | 162.37 ± 8.53 | 0.94 |
| Body weight (kg) | 59.11 ± 9.77 | 59.19 ± 10.44 | 0.98 |
| BMI (kg/m2) | 22.26 ± 2.41 | 22.30 ± 2.31 | 0.95 |
| Systolic blood pressure (mmHg) | 113.8 ± 14.9 | 115.7 ± 11.1 | 0.65 |
| Diastolic blood pressure (mmHg) | 75.0 ± 10.6 | 79.3 ± 8.8 | 0.71 |
| Pulse (bpm) | 78.8 ± 13.4 | 77.3 ± 11.5 | 0.72 |
Gender is indicated by the number of subjects, and other values are indicated as means ± standard deviation.
*Placebo and T21 groups were compared by the two-sample t-test (χ2 test for gender) between groups.
Analysis of data on primary endpoints and secondary endpoints
The primary endpoints of skin measurements (stratum corneum water content, TEWL, skin brightness (L*), and redness (a*)) and QOL questionnaires (SKINDEX16, DLQI, SF-36, VAS questionnaire) were evaluated for changes after the intervention in both groups.
No significant differences in stratum corneum water content were observed between groups at any of the test weeks. In an intragroup comparison, the T21 group showed a significant decrease (deterioration) in stratum corneum water content on the upper arms at week 4 compared with before intake. The placebo group showed a significant increase (improvement) in stratum corneum water content on the cheek at week 8 and a significant decrease (deterioration) on the shin at week 4 (data not shown).
Table 2A shows the amount of change in TEWL. In the neck, the T21 group showed a significant decrease (improvement) in TEWL compared with the placebo group at all time points. In the shin, the T21 group showed a significant increase (deterioration) compared with the placebo group at week 4. In intragroup comparisons, the T21 group showed a significant decrease (improvement) in TEWL at week 8 on the neck and a significant increase (deterioration) in TEWL at all time points in the upper arms and shin. The placebo group showed a significant increase (deterioration) in TEWL at week 8 in the neck and shin and a significant increase (deterioration) at all time points in the upper arm.
Table 2. Skin measurements and changes in the placebo and T21 groups.
| (A) TEWL | ||||||
|---|---|---|---|---|---|---|
| Measurement site | Intervention period | Placebo group (n=20) | T21 group (n=20) | p-value | ||
| (weeks) | ||||||
| Measurement (g/h • m2) | Change value | Measurement (g/h • m2) | Change value | (Between-group) | ||
| Cheek | 0 | 18.25 ± 1.38 | - | 16.91 ± 1.38 | - | - |
| 4 | 18.97 ± 1.28 | 0.73 ± 1.11 | 18.20 ± 1.59 | 1.28 ± 0.82 | 0.69 | |
| 8 | 17.86 ± 0.87 | −0.39 ± 1.06 | 16.68 ± 1.06 | −0.23 ± 0.71 | 0.90 | |
| Neck | 0 | 9.64 ± 0.61 | - | 12.27 ± 1.23 | - | - |
| 4 | 10.22 ± 0.67 | 0.58 ± 0.50 | 10.60 ± 0.63 | −1.67 ± 0.81 | 0.02* | |
| 8 | 11.07 ± 0.80# | 1.43 ± 0.55 | 10.92 ± 0.90# | −1.35 ± 0.51 | <0.01** | |
| Upper arm | 0 | 8.79 ± 0.36 | - | 8.97 ± 0.48 | - | - |
| 4 | 9.97 ± 0.40# | 1.18 ± 0.49 | 9.84 ± 0.48# | 0.87 ± 0.36 | 0.61 | |
| 8 | 10.21 ± 0.30## | 1.42 ± 0.38 | 10.61 ± 0.49## | 1.65 ± 0.33 | 0.65 | |
| Shin | 0 | 9.72 ± 0.31 | - | 9.34 ± 0.25 | - | - |
| 4 | 9.93 ± 0.31 | 0.21 ± 0.33 | 10.63 ± 0.35## | 1.29 ± 0.24 | 0.01* | |
| 8 | 10.75 ± 0.27## | 1.02 ± 0.35 | 10.50 ± 0.32## | 1.16 ± 0.27 | 0.76 | |
| (B) Brightness (L*) | ||||||
| Measurement site | Intervention period | Placebo group (n=20) | T21 group (n=20) | p-value | ||
| (weeks) | ||||||
| Measurement | Change value | Measurement | Change value | (Between-group) | ||
| Cheek | 0 | 63.06 ± 0.63 | - | 63.44 ± 0.78 | - | - |
| 4 | 63.12 ± 0.63 | 0.06 ± 0.05 | 63.67 ± 0.71 | 0.24 ± 0.13 | 0.20 | |
| 8 | 63.35 ± 0.63## | 0.29 ± 0.06 | 63.96 ± 0.72## | 0.52 ± 0.11 | 0.08 | |
| Neck | 0 | 61.92 ± 0.70 | - | 61.68 ± 0.71 | - | - |
| 4 | 61.94 ± 0.66 | 0.02 ± 0.07 | 61.96 ± 0.69## | 0.28 ± 0.09 | 0.03* | |
| 8 | 61.98 ± 0.70 | 0.07 ± 0.12 | 62.09 ± 0.72## | 0.41 ± 0.12 | 0.04* | |
| Upper arm | 0 | 66.87 ± 0.41 | - | 66.40 ± 0.57 | - | - |
| 4 | 66.98 ± 0.40 | 0.11 ± 0.10 | 66.66 ± 0.54 | 0.25 ± 0.14 | 0.43 | |
| 8 | 67.24 ± 0.39## | 0.37 ± 0.13 | 66.90 ± 0.53## | 0.50 ± 0.16 | 0.55 | |
| Shin | 0 | 68.55 ± 0.79 | - | 68.49 ± 0.70 | - | - |
| 4 | 68.48 ± 0.77 | −0.07 ± 0.10 | 68.67 ± 0.71 | 0.17 ± 0.10 | 0.16 | |
| 8 | 68.75 ± 0.76 | 0.20 ± 0.15 | 68.87 ± 0.71## | 0.37 ± 0.11 | 0.35 | |
| (C) Redness (a*) | ||||||
| Measurement site | Intervention period | Placebo group (n=20) | T21 group (n=20) | p-value | ||
| (weeks) | ||||||
| Measurement | Change value | Measurement | Change value | (Between-group) | ||
| Cheek | 0 | 8.87 ± 0.41 | - | 9.26 ± 0.50 | - | - |
| 4 | 8.87 ± 0.34 | 0.01 ± 0.20 | 9.35 ± 0.52 | 0.09 ± 0.18 | 0.75 | |
| 8 | 8.85 ± 0.40 | −0.01 ± 0.12 | 9.01 ± 0.59 | −0.24 ± 0.18 | 0.29 | |
| Neck | 0 | 8.00 ± 0.54 | - | 7.59 ± 0.48 | - | - |
| 4 | 8.55 ± 0.53## | 0.55 ± 0.14 | 8.11 ± 0.52## | 0.52 ± 0.21 | 0.91 | |
| 8 | 8.51 ± 0.69 | 0.51 ± 0.27 | 7.68 ± 0.64 | 0.09 ± 0.27 | 0.28 | |
| Upper arm | 0 | 5.01 ± 0.30 | - | 4.91 ± 0.28 | - | - |
| 4 | 5.04 ± 0.26 | 0.03 ± 0.18 | 5.22 ± 0.33 | 0.31 ± 0.19 | 0.29 | |
| 8 | 4.76 ± 0.25 | −0.24 ± 0.17 | 5.02 ± 0.28 | 0.12 ± 0.17 | 0.14 | |
| Shin | 0 | 3.16 ± 0.37 | - | 3.13 ± 0.32 | - | - |
| 4 | 3.33 ± 0.37 | 0.17 ± 0.13 | 3.14 ± 0.34 | 0.01 ± 0.09 | 0.34 | |
| 8 | 3.33 ± 0.37 | 0.17 ± 0.18 | 2.89 ± 0.34 | −0.23 ± 0.12 | 0.07 | |
The “changes values Δ” are obtained by subtracting the value at the observation before ingestion from the value at 4 or 8 weeks after ingestion.
Values indicated as means ± standard error.
Between-group comparisons: *p<0.05, **p<0.01.
Intra-group comparisons: #p<0.05, ##p<0.01.
TEWL: trans-epidermal water loss.
Table 2B and 2C show the amount of change in skin brightness (L*) and redness (a*). In terms of the change in skin brightness (L*), the T21 group showed a significant increase (improvement) at all time points on the neck compared with the placebo group. In intragroup comparisons, the T21 group showed an increase (improvement) at all time points on the neck, as well as a significant increase (improvement) at week 8 on the cheeks, upper arms, and shin. The placebo group showed a significant increase (improvement) at week 8 in the cheeks and upper arms. Comparison of the change in skin redness (a*) showed no significant difference between the two groups at all time points. In an intragroup comparison relative to before intake, both groups showed a significant increase (deterioration) at week 4 on the neck.
No significant differences were found between the T21 group and placebo group in any of the QOL questionnaires (SKINDEX16, DLQI, SF-36, or VAS). Each of the questionnaires included items that showed significant improvement in the T21 group and placebo group in intragroup comparisons relative to before intake (data not shown).
In the secondary endpoint, i.e., inflammation-related markers, the two groups were compared in terms of change due to the intervention: the T21 group showed a significant increase in TARC compared with the placebo group, whereas no significant differences were observed in the other items. In intragroup comparisons relative to before intake, LDH in the T21 group was significantly reduced (improved) at week 8, but no significant changes were observed in the other items. No significant changes were observed in the placebo group (Table 3).
Table 3. Inflammation-related markers and changes in the placebo and T21 groups.
| Item | Intervention period | Placebo group (n=20) | T21 group (n=20) | p-value | ||
|---|---|---|---|---|---|---|
| (weeks) | ||||||
| Measurement | Change value | Measurement | Change value | (Between-group) | ||
| SCCA2 (ng/mL) | 0 | 0.61 ± 0.08 | - | 0.92 ± 0.22 | - | - |
| 4 | 0.59 ± 0.10 | −0.03 ± 0.04 | 0.81 ± 0.15 | −0.11 ± 0.08 | 0.32 | |
| 8 | 0.51 ± 0.12 | −0.11 ± 0.05 | 0.81 ± 0.27 | −0.11 ± 0.08 | 0.96 | |
| Th1/Th2 | 0 | 9.92 ± 1.65 | - | 10.34 ± 1.17 | - | - |
| 4 | 8.19 ± 1.12 | −1.73 ± 1.00 | 10.47 ± 1.26 | 0.13 ± 0.81 | 0.16 | |
| 8 | 9.56 ± 1.35 | −0.36 ± 0.94 | 11.21 ± 0.98 | 0.87 ± 1.00 | 0.38 | |
| Peripheral blood | 0 | 119.0 ± 15.5 | - | 150.0 ± 30.5 | - | - |
| eosinophil count (/μL) | 4 | 116.5 ± 14.7 | −2.5 ± 11.7 | 146.5 ± 33.7 | −3.5 ± 15.5 | 0.96 |
| 8 | 119.0 ± 14.0 | 0.0 ± 10.6 | 168.5 ± 35.4 | 18.5 ± 17.6 | 0.37 | |
| TGF-β1 (pg/mL) | 0 | 5,651.0 ± 667.8 | - | 3,642.6 ± 357.6 | - | - |
| 4 | 5,219.6 ± 728.5 | −431.4 ± 465.6 | 4,024.2 ± 416.6 | 381.6 ± 255.0 | 0.13 | |
| 8 | 5,327.3 ± 658.6 | −323.7 ± 468.0 | 3,453.9 ± 348.8 | −188.8 ± 335.5 | 0.82 | |
| TARC (pg/mL) | 0 | 255.1 ± 32.7 | - | 374.3 ± 74.8 | - | - |
| 4 | 316.2 ± 98.8 | 61.1 ± 80.1 | 343.0 ± 49.7 | −31.4 ± 31.2 | 0.29 | |
| 8 | 216.2 ± 23.6 | −38.9 ± 19.1 | 393.7 ± 82.2 | 19.4 ± 15.3 | 0.02* | |
| Total IgE (IU/mL) | 0 | 155.5 ± 53.4 | - | 401.9 ± 189.2 | - | - |
| 4 | 147.8 ± 47.5 | −7.7 ± 6.5 | 360.3 ± 168.1 | −41.6 ± 24.2 | 0.18 | |
| 8 | 148.4 ± 49.0 | −7.1 ± 7.0 | 349.1 ± 157.5 | −52.8 ± 35.0 | 0.21 | |
| LDH (U/L) | 0 | 180.5 ± 5.5 | - | 178.1 ± 6.9 | - | - |
| 4 | 177.5 ± 4.6 | −3.0 ± 4.4 | 172.9 ± 6.3 | −5.2 ± 2.9 | 0.67 | |
| 8 | 170.0 ± 5.8 | −10.5 ± 5.4 | 166.7 ± 8.5## | −11.5 ± 3.0 | 0.87 | |
The “changes values Δ” are obtained by subtracting the value at the observation before ingestion from the value at 4 or 8 weeks after ingestion.
Values indicated as means ± standard error.
Between-group comparisons: *p<0.05.
Intra-group comparisons: ##p<0.01.
SCCA2: squamous cell carcinoma antigen 2; TGF-β1: transforming growth factor-β1; TARC: thymus and activation-regulated chemokine; LDH: lactate dehydrogenase.
Subgroup analysis
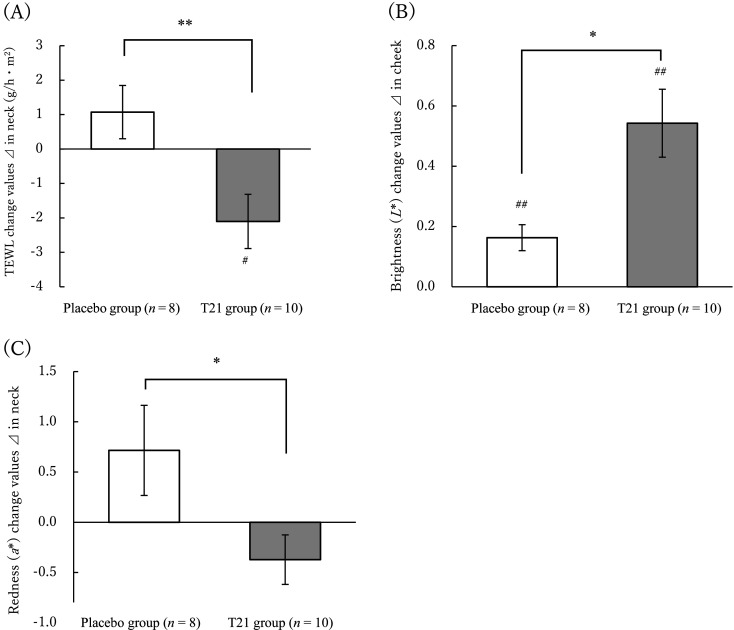
The efficacy of the intervention was assessed in the population with a history of atopic dermatitis themselves or among blood relatives, as they were more prone to dry skin. This population consisted of 10 and 8 subjects from the T21 and placebo groups, respectively. As with the overall population, the T21 group showed a significant decrease (improvement) compared with the placebo group in the amount of change in TEWL on the neck, and a significant improvement was seen in the T21 group in an intragroup comparison relative to before intake (Fig. 2A). The skin brightness (L*) was significantly increased (improved) in the T21 group compared with the placebo group on the cheeks, and intragroup comparisons showed significant improvement in both groups compared with the before intake levels (Fig. 2B). In terms of the amount of change in skin redness (a*), the T21 group showed a significant decrease (improvement) on the neck compared with the placebo group (Fig. 2C). In addition, on the DLQI, a QOL questionnaire, the T21 group showed a significant decrease (improvement) in scores on the leisure-related items compared with those of the placebo group. In an intragroup comparison relative to before intake, there were significant improvements in the T21 group in the daily life DLQI total score (Fig. 3).
Fig. 2.
Changes in the skin measurements of the placebo and T21 groups (change value Δ).
Statistical analysis was performed using the subjects with a history of atopic dermatitis in themselves or in blood relatives. The “changes values Δ” are obtained by subtracting the value at the observation before ingestion from the value at 8 weeks after ingestion.
(A) Trans-epidermal water loss (TEWL) change in values in neck, (B) Brightness (L*) change in values in cheek, (C) Redness (a*) change values in neck.
The Δ values are means ± standard error.
Between-group comparisons: *p<0.05, **p<0.01.
Intra-group comparisons: #p<0.05, ##p<0.01.
Fig. 3.
Changes in the dermatology life quality index (DLQI) scores of the placebo and T21 groups (score change Δ).
Statistical analysis was performed using the subjects with a history of atopic dermatitis in themselves or in blood relatives. The “score changes Δ” are obtained by subtracting the score at the observation before ingestion from the score at 8 weeks after ingestion.
The Δ values are means ± standard error.
Between-group comparisons: *p<0.05.
intra-group comparisons: #p<0.05, ##p<0.01.
Safety assessment
There were 44 subjects for the adverse event analysis and 42 subjects for safety measurements and laboratory analyses who completed the prescribed schedule and study content. During the intervention period, adverse events, such as headache, myalgia, and corona virus desease 2019 (COVID-19) infection symptoms, were identified in 4 subjects (8 events) in the placebo group and 10 subjects (16 events) in the T21 group. These adverse events were determined by the principal investigator to be unrelated to the test foods, i.e., not a side effect of the test foods. In the measurements and laboratory values, significant changes were observed in several items before and after intake in each group, but the principal investigator considered all changes to be minor fluctuations within the reference range and not clinically problematic (Supplementary Table 1).
DISCUSSION
To investigate the effects of T21-containing foods on the perceived skin condition, skin measurements, and inflammation-related markers during 8 consecutive weeks of test food intake, a randomized, double-blind, placebo-controlled parallel-group study was conducted in healthy male and female subjects aged 20 to 64 years who had dry, itchy skin and a predisposition to atopy. The subjects were asked to take two tablets per day of L. lactis strain T21 (25 mg/tablet)-containing food or a placebo for 8 consecutive weeks. Skin measurements on 4 areas (cheeks, neck, upper arms, and shins) showed that the TEWL on the neck of the T21 group significantly decreased after 8 weeks of intake, and the amount of change in L* value, which indicates skin brightness, increased. These results suggest that the skin barrier function on the neck is improved and that the skin brightness increases with the intake of food containing the T21 strain. In the analysis of a population with a history of atopic dermatitis themselves or among their blood relatives, as they were considered to be prone to dry skin, the TEWL at the neck area and certain indicators of the DLQI in the T21 group were significantly improved after 8 weeks of intake. Furthermore, an increase in the amount of change in L* value on the neck was observed, while the a* value, which indicates redness, decreased in the cheeks. These results suggest that the skin barrier function improves, skin brightness increases, and redness (a* value), which is considered to indicate a rough skin condition, improves, thereby alleviating skin dryness and improving the roughness of the skin. Furthermore, the DLQI, which is a subjective evaluation, correlates well with dry skin conditions such as atopic dermatitis [19], and the relief of discomfort caused by dry skin was suggested.
In this study, 4 sites (cheek, neck, upper arm, and shin) were selected to evaluate the overall changes in the body due to intake; however, the overall analysis showed improvement only in the neck. Because the skin rash caused by atopic dermatitis tends to be characterized by an intense skin rash on the face, neck, chest, back, and other parts after puberty [16], it is considered that the effect of the T21 on the upper arms and shins could not be detected in the study. In the cheeks, external influences such as friction and ventilation caused by wearing masks to prevent COVID-19 infection may have contributed to noise. Intestinal bacteria are greatly involved in the immune response of living organisms and are associated with various diseases. Many studies have demonstrated their association with allergic diseases, such as atopic dermatitis, and the prevention of onset and treatment of allergic diseases have also been evaluated. A comparison of the intestinal bacteria in children with allergic diseases and healthy children showed a significant decrease in lactobacilli in children with allergic diseases [20]. Prevention of the onset of allergic diseases and treatment after onset by an intervention to address intestinal bacteria is also being studied [21]. Some reports suggest that the intestinal regulation effect of Lactobacillus intake improves skin function in humans [2]. Recently, it has been reported that intake of lactic acid bacteria improves skin function, including atopic dermatitis [22,23,24]. Th cells, which play important roles in the immune system, are classified into Th1 cells and Th2 cells according to the cytokines they produce: Th1 cells produce cytokines such as interferon (IFN)-γ, which are mainly related to cellular immunity, while Th2 cells produce cytokines such as IL-4, which are mainly related to humoral immunity. IL-4 produced by Th2 cells promotes IgE production from B cells, while IFN-γ produced by Th1 cells suppresses IgE production. Th1 and Th2 cells are antagonistic to each other, preserving a balance to maintain homeostasis of the immune system. However, when Th1 cells become dominant, autoimmune diseases occur; by contrast, when Th2 cells become dominant, IgE production increases and allergies develop [25]. In addition, in experiments using mice, the oral administration of lactic acid bacteria itself acts on the Peyer’s patches of the intestinal tract and induces the production of IL-12, which induces undifferentiated T cells to become Th1 cells. This triggers the production of IFN-γ, which consequently suppresses Th2 cells and IgE production and exhibits an antiallergic effect [26]. Other reports indicate that lactic acid bacteria suppress Th2 cytokines and increase anti-inflammatory cytokines [27] and that the strain T21 promotes IL-12 production [6]. In the evaluation of inflammation-related markers in this study, which was conducted to confirm the mechanism of action, an increase in TARC was observed in the T21 group compared with the placebo group, but the change was within the reference range for normal adult subjects. Moreover, no significant differences between groups were observed in other interrelated inflammation-related markers. It has been reported that intake of the T21 alleviates the symptoms of pollinosis and perennial rhinitis, lowers the amount of specific IgE in serum, and is involved in suppressing the increase in eosinophils [7]. Therefore, we anticipated a mechanism by which blood IgE levels and the percentage of eosinophils would change; however, we were unable to obtain definitive results demonstrating a correlation between them. On the other hand, a comparison of total IgE levels in the blood before and 8 weeks after intake revealed a decrease of 7.1 IU/mL in the placebo group and 52.8 IU/mL in the T21 group (Table 3). The lack of significant differences when compared with the placebo group is thought to be due to the large individual variations in baseline values (155.5 IU/mL in the placebo group and 401.9 IU/mL in the T21 group) and the broad range of variability. Based on the findings of this study, we aim to conduct a clinical trial to further elucidate the mechanism of action of T21 by selecting subjects with high total IgE concentrations in the blood and ensuring no initial differences between groups.
In the safety assessment, 16 adverse events occurred in 10 of the 22 subjects in the T21 group, and 8 adverse events occurred in 4 of the 22 subjects in the placebo group. However, all events were deemed by the principal investigator to be “not related” to the test foods. The principal investigator also concluded that the changes in clinical laboratory test results, physical measurements, and physical examinations before and after intake were not clinically problematic. Based on these results, the principal investigator determined that no safety issues were found for the food product containing T21 tested under these study conditions.
In this study, healthy adults with atopic predispositions were given food containing T21 for 8 weeks, and skin measurements were taken at 4 sites (cheek, neck, upper arm, and shin). The results showed improvements in the skin barrier function and increased brightness of the skin on the neck. Furthermore, in healthy adults with a history of atopic dermatitis themselves or among blood relatives, T21 improved rough skin and reduced discomfort caused by dryness. Additionally, ingestion of the T21-containing food was demonstrated to be safe.
CONFLICT OF INTEREST
Nissin Food Holdings Co., Ltd. provided the research funds and test foods (T21 and placebo) for this study, which was properly conducted by a third-party organization, EP Mediate Co., Ltd. Sumio Kondo at Fukushima Health Care Center was the principal investigator of this study; he has no conflicts of interest to disclose despite having performed commissioned work. There were no other conflicts of interest to be noted.
Supplementary Material
REFERENCES
- 1.Tanemono S, Sujino T, Kanai T. 2017. Relationship between intestinal flora and immunity. Jpn J Clin Immunol 40: 408–415. [Google Scholar]
- 2.Izawa Y, Noma T, Yamamoto M, Kimura K, Ito H, Taketomo N, Numano K, Kawashima M. 2008. Verification of the effect of yogurt using LB81 lactobacillus on skin function improvement. Chonai Saikingaku Zasshi 22: 1–5. [Google Scholar]
- 3.Yoshikawa K, Aota K, Takada Y, Nakamura T, Hoshino T, Yamashita S, Takara T. 2018. Effect of Lactobacillus brevis SBC8803 (SBL88TM lactobacillus) intake on improving sleep quality—a randomized, placebo-controlled, double-blind, parallel-group study. Jpn Pharmacol Ther 46: 1723–1738. [Google Scholar]
- 4.Nakagawa K, Koda T, Hamada K, Sugaya T, Saito T. 2020. Application of Lactobacillus B240 as a conditioning food for mucosal immune support and anti-infective effect. J Nutr Sci Vitaminol (Tokyo) 73: 55–60. [Google Scholar]
- 5.Suzuki H. 2015. Effects of consumption of a beverage containing Lactococcus lactis subsp. lactis JCM5805 on antiviral immune response and maintenance of physical condition—a placebo-controlled, randomized, double-blind, parallel-group comparison study—. Jpn Pharmacol Ther 43: 1465–1472. [Google Scholar]
- 6.Nissin Foods Holdings Co. Ltd. – Oki A, Sunada Y, Uehara K, Okada S, Tanaka N: New Lactobacillus, Patent No. 6345642, 2018-6-20.
- 7.Sunada Y. 2016. Anti-allergic action of Lactococcus lactis strain T-21, Keynote of the 2016 Annual Meeting of the Japan Society for Bioscience, Biotechnology, and Agrochemistry, p 1135, p 2369. [Google Scholar]
- 8.McAuliffe O. 2018. Symposium review: Lactococcus lactis from nondairy sources: their genetic and metabolic diversity and potential applications in cheese. J Dairy Sci 101: 3597–3610. [DOI] [PubMed] [Google Scholar]
- 9.Hayashida M, Kaneko T, Takano T, Eguchi R, Yanai K, Mizukami H, Zaitsu Y, Matsumoto K, Shimofuji T, Murayama N, et al. 2019. Improvement of atopic skin by MRE bacteria fermentation decomposition substance beverage. Med Consult New Rem 56: 117–126. [Google Scholar]
- 10.Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. 1996. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol 107: 707–713. [DOI] [PubMed] [Google Scholar]
- 11.Finlay AY, Khan GK. 1994. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi N, Suzukamo Y, Nakamura M, Miyachi Y, Green J, Ohya Y, Finlay AY, Fukuhara S, Acne QOL Questionnaire Development Team. 2006. Japanese version of the Dermatology Life Quality Index: validity and reliability in patients with acne. Health Qual Life Outcomes 4: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. 1998. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol 51: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 14.Fukuhara S, Ware JE, Jr, Kosinski M, Wada S, Gandek B. 1998. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J Clin Epidemiol 51: 1045–1053. [DOI] [PubMed] [Google Scholar]
- 15.Final report of the committee for the evaluation of antibiotic safety criteria, Japanese Society of Chemotherapy (confirmed version): antimicrobial safety evaluation criteria. 2010. Japanese J Chemother 58: 483–493. [Google Scholar]
- 16.Saeki H, Oya Y, Furuta J, Arakawa H, Ichikawa S, Katsunuma T, Kato M, Tanaka A, Tsunemi Y, Nakahara T, et al. 2021. Japanese J Dermatol 131: 2691–2777. [Google Scholar]
- 17.Caproni M, Torchia D, Antiga E, Terranova M, Volpi W, del Bianco E, D’Agata A, Fabbri P. 2007. The comparative effects of tacrolimus and hydrocortisone in adult atopic dermatitis: an immunohistochemical study. Br J Dermatol 156: 312–319. [DOI] [PubMed] [Google Scholar]
- 18.Simon D, Vassina E, Yousefi S, Kozlowski E, Braathen LR, Simon HU. 2004. Reduced dermal infiltration of cytokine-expressing inflammatory cells in atopic dermatitis after short-term topical tacrolimus treatment. J Allergy Clin Immunol 114: 887–895. [DOI] [PubMed] [Google Scholar]
- 19.Uchi H, Uchi K, Chishaki A, Furue M. 2012. Quality of life in atopic dermatitis—using DLQI and Skindex-16. Jpn J Pediatr Allergy Clin Immunol 26: 59–66. [Google Scholar]
- 20.Björkstén B, Naaber P, Sepp E, Mikelsaar M. 1999. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy 29: 342–346. [DOI] [PubMed] [Google Scholar]
- 21.Kim SO, Ah YM, Yu YM, Choi KH, Shin WG, Lee JY. 2014. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol 113: 217–226. [DOI] [PubMed] [Google Scholar]
- 22.Ai J, Ma W, Pan Z, Mao B, Tang X, Zhang Q, Zhao J, Chen W, Cui S. 2024. Ameliorative effect of Lactobacillus plantarum CCFM8661 on oleic acid-induced acne: integrated gut microbiota link to acne pathogenesis. J Sci Food Agric 104: 328–339. [DOI] [PubMed] [Google Scholar]
- 23.Colombo D, Rigoni C, Cantù A, Carnevali A, Filippetti R, Franco T, Grassi A, Loi C, Mazzotta A, Patroi I, et al. Young Dermatologists Italian Network2023. Probiotics and prebiotics orally assumed as disease modifiers for stable mild atopic dermatitis: an Italian real-life, multicenter, retrospective, observational study. Medicina (Kaunas) 59: 2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo YR, Kim HS. 2024. Interaction between the microbiota and the skin barrier in aging skin: a comprehensive review. Front Physiol 15: 1322205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimoto H, Mizumachi K, Okamoto T, Kurisaki J. 2004. New Lactococcus strain with immunomodulatory activity: enhancement of Th1-type immune response. Microbiol Immunol 48: 75–82. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida S, Ohata E, Masuda T, Okada S, Miyazaki Y, Yamashita T, Yasui H. 2010. Suppression of allergy by Lactobacillus plantarum FG4-4 in a mouse model of atopic dermatitis. Nihon Nyusankin Gakkaishi 21: 214–220. [Google Scholar]
- 27.Miyoshi M. 2010. Research on immunomodulatory function of lactic acid bacteria. Milk Science 69: 187–191. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.